Pan-Cancer Analysis and Experimental Validation Identify ACOT7 as a Novel Oncogene and Potential Therapeutic Target in Lung Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
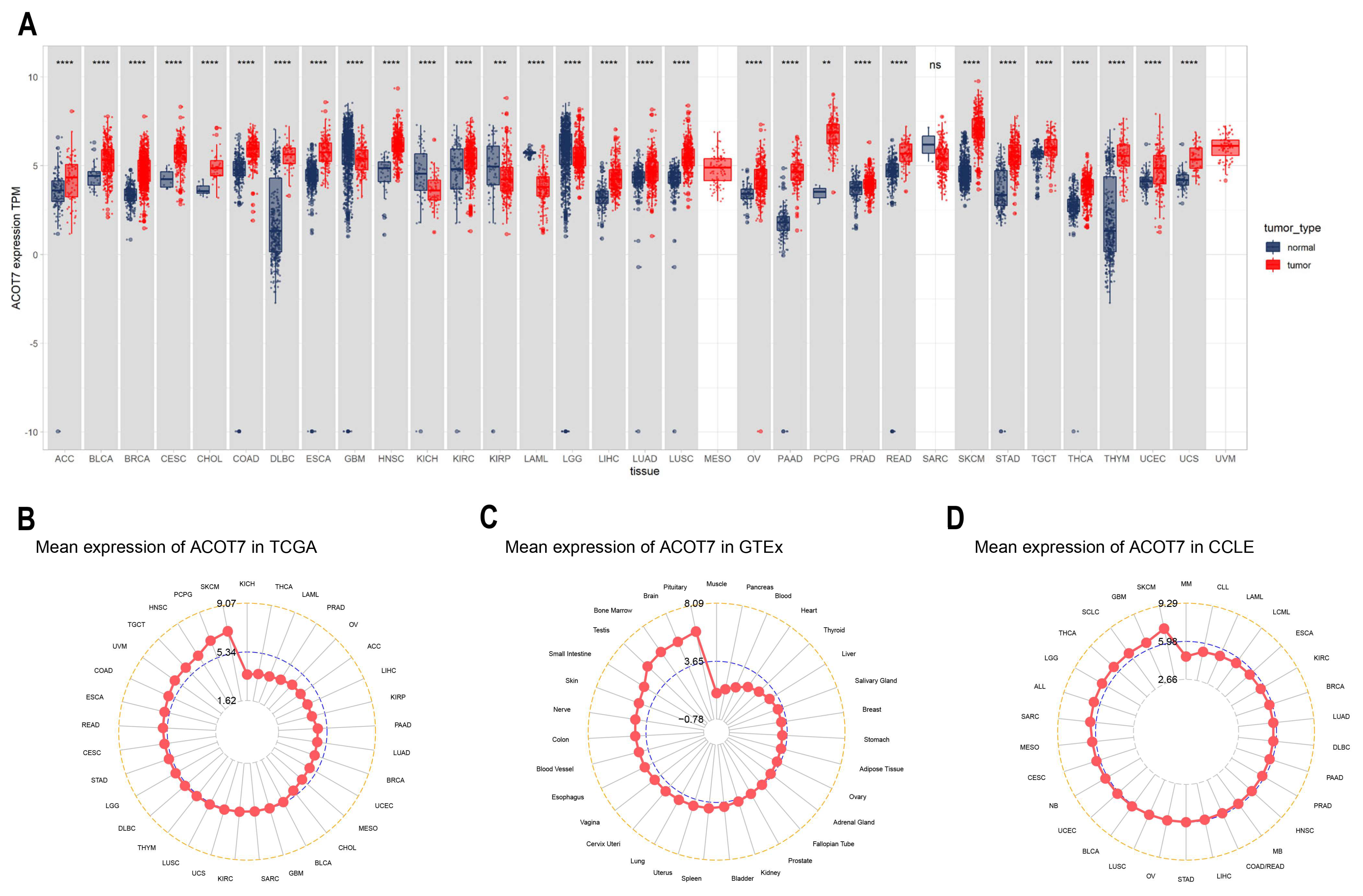
2.1. Differential Expression of ACOT7 in Pan-Cancer
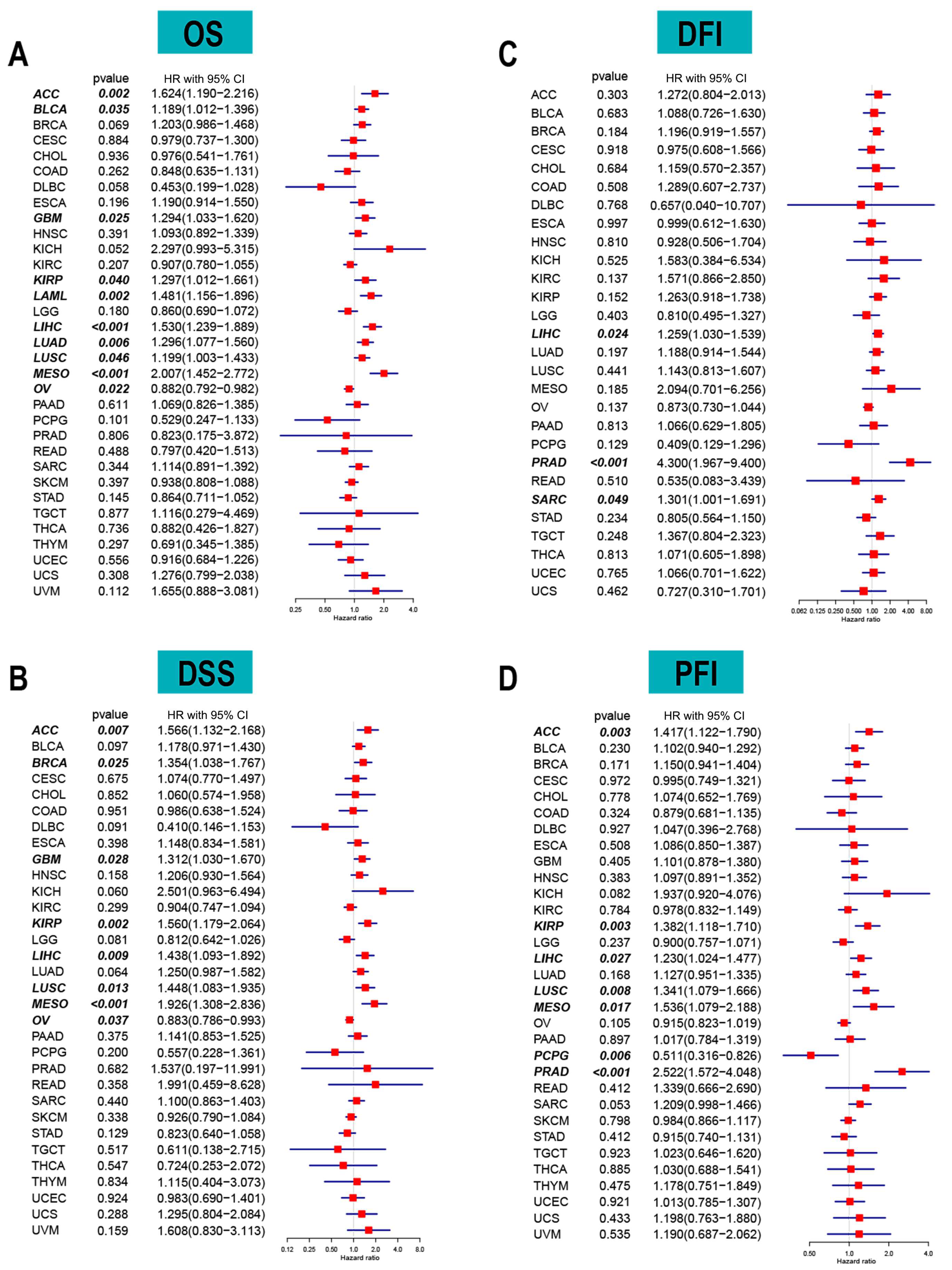
2.2. ACOT7 and Prognosis Analyses in Pan-Cancer
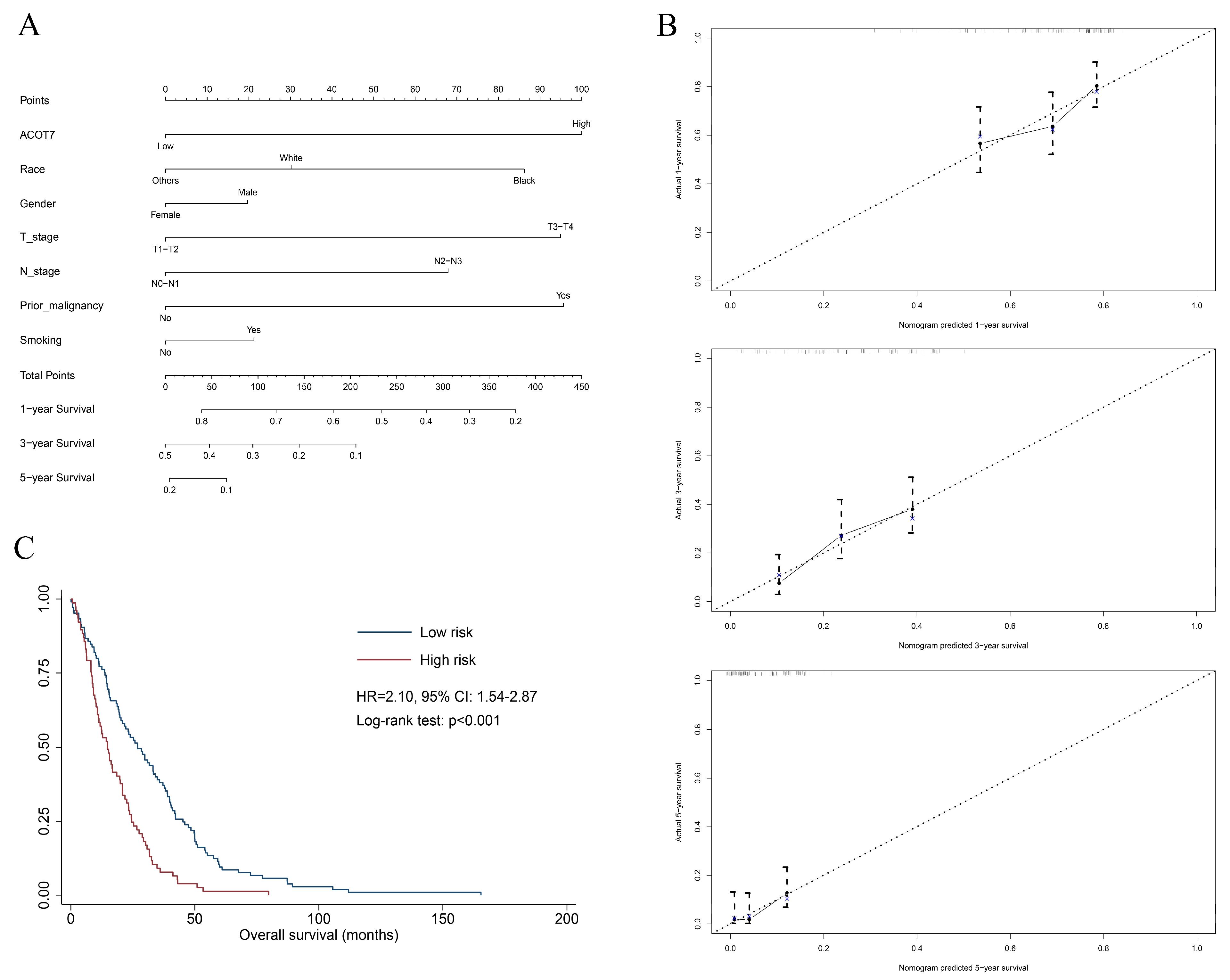
2.3. Construction of Nomogram in LUAD
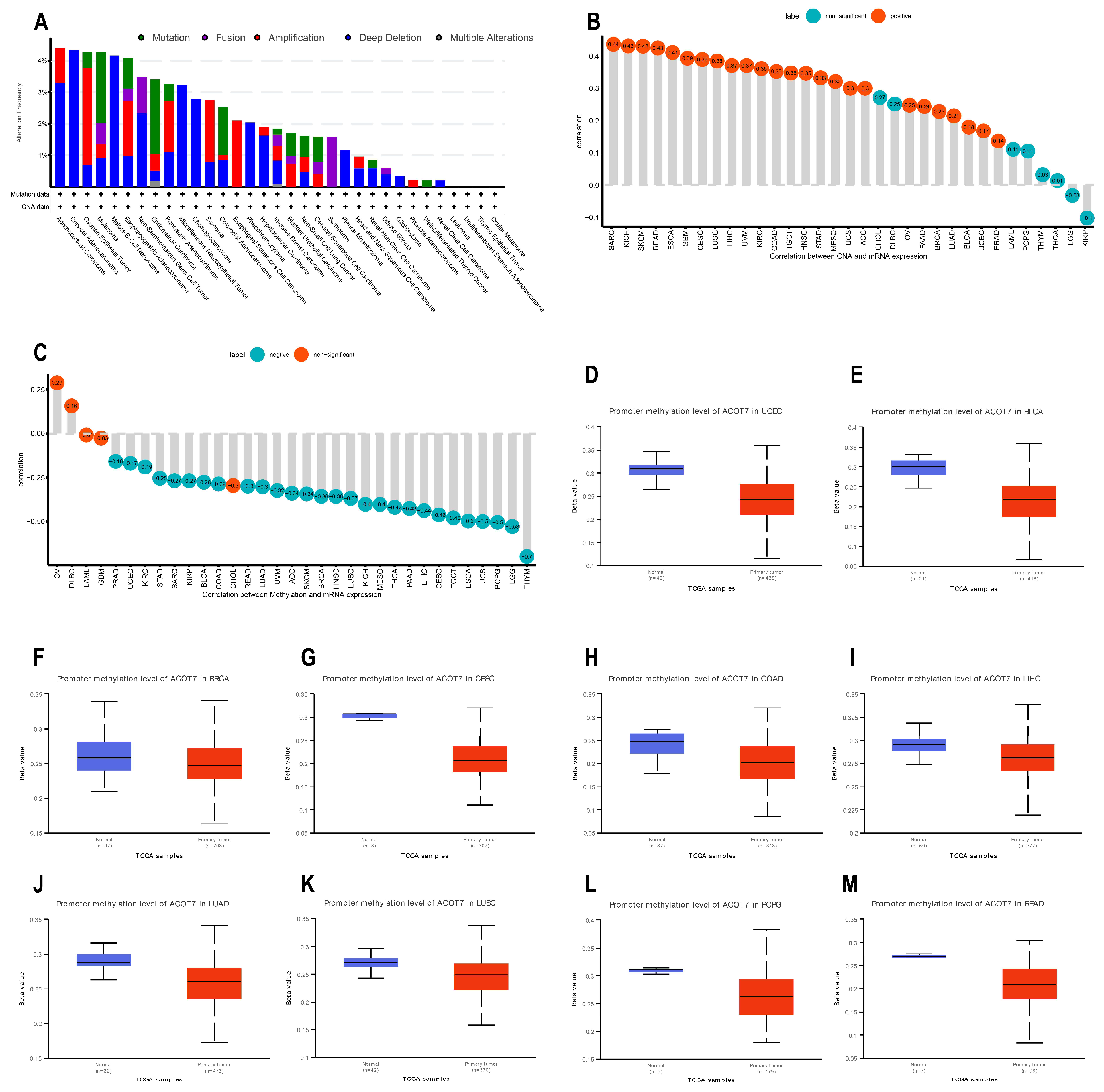
2.4. Genetic Alteration Analysis of ACOT7
2.5. Correlation between ACOT7 Expression and TME
2.6. Drug Screening of ACOT7
2.7. Cell Culture and siRNAs Transfection
2.8. RNA Extraction
2.9. Cell Proliferation and Migration/Invasion Assays
2.10. Statistical Analysis
3. Results
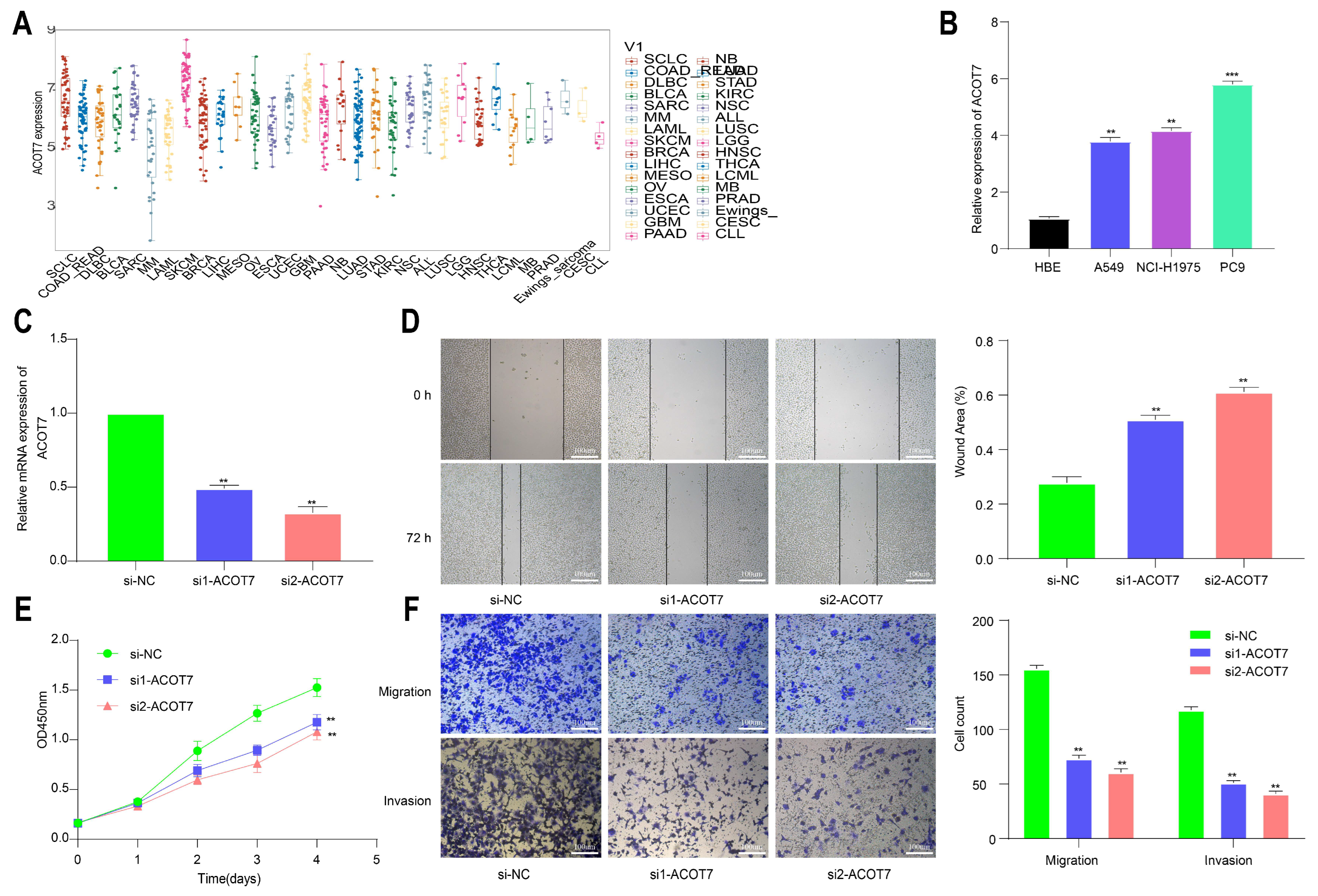
3.1. Expression Levels of ACOT7 in Pan-Cancer
3.2. ACOT7 as a Prognostic Indicator in Cancers
3.3. Genetic Alteration Analyses of ACOT7
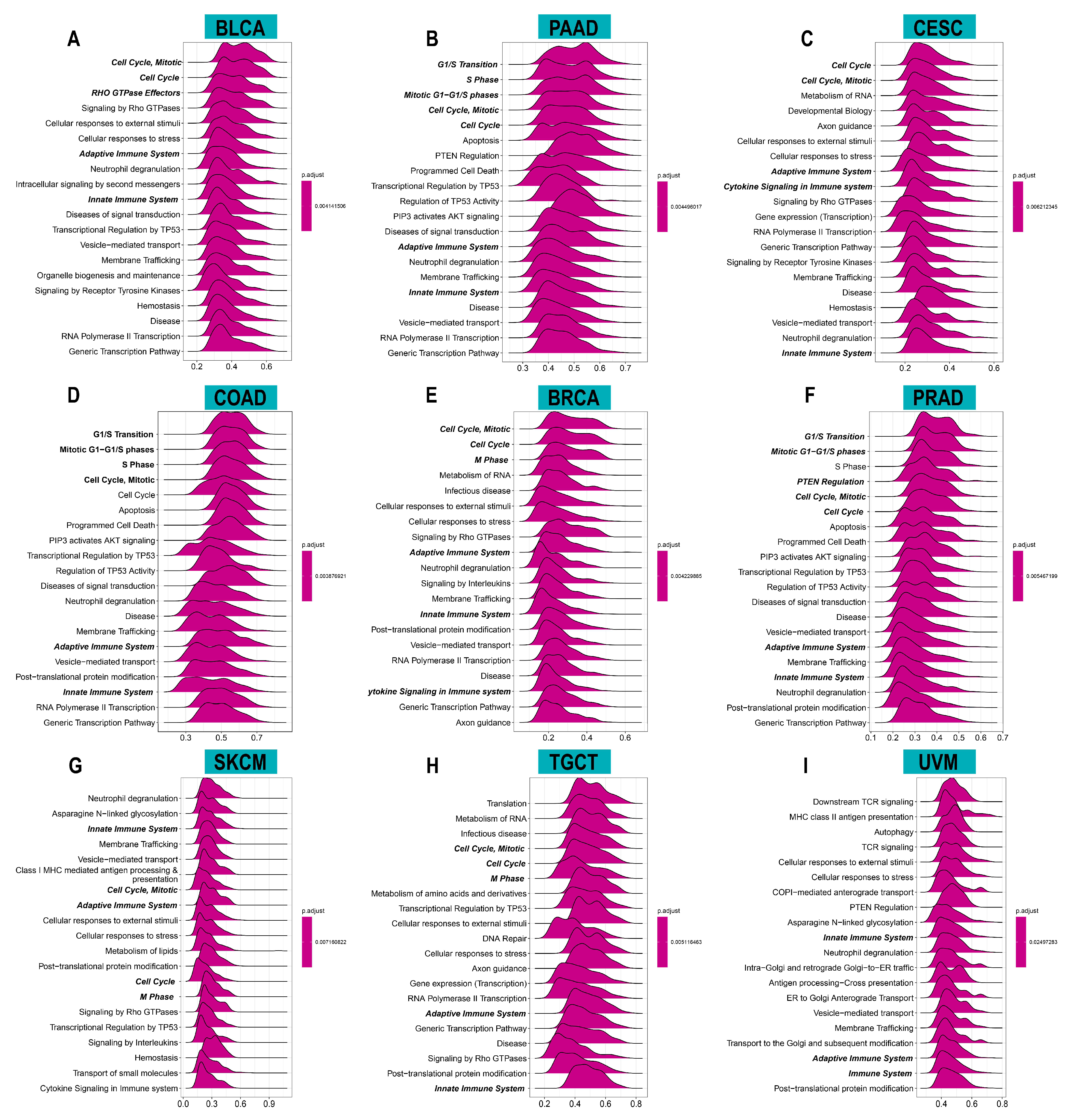
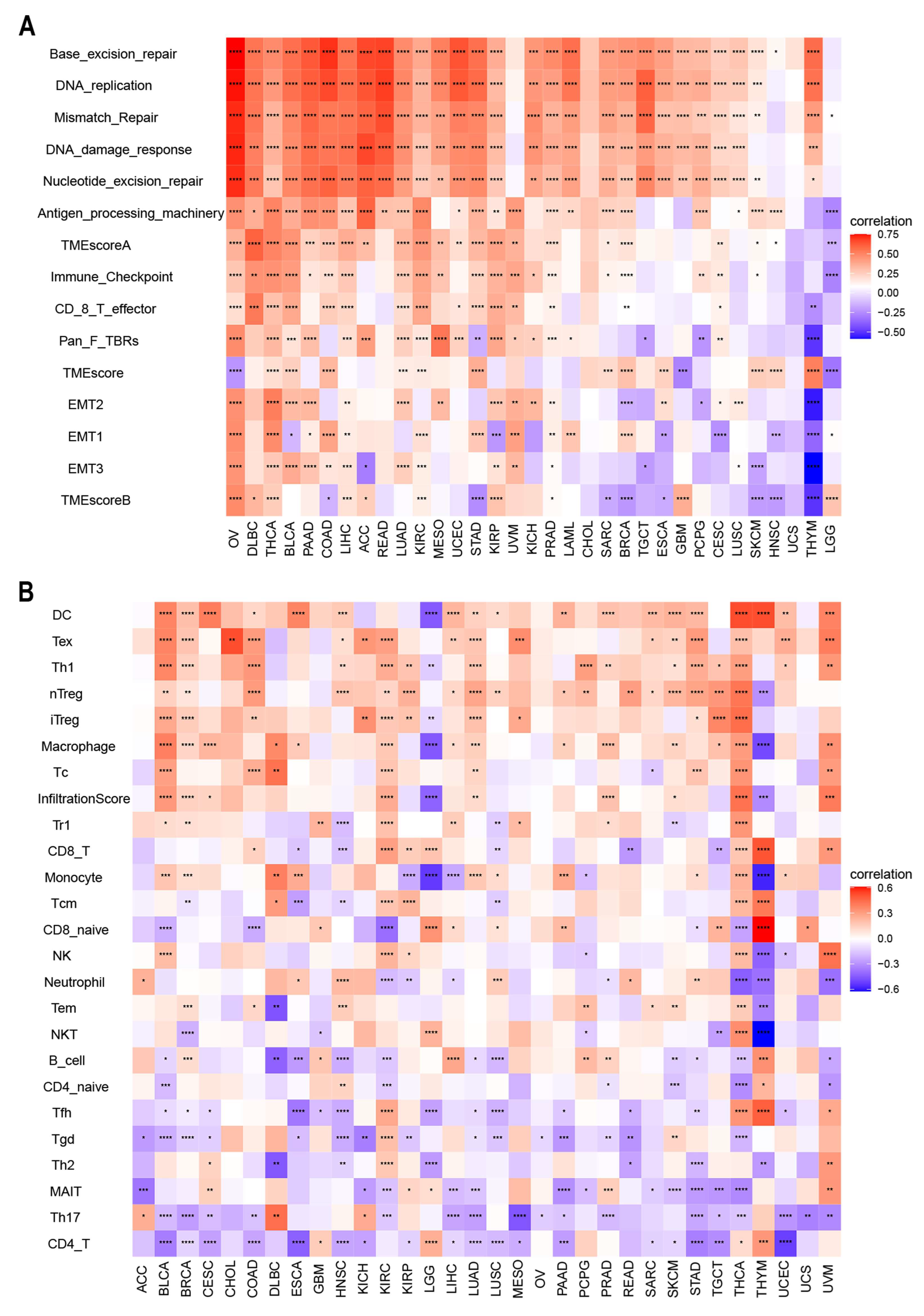
3.4. Relationship between ACOT7 Expression and TME
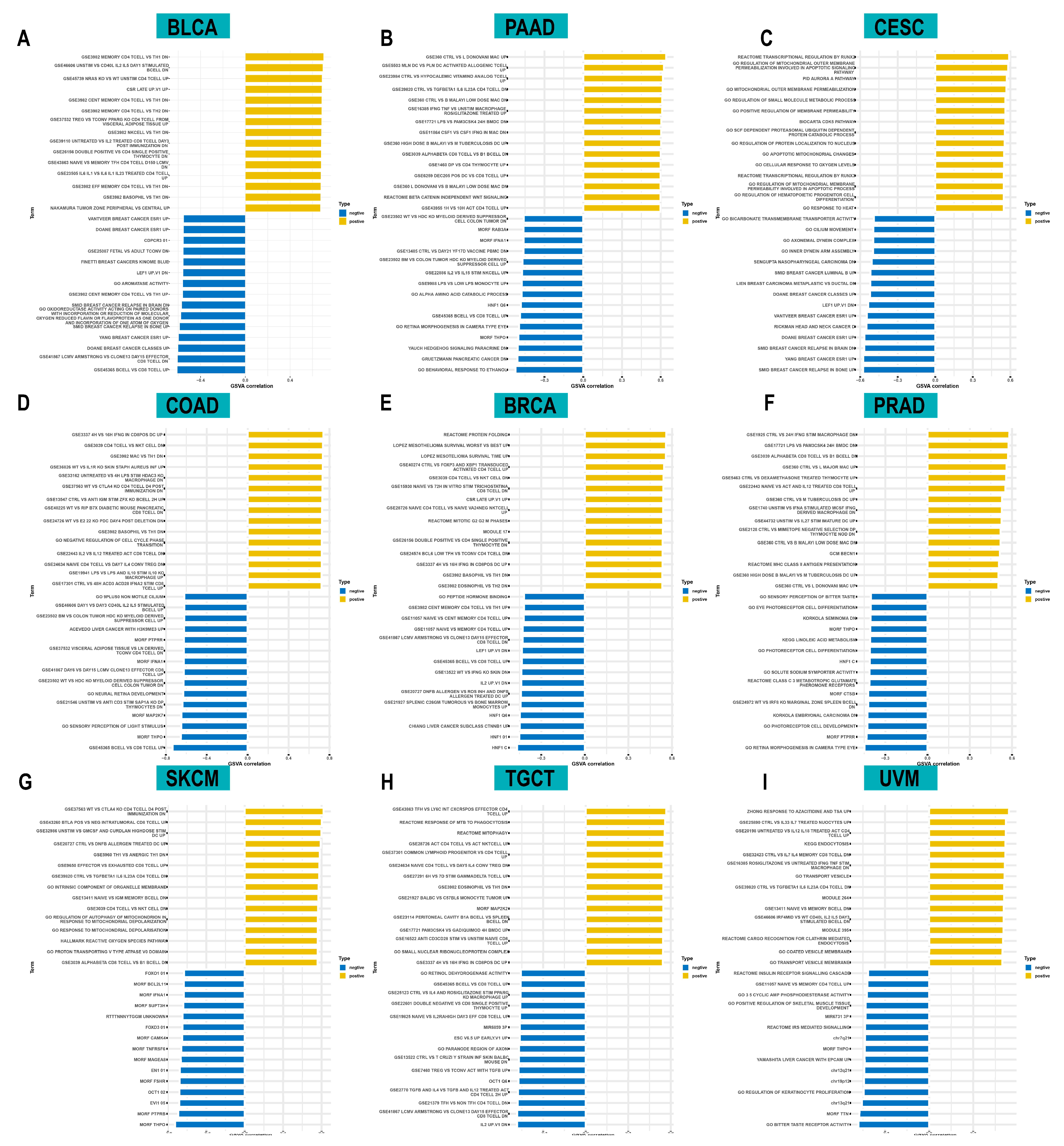
3.5. Drug Screening of ACOT7 in the GDSC Database
3.6. Knockdown of ACOT7 Inhibits Proliferation and Progression of Lung Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forwood, J.; Thakur, A.; Guncar, G.; Marfori, M.; Mouradov, D.; Meng, W.; Robinson, J.; Huber, T.; Kellie, S.; Martin, J.; et al. Structural basis for recruitment of tandem hotdog domains in acyl-CoA thioesterase 7 and its role in inflammation. Proc. Natl. Acad. Sci. USA 2007, 104, 10382–10387. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; Greene, S.; Hultenby, K.; Svensson, L.; Engberg, S.; Alexson, S. Alternative exon usage selectively determines both tissue distribution and subcellular localization of the acyl-CoA thioesterase 7 gene products. Cell. Mol. Life Sci. CMLS 2007, 64, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J. Long-chain acyl-CoA hydrolase in the brain. Amino Acids 2005, 28, 273–278. [Google Scholar] [CrossRef]
- Sakuma, S.; Fujimoto, Y.; Sawada, T.; Saeki, K.; Akimoto, M.; Fujita, T. Existence of acyl-CoA hydrolase-mediated pathway supplying arachidonic acid for prostaglandin synthesis in microsomes from rabbit kidney medulla. Prostaglandins Other Lipid Mediat. 1999, 57, 63–72. [Google Scholar] [CrossRef]
- Jenkins, C.; Cedars, A.; Gross, R. Eicosanoid signalling pathways in the heart. Cardiovasc. Res. 2009, 82, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, H.; Hwang, H.; Park, H.; Moon, Y.; Kim, B.; Lee, H.; Kim, K.; Kim, Y.; Lee, B.; et al. Acyl-CoA thioesterase 7 is involved in cell cycle progression via regulation of PKCζ-p53-p21 signaling pathway. Cell Death Dis. 2017, 8, e2793. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Farkona, S.; Diamandis, E.; Blasutig, I. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Wang, P.; Zenklusen, J. SnapShot: TCGA-Analyzed Tumors. Cell 2018, 173, 530. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Jurasz, P. The role of platelets in the tumor microenvironment: From solid tumors to leukemia. Biochim. Biophys. Acta 2016, 1863, 392–400. [Google Scholar] [CrossRef]
- Szkandera, J.; Gerger, A.; Liegl-Atzwanger, B.; Absenger, G.; Stotz, M.; Friesenbichler, J.; Trajanoski, S.; Stojakovic, T.; Eberhard, K.; Leithner, A.; et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int. J. Cancer 2014, 135, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (Amst. Neth.) 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L. Classification of Advanced Human Cancers Based on Tumor Immunity in the MicroEnvironment (TIME) for Cancer Immunotherapy. JAMA Oncol. 2016, 2, 1403–1404. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Gosney, J.; Skov, B.; Adam, J.; Motoi, N.; Bloom, K.; Dietel, M.; Longshore, J.; López-Ríos, F.; Penault-Llorca, F.; et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin. Oncol. 2015, 42, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Maute, R.; Dulken, B.; Hutter, G.; George, B.; McCracken, M.; Gupta, R.; Tsai, J.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.; Drake, C.; Pardoll, D. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Jin, Z.; Yang, S.; Xu, Y.; Zhu, Y.; Ji, Y. TCGA-assembler 2: Software pipeline for retrieval and processing of TCGA/CPTAC data. Bioinformatics (Oxf. Engl.) 2018, 34, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [CrossRef] [PubMed]
- Goldman, M.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Thul, P.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. A Publ. Protein Soc. 2018, 27, 233–244. [Google Scholar] [CrossRef]
- Collisson, E.A.; Campbell, J.D.; Brooks, A.N.; Berger, A.H.; Lee, W.; Chmielecki, J.; Beer, D.G.; Cope, L.; Creighton, C.J.; Danilova, L.; et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Parsons, A.; Daley, A.; Begh, R.; Aveyard, P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 2010, 340, b5569. [Google Scholar] [CrossRef]
- Saeed, A.M.; Toonkel, R.; Glassberg, M.K.; Nguyen, D.; Hu, J.J.; Zimmers, T.A.; Robbins, D.J.; Koniaris, L.G.; Lally, B.E. The influence of Hispanic ethnicity on nonsmall cell lung cancer histology and patient survival: An analysis of the Survival, Epidemiology, and End Results database. Cancer 2012, 118, 4495–4501. [Google Scholar] [CrossRef]
- Tong, B.C.; Kosinski, A.S.; Burfeind, W.R., Jr.; Onaitis, M.W.; Berry, M.F.; Harpole, D.H., Jr.; D’Amico, T.A. Sex differences in early outcomes after lung cancer resection: Analysis of the Society of Thoracic Surgeons General Thoracic Database. J. Thorac. Cardiovasc. Surg. 2014, 148, 13–18. [Google Scholar] [CrossRef]
- Bryant, A.S.; Cerfolio, R.J. Impact of race on outcomes of patients with non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 711–715. [Google Scholar] [CrossRef]
- Fu, J.B.; Kau, T.Y.; Severson, R.K.; Kalemkerian, G.P. Lung cancer in women: Analysis of the national Surveillance, Epidemiology, and End Results database. Chest 2005, 127, 768–777. [Google Scholar] [CrossRef]
- Camp, R.; Dolled-Filhart, M.; Rimm, D. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, l1. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Newman, A.; Steen, C.; Liu, C.; Gentles, A.; Chaudhuri, A.; Scherer, F.; Khodadoust, M.; Esfahani, M.; Luca, B.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Ru, B.; Wong, C.; Tong, Y.; Zhong, J.; Zhong, S.; Wu, W.; Chu, K.; Wong, C.; Lau, C.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics (Oxf. Engl.) 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Soares, J.; Greninger, P.; Edelman, E.; Lightfoot, H.; Forbes, S.; Bindal, N.; Beare, D.; Smith, J.; Thompson, I.; et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013, 41, D955–D961. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Edelman, E.J.; Heidorn, S.J.; Greenman, C.D.; Dastur, A.; Lau, K.W.; Greninger, P.; Thompson, I.R.; Luo, X.; Soares, J.; et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012, 483, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego Calif.) 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, D.; Esteve-Codina, A.; Piferrer, F. Consistent inverse correlation between DNA methylation of the first intron and gene expression across tissues and species. Epigenetics Chromatin 2018, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, Y.; Li, J.; Du, W.; Li, R.; Yang, J.; Li, J.; Li, F.; Tan, H. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Charoentong, P.; Finotello, F.; Angelova, M.; Mayer, C.; Efremova, M.; Rieder, D.; Hackl, H.; Trajanoski, Z. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017, 18, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Vaddepally, R.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [PubMed]
- Schaub, F.; Dhankani, V.; Berger, A.; Trivedi, M.; Richardson, A.; Shaw, R.; Zhao, W.; Zhang, X.; Ventura, A.; Liu, Y.; et al. Pan-cancer Alterations of the MYC Oncogene and Its Proximal Network across the Cancer Genome Atlas. Cell Syst. 2018, 6, 282–300.e282. [Google Scholar] [CrossRef]
- Bailey, M.; Tokheim, C.; Porta-Pardo, E.; Sengupta, S.; Bertrand, D.; Weerasinghe, A.; Colaprico, A.; Wendl, M.; Kim, J.; Reardon, B.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 173, 371–385.e318. [Google Scholar] [CrossRef] [PubMed]
- Schlomm, T. [Results of the CGC/TCGA Pan-Cancer Analysis of the Whole Genomes (PCAWG) Consortium]. Der Urologe. Ausg. A 2020, 59, 1552–1553. [Google Scholar] [CrossRef]
- Calabrese, C.; Davidson, N.; Demircioğlu, D.; Fonseca, N.; He, Y.; Kahles, A.; Lehmann, K.; Liu, F.; Shiraishi, Y.; Soulette, C.; et al. Genomic basis for RNA alterations in cancer. Nature 2020, 578, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Czech, T.; Yamada, J.; Csaszar, E.; Baumgartner, C.; Slavc, I.; Lubec, G. Aberrant cytosolic acyl-CoA thioester hydrolase in hippocampus of patients with mesial temporal lobe epilepsy. Amino Acids 2004, 27, 269–275. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Feng, S.; Zuo, S.; Zhao, X.; Li, H. Acyl-CoA Thioesterase 7 is Transcriptionally Activated by Krüppel-Like Factor 13 and Promotes the Progression of Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, X. Interaction between ACOT7 and LncRNA NMRAL2P via Methylation Regulates Gastric Cancer Progression. Yonsei Med. J. 2020, 61, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.; Lee, C.; Shoushtari, A.; Hellmann, M.; Shen, R.; Janjigian, Y.; Barron, D.; Zehir, A.; Jordan, E.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.; de Sauvage, F. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.; Iida, N.; Roberts, E.; Sangaletti, S.; Wong, M.; Yull, F.; Coussens, L.; DeClerck, Y. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Powell, J.D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Baudoin, N.C.; Bloomfield, M. Karyotype Aberrations in Action: The Evolution of Cancer Genomes and the Tumor Microenvironment. Genes 2021, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.R.; Danai, L.V.; Lewis, C.A.; Chan, S.H.; Gui, D.Y.; Kunchok, T.; Dennstedt, E.A.; Vander Heiden, M.G.; Muir, A. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Wu, W.; Wang, Y.; Alexander, P.B.; Sun, C.; Gong, Z.; Cheng, J.N.; Sun, H.; Guan, Y.; Xia, X.; et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat. Commun. 2018, 9, 5361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhen, H.; Zhao, H.; Zhao, L.; Cao, B. Efficacy and safety of adjuvant EGFR-TKIs for resected non-small cell lung cancer: A systematic review and meta-analysis based on randomized control trials. BMC Cancer 2022, 22, 328. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shih, J. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 2018, 17, 38. [Google Scholar] [CrossRef] [PubMed]



| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR with 95% CI | p Value | HR (95%) | p Value | |
| ACOT7 (high vs. low) | 1.59 (1.07–2.36) | 0.021 | 1.72 (1.17–2.60) | 0.007 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.22 (0.75–2.00) | 0.422 | 1.39 (0.82–2.30) | 0.197 |
| Others | 0.87 (0.54–1.39) | 0.552 | 0.79 (0.48–1.30) | 0.363 |
| Age (≥65 vs. <65, y) | 0.89 (0.67–1.21) | 0.475 | ||
| Gender (male vs. female) | 1.17 (0.87–1.57) | 0.291 | 1.11 (0.81–1.52) | 0.526 |
| T stage (T3–T4 vs. T1–T2) | 1.85 (1.26–2.72) | 0.002 | 1.71 (1.12–2.13) | 0.013 |
| N stage (N2–N3 vs. N0–N1) | 1.57 (1.09–2.42) | 0.014 | 1.46 (0.99–2.13) | 0.052 |
| M stage (M1 vs. M0) | 1.10 (0.64–1.88) | 0.732 | ||
| Prior malignancy | 1.56 (1.04–2.33) | 0.031 | 1.75 (1.15–2.66) | 0.009 |
| Smoking | 1.05 (0.77–1.43) | 0.772 | 1.16 (0.83–1.62) | 0.390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Zhang, G.; Xie, K.; Diao, Y.; Luo, C.; Wang, Y.; Shen, Y.; Xue, Q. Pan-Cancer Analysis and Experimental Validation Identify ACOT7 as a Novel Oncogene and Potential Therapeutic Target in Lung Adenocarcinoma. Cancers 2022, 14, 4522. https://doi.org/10.3390/cancers14184522
Zheng C, Zhang G, Xie K, Diao Y, Luo C, Wang Y, Shen Y, Xue Q. Pan-Cancer Analysis and Experimental Validation Identify ACOT7 as a Novel Oncogene and Potential Therapeutic Target in Lung Adenocarcinoma. Cancers. 2022; 14(18):4522. https://doi.org/10.3390/cancers14184522
Chicago/Turabian StyleZheng, Chao, Guochao Zhang, Kai Xie, Yifei Diao, Chao Luo, Yanqing Wang, Yi Shen, and Qi Xue. 2022. "Pan-Cancer Analysis and Experimental Validation Identify ACOT7 as a Novel Oncogene and Potential Therapeutic Target in Lung Adenocarcinoma" Cancers 14, no. 18: 4522. https://doi.org/10.3390/cancers14184522
APA StyleZheng, C., Zhang, G., Xie, K., Diao, Y., Luo, C., Wang, Y., Shen, Y., & Xue, Q. (2022). Pan-Cancer Analysis and Experimental Validation Identify ACOT7 as a Novel Oncogene and Potential Therapeutic Target in Lung Adenocarcinoma. Cancers, 14(18), 4522. https://doi.org/10.3390/cancers14184522




