Adrenal Insufficiency with Anticancer Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor Receptor: Analysis of the FDA Adverse Event Reporting System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Design
2.2. Definition of Cases and Drugs of Interest
2.3. Descriptive Analysis
2.4. Disproportionality Analysis
- (a)
- the frequentist reporting odds ratio (ROR), deemed statistically significant by the lower limit of the 95% of the Confidence Interval (CI) >1, with at least five cases, using the Bonferroni correction to reduce the likelihood of detecting spurious associations related to multiple testing [20].
- (b)
- the Bayesian Information Component (IC), deemed significant by the 95% credibility interval, IC025 > 0, which is more accurate in case of low number of cases [24].
2.5. Exploring the Underlying Pharmacological Basis
2.6. Global Assessment of the Evidence
3. Results
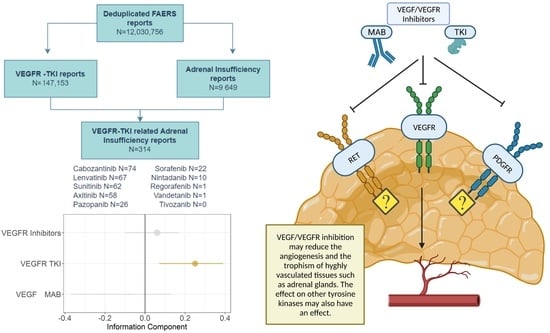
3.1. Descriptive Analysis
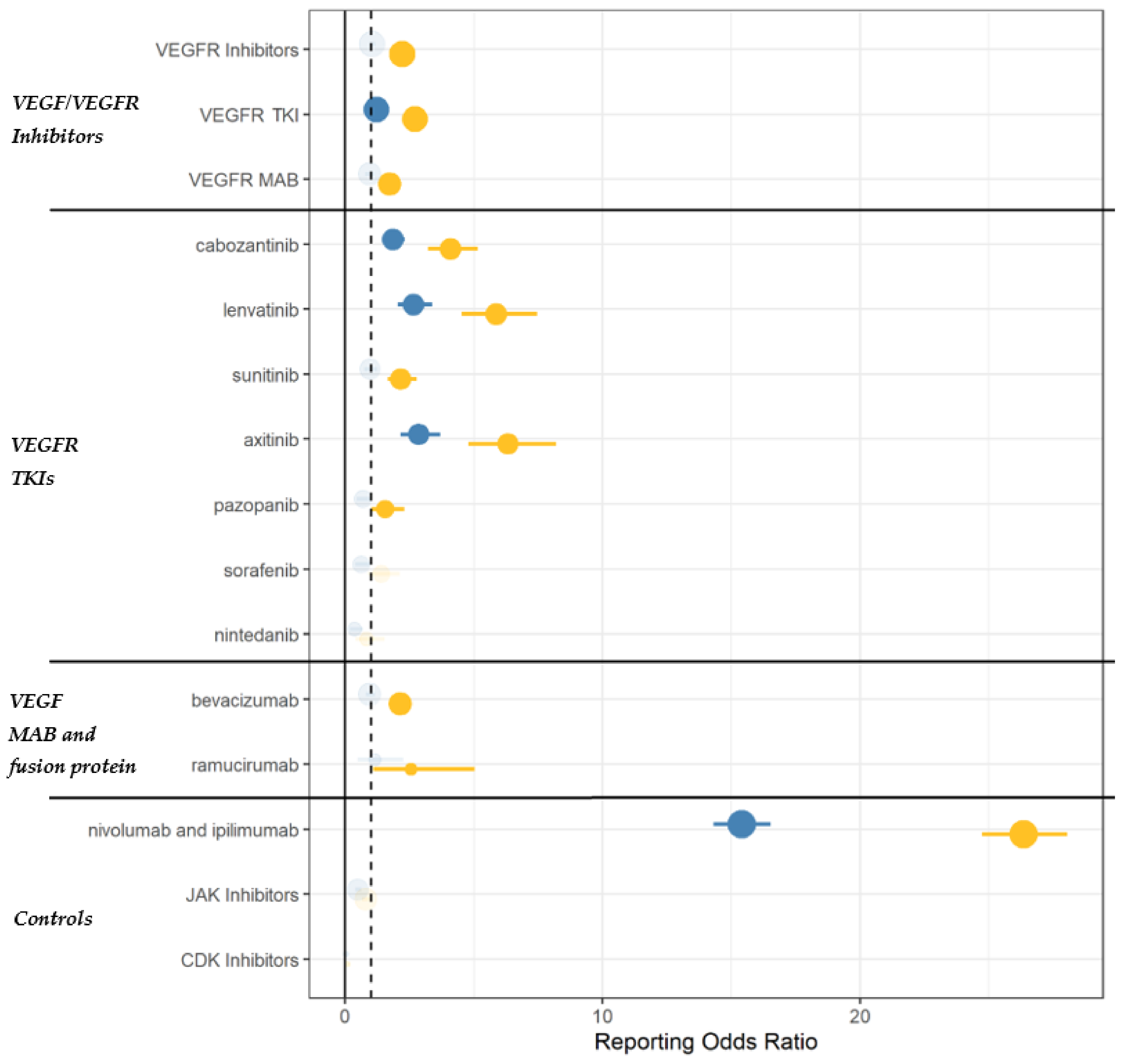
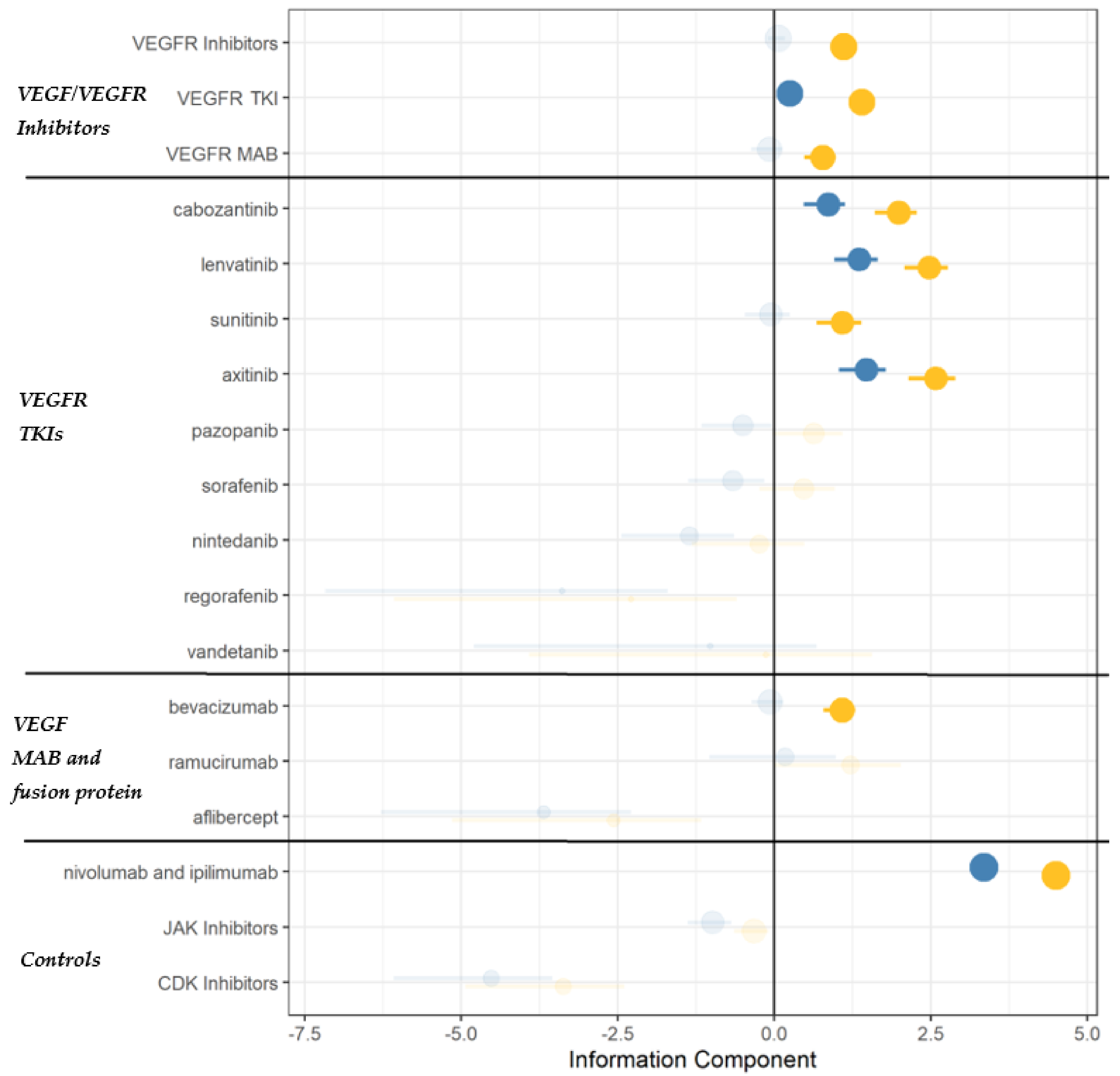
3.2. Disproportionality Analysis
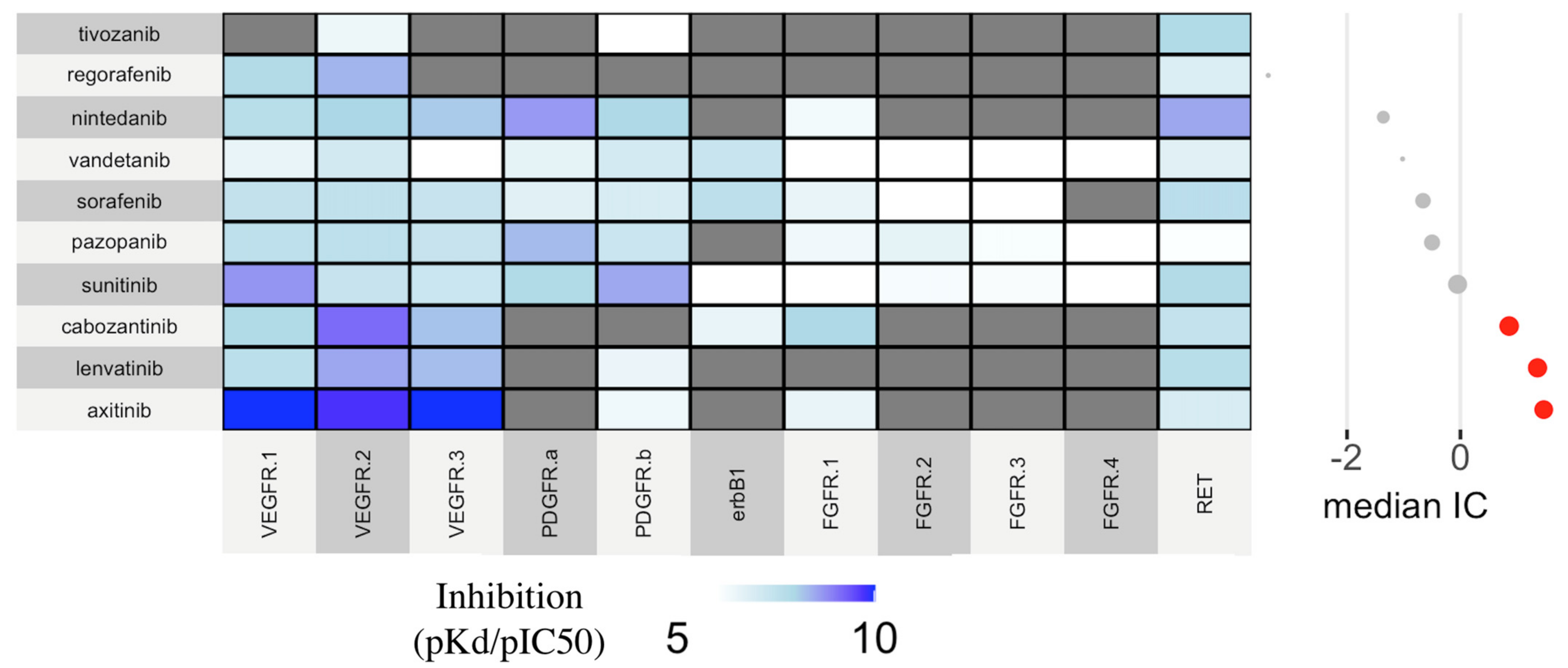
3.3. Global Assessment and Pharmacological Basis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrara, N.; Adamis, A.P. Ten Years of Anti-Vascular Endothelial Growth Factor Therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Levitzki, A. Tyrosine Kinase Inhibitors: Views of Selectivity, Sensitivity, and Clinical Performance. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 161–185. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. VEGF-Targeted Cancer Therapeutics-Paradoxical Effects in Endocrine Organs. Nat. Rev. Endocrinol. 2014, 10, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, A.; Senes, P.; Scoppola, A.; Chiefari, A.; Iannantuono, G.M.; Appetecchia, M.; Torino, F. Endocrine Toxicities of Antineoplastic Therapy: The Adrenal Topic. Cancers 2022, 14, 593. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Camastra, S.; Mazzi, V.; Miccoli, M.; Benvenga, S.; Antonelli, A. Therapy of endocrine Disease: Endocrine-Metabolic Effects of Treatment with Multikinase Inhibitors. Eur. J. Endocrinol. 2021, 184, R29–R40. [Google Scholar] [CrossRef] [PubMed]
- Smolenschi, C.; Tazdait, M.; Kuhn, E.; Boige, V. Bilateral Adrenal Haematoma Complicated by Adrenal Insufficiency in a Patient Treated with Bevacizumab. BMJ Case Rep. 2021, 14, e239689. [Google Scholar] [CrossRef]
- Voss, M.; Batarfi, A.; Steidl, E.; Wagner, M.; Forster, M.-T.; Steinbach, J.P.; Rödel, C.M.; Bojunga, J.; Ronellenfitsch, M.W. Adrenal Insufficiency in Patients with Corticosteroid-Refractory Cerebral Radiation Necrosis Treated with Bevacizumab. J. Clin. Med. 2019, 8, E1608. [Google Scholar] [CrossRef]
- Yoshino, T.; Kawai, K.; Miyazaki, J.; Kimura, T.; Ikeda, A.; Takaoka, E.; Suetomi, T.; Oikawa, T.; Kojima, T.; Iwasaki, H.; et al. A Case of Acute Adrenal Insufficiency Unmasked during Sunitinib Treatment for Metastatic Renal Cell Carcinoma. Jpn. J. Clin. Oncol. 2012, 42, 764–766. [Google Scholar] [CrossRef][Green Version]
- Javaid, A.; Mathai, J.; Song, D.; Brown, S. Sunitinib-Induced Adrenal Crisis in a Patient with Pre-Existing Immunotherapy-Related Hypopituitarism. Case Rep. Oncol. 2022, 15, 1–6. [Google Scholar] [CrossRef]
- Elshimy, G.; Gandhi, A.; Guo, R.; Correa, R. Tyrosine Kinase Inhibitors’ Newly Reported Endocrine Side Effect: Pazopanib-Induced Primary Adrenal Insufficiency in a Patient with Metastatic Renal Cell Cancer. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620936808. [Google Scholar] [CrossRef]
- Monti, S.; Presciuttini, F.; Deiana, M.G.; Motta, C.; Mori, F.; Renzelli, V.; Stigliano, A.; Toscano, V.; Pugliese, G.; Poggi, M. Cortisol Deficiency in Lenvatinib Treatment of Thyroid Cancer: An Underestimated Common Adverse Event. Thyroid Off. J. Am. Thyroid Assoc. 2022, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Brassard, M.; Neraud, B.; Trabado, S.; Salenave, S.; Brailly-Tabard, S.; Borget, I.; Baudin, E.; Leboulleux, S.; Chanson, P.; Schlumberger, M.; et al. Endocrine Effects of the Tyrosine Kinase Inhibitor Vandetanib in Patients Treated for Thyroid Cancer. J. Clin. Endocrinol. Metab. 2011, 96, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; De Leo, S.; Di Stefano, M.; Vannucchi, G.; Persani, L.; Fugazzola, L. Primary Adrenal Insufficiency During Lenvatinib or Vandetanib and Improvement of Fatigue After Cortisone Acetate Therapy. J. Clin. Endocrinol. Metab. 2019, 104, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Gatti, M.; Gelsomino, F.; Ardizzoni, A.; Poluzzi, E.; De Ponti, F. Lessons to Be Learnt from Real-World Studies on Immune-Related Adverse Events with Checkpoint Inhibitors: A Clinical Perspective from Pharmacovigilance. Target. Oncol. 2020, 15, 449–466. [Google Scholar] [CrossRef]
- Cornet, L.; Khouri, C.; Roustit, M.; Guignabert, C.; Chaumais, M.-C.; Humbert, M.; Revol, B.; Despas, F.; Montani, D.; Cracowski, J.-L. Pulmonary Arterial Hypertension Associated with Protein Kinase Inhibitors: A Pharmacovigilance-Pharmacodynamic Study. Eur. Respir. J. 2019, 53, 1802472. [Google Scholar] [CrossRef]
- Mahé, J.; de Campaigno, E.P.; Chené, A.-L.; Montastruc, J.-L.; Despas, F.; Jolliet, P. Pleural Adverse Drugs Reactions and Protein Kinase Inhibitors: Identification of Suspicious Targets by Disproportionality Analysis from VigiBase. Br. J. Clin. Pharmacol. 2018, 84, 2373–2383. [Google Scholar] [CrossRef]
- Patras de Campaigno, E.; Bondon-Guitton, E.; Laurent, G.; Montastruc, F.; Montastruc, J.-L.; Lapeyre-Mestre, M.; Despas, F. Identification of Cellular Targets Involved in Cardiac Failure Caused by PKI in Oncology: An Approach Combining Pharmacovigilance and Pharmacodynamics. Br. J. Clin. Pharmacol. 2017, 83, 1544–1555. [Google Scholar] [CrossRef]
- Goldman, A.; Bomze, D.; Dankner, R.; Fourey, D.; Boursi, B.; Arad, M.; Maor, E. Cardiovascular Toxicities of Antiangiogenic Tyrosine Kinase Inhibitors: A Retrospective, Pharmacovigilance Study. Target. Oncol. 2021, 16, 471–483. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Z.; Song, H. Thyroid Dysfunction Related to Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors: A Real-World Study Based on FAERS. J. Clin. Pharm. Ther. 2021, 46, 1418–1425. [Google Scholar] [CrossRef]
- Raschi, E.; Fusaroli, M.; Massari, F.; Mollica, V.; Repaci, A.; Ardizzoni, A.; Poluzzi, E.; Pagotto, U.; Di Dalmazi, G. The Changing Face of Drug-Induced Adrenal Insufficiency in the Food and Drug Administration Adverse Event Reporting System. J. Clin. Endocrinol. Metab. 2022, 107, dgac359. [Google Scholar] [CrossRef]
- Grouthier, V.; Lebrun-Vignes, B.; Moey, M.; Johnson, D.B.; Moslehi, J.J.; Salem, J.-E.; Bachelot, A. Immune Checkpoint Inhibitor-Associated Primary Adrenal Insufficiency: WHO VigiBase Report Analysis. Oncologist 2020, 25, 696–701. [Google Scholar] [CrossRef]
- Faillie, J.-L. Case-Non-Case Studies: Principle, Methods, Bias and Interpretation. Therapies 2019, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Poluzzi, E.; Salvo, F.; Pariente, A.; De Ponti, F.; Marchesini, G.; Moretti, U. Pharmacovigilance of Sodium-Glucose Co-Transporter-2 Inhibitors: What a Clinician Should Know on Disproportionality Analysis of Spontaneous Reporting Systems. Nutr. Metab. Cardiovasc. Dis. NMCD 2018, 28, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, R.; DuMouchel, W.; LePendu, P.; Bauer-Mehren, A.; Ryan, P.; Shah, N.H. Performance of Pharmacovigilance Signal-Detection Algorithms for the FDA Adverse Event Reporting System. Clin. Pharmacol. Ther. 2013, 93, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, M.; Raschi, E.; Giunchi, V.; Menchetti, M.; Rimondini Giorgini, R.; De Ponti, F.; Poluzzi, E. Impulse Control Disorders by Dopamine Partial Agonists: A Pharmacovigilance-Pharmacodynamic Assessment through the FDA Adverse Event Reporting System. Int. J. Neuropsychopharmacol. 2022, pyac031. [Google Scholar] [CrossRef]
- Raschi, E.; Fusaroli, M.; Ardizzoni, A.; Poluzzi, E.; De Ponti, F. Cyclin-Dependent Kinase 4/6 Inhibitors and Interstitial Lung Disease in the FDA Adverse Event Reporting System: A Pharmacovigilance Assessment. Breast Cancer Res. Treat. 2021, 186, 219–227. [Google Scholar] [CrossRef]
- Anderson, N.; Borlak, J. Correlation versus Causation? Pharmacovigilance of the Analgesic Flupirtine Exemplifies the Need for Refined Spontaneous ADR Reporting. PLoS ONE 2011, 6, e25221. [Google Scholar] [CrossRef]
- Huinen, Z.R.; Huijbers, E.J.M.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-Angiogenic Agents-Overcoming Tumour Endothelial Cell Anergy and Improving Immunotherapy Outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [CrossRef]
- Enokida, T.; Tahara, M. Management of VEGFR-Targeted TKI for Thyroid Cancer. Cancers 2021, 13, 5536. [Google Scholar] [CrossRef]
- Shu, Y.; Ding, Y.; Dai, B.; Zhang, Q. A Real-World Pharmacovigilance Study of Axitinib: Data Mining of the Public Version of FDA Adverse Event Reporting System. Expert Opin. Drug Saf. 2022, 21, 563–572. [Google Scholar] [CrossRef]
- Rathmell, W.K.; Rumble, R.B.; Van Veldhuizen, P.J.; Al-Ahmadie, H.; Emamekhoo, H.; Hauke, R.J.; Louie, A.V.; Milowsky, M.I.; Molina, A.M.; Rose, T.L.; et al. Management of Metastatic Clear Cell Renal Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, JCO2200868. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.; Mortazavi, A.; Cordes, L.; Salabao, C.; Vandlik, S.; Apolo, A.B. Management of Adverse Events Associated with Cabozantinib plus Nivolumab in Renal Cell Carcinoma: A Review. Cancer Treat. Rev. 2022, 103, 102333. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, W.; Sun, X.; Zhao, Z.; Liu, L. Anti-Angiogenic Agent-Associated Adrenal Insufficiency in Immune Checkpoint Inhibitors-Treated Patients. Eur. J. Cancer Oxf. Engl. 1990 2021, 157, 358–360. [Google Scholar] [CrossRef]
- Puliani, G.; Appetecchia, M. Endocrine Toxicities of Antineoplastic Therapy. Cancers 2021, 13, 294. [Google Scholar] [CrossRef]
- Fogli, S.; Porta, C.; Del Re, M.; Crucitta, S.; Gianfilippo, G.; Danesi, R.; Rini, B.I.; Schmidinger, M. Optimizing Treatment of Renal Cell Carcinoma with VEGFR-TKIs: A Comparison of Clinical Pharmacology and Drug-Drug Interactions of Anti-Angiogenic Drugs. Cancer Treat. Rev. 2020, 84, 101966. [Google Scholar] [CrossRef]
| AI (Cases) (314) | Other Reports (Non-Cases) (146,839) | p-Value | |||
|---|---|---|---|---|---|
| Demographic Features | N | % | N | % | |
| Sex | 0.687 | ||||
| Female | 107 | 37.4 | 48,813 | 37.8 | |
| Male | 179 | 62.6 | 87,591 | 64.2 | |
| Missing | 28 | (-) 1 | 10,435 | (-) 1 | |
| Reporter Country | 0.003 | ||||
| North America | 122 | 38.9 | 87,023 | 59.3 | |
| Europe | 72 | 22.9 | 24,194 | 16.5 | |
| Asia | 106 | 33.8 | 27,499 | 18.8 | |
| South America | 10 | 3.2 | 6151 | 4.2 | |
| Oceania | 4 | 1.3 | 1282 | 0.9 | |
| Africa | 0 | - | 535 | 0.4 | |
| Missing | 0 | (-) 1 | 155 | (-) 1 | |
| Reporter Qualification | 0.003 | ||||
| Consumer | 42 | 13.9 | 58,546 | 41.3 | |
| Healthcare professionals | 33 | 10.9 | 7098 | 5.0 | |
| Lawyer | 0 | - | 12 | <0.1 | |
| Medical doctor | 175 | 57.8 | 47,730 | 33.6 | |
| Other | 41 | 13.5 | 18,172 | 12.8 | |
| Pharmacists | 12 | 4.0 | 10,368 | 7.3 | |
| Missing | 11 | (-) 1 | 4913 | (-) 1 | |
| Age | 0.687 | ||||
| Median years [IQR] 2 | 66 [60–73] | 66 [58–73] | |||
| Missing | 71 | 40,354 | |||
| Reporting Year | 0.003 | ||||
| ≤2015 | 35 | 13.3 | 31,721 | 26.8 | |
| 2016 | 11 | 4.2 | 10,520 | 8.9 | |
| 2017 | 18 | 6.8 | 12,654 | 10.7 | |
| 2018 | 31 | 11.8 | 15,394 | 13.0 | |
| 2019 | 43 | 16.4 | 15,694 | 13.3 | |
| 2020 | 54 | 20.5 | 15,209 | 12.9 | |
| 2021 | 71 | 27.0 | 16,951 | 14.3 | |
| Missing | 51 | 28,666 | |||
| AI (Cases) (314) | Other Reports (Non-Cases) (146,839) | p-Value | |||
|---|---|---|---|---|---|
| Clinical Features | |||||
| Outcome | 0.003 | ||||
| Death | 41 | 13.1 | 31,973 | 21.8 | |
| Life-threatening | 27 | 8.6 | 3376 | 2.3 | |
| Disability | 6 | 1.9 | 1412 | 1.0 | |
| Required intervention | 1 | 0.3 | 87 | 0.1 | |
| Hospitalization | 141 | 44.9 | 39,050 | 26.6 | |
| Congenital anomaly | 0 | 0 | 9 | <0.1 | |
| Other Serious | 89 | 28.3 | 30,572 | 20.8 | |
| Non serious | 9 | 2.9 | 40,360 | 27.5 | |
| Time to onset | 0.007 | ||||
| Median days [IQR] 1 | 72 [14–201] | 37 [9–139] | |||
| Missing | 125 | 74,543 | |||
| Co-reported events | <0.001 | ||||
| Median N. [IQR] 1 | 4 [2–8] | 2 [1–5] | |||
| Concomitant drugs | <0.001 | ||||
| Median N. [IQR] 1 | 5 [2–12] | 2 [1–6] | |||
| Concomitant Immunotherapy | 134 | 42.7 | 8795 | 6.0 | <0.001 |
| Criteria | Description | Source/Method |
|---|---|---|
| Strength of the association | Although ROR and IC are not measures of risk, the strength of the disproportionality both in primary (vs. all other drugs) and secondary (vs. other anticancer drugs) analyses suggests a robust signal (the impact of unmeasurable confounders is likely to be negligible) | Disproportionality |
| Analogy | The association was also demonstrated for other anticancer drugs used as a positive control (immune checkpoint inhibitors) | Disproportionality and Literature |
| Biological plausibility/empirical evidence | The interpolation between disproportionality results and pharmacodynamic data (affinity for VEGFR) supports the mechanistic basis | Disproportionality and Pharmacodynamics |
| Consistency | Results of disproportionality approaches were consistent in sensitivity analyses | Disproportionality |
| Coherence | Case reports/series have been published recently, suggesting a potential association. A recent pharmacovigilance study on axitinib found a disproportionality signal for AI | Literature |
| Exclusion of biases/confounders 1 | The association (statistically significant disproportionality) persisted across sensitivity analysis accounting for confounding by indication and potential co-reported bias | Disproportionality |
| Specificity | Pharmacovigilance data suggest TKIs to carry a stronger association as compared to mAbs. Moreover, a drug-specific effect (rather than a class-effect) cannot be excluded | Disproportionality |
| Temporal relationship | Notwithstanding missing data, the time to onset (delay from the first administration of the drug to the date the event was recorded) is coherent with biological and clinical notions. | Descriptive |
| Reversibility | Data on discontinuation and dechallenge support the reversibility | Descriptive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raschi, E.; Fusaroli, M.; Giunchi, V.; Repaci, A.; Pelusi, C.; Mollica, V.; Massari, F.; Ardizzoni, A.; Poluzzi, E.; Pagotto, U.; et al. Adrenal Insufficiency with Anticancer Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor Receptor: Analysis of the FDA Adverse Event Reporting System. Cancers 2022, 14, 4610. https://doi.org/10.3390/cancers14194610
Raschi E, Fusaroli M, Giunchi V, Repaci A, Pelusi C, Mollica V, Massari F, Ardizzoni A, Poluzzi E, Pagotto U, et al. Adrenal Insufficiency with Anticancer Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor Receptor: Analysis of the FDA Adverse Event Reporting System. Cancers. 2022; 14(19):4610. https://doi.org/10.3390/cancers14194610
Chicago/Turabian StyleRaschi, Emanuel, Michele Fusaroli, Valentina Giunchi, Andrea Repaci, Carla Pelusi, Veronica Mollica, Francesco Massari, Andrea Ardizzoni, Elisabetta Poluzzi, Uberto Pagotto, and et al. 2022. "Adrenal Insufficiency with Anticancer Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor Receptor: Analysis of the FDA Adverse Event Reporting System" Cancers 14, no. 19: 4610. https://doi.org/10.3390/cancers14194610
APA StyleRaschi, E., Fusaroli, M., Giunchi, V., Repaci, A., Pelusi, C., Mollica, V., Massari, F., Ardizzoni, A., Poluzzi, E., Pagotto, U., & Di Dalmazi, G. (2022). Adrenal Insufficiency with Anticancer Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor Receptor: Analysis of the FDA Adverse Event Reporting System. Cancers, 14(19), 4610. https://doi.org/10.3390/cancers14194610