The Effect of Atm Loss on Radiosensitivity of a Primary Mouse Model of Pten-Deleted Brainstem Glioma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
| Genotype | End Point | No. Mice | Figure |
|---|---|---|---|
| nPtenA-FL/+ | Time to tumor detection (This group includes mice that, upon tumor detection, were subjected to tissue analyses or survival experiments below) | 45 | Figure 1D |
| nPtenA-FL/FL | Time to tumor detection (This group includes mice that, upon tumor detection, were subjected to tissue analyses or survival experiments below) | 56 | Figure 1D |
| nPtenA-FL/+ | Brain tissue collected within 24 h of tumor detection via in vivo imaging for HE, HA IHC, PTEN IHC, Ki67 IHC | 3 | Figure 1C and Figure 2B–E |
| nPtenA-FL/FL | Brain tissue collected within 24 h of tumor detection via in vivo imaging for HE, HA IHC, PTEN IHC, Ki67 IHC | 3 | Figure S1B–E and Figure 1C |
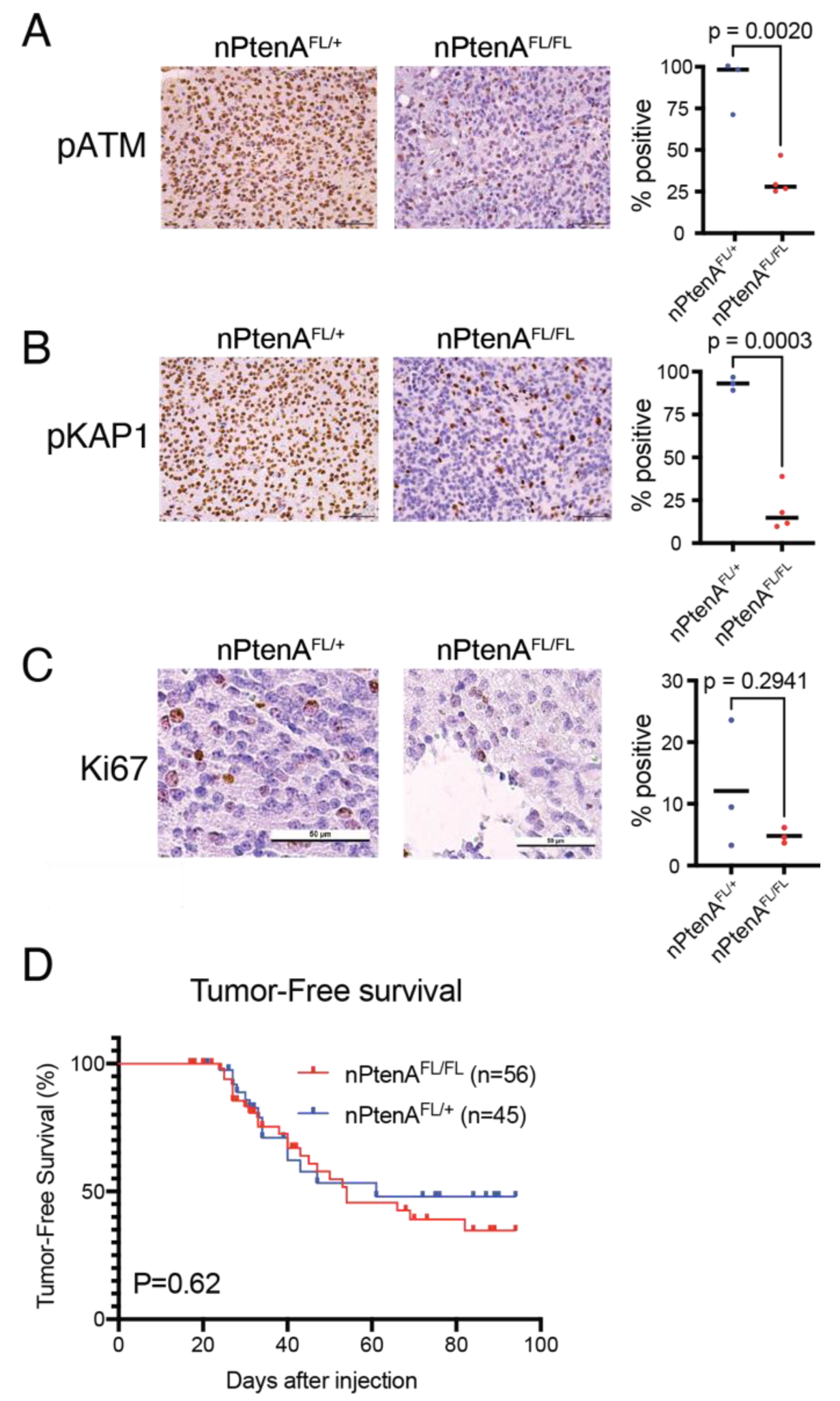
| nPtenA-FL/+ | Brain tissue collected 1 h after 10 Gy × 1 delivered within 24 h of tumor detection via in vivo imaging and subjected to pATM IHC, pKAP1 IHC | 3 | Figure 1A,B |
| nPtenA-FL/FL | Brain tissue collected 1 h after 10 Gy × 1 delivered within 24 h of tumor detection via in vivo imaging and subjected to pATM IHC, pKAP1 IHC | 4 | Figure 1A,B |
| nPtenA-FL/+ | Survival after tumor detection without brain treatment | 14 | Figure 3F |
| nPtenA-FL/+ | Survival after 10 Gy × 3 delivered following tumor detection * | 17 | Figure 3F |
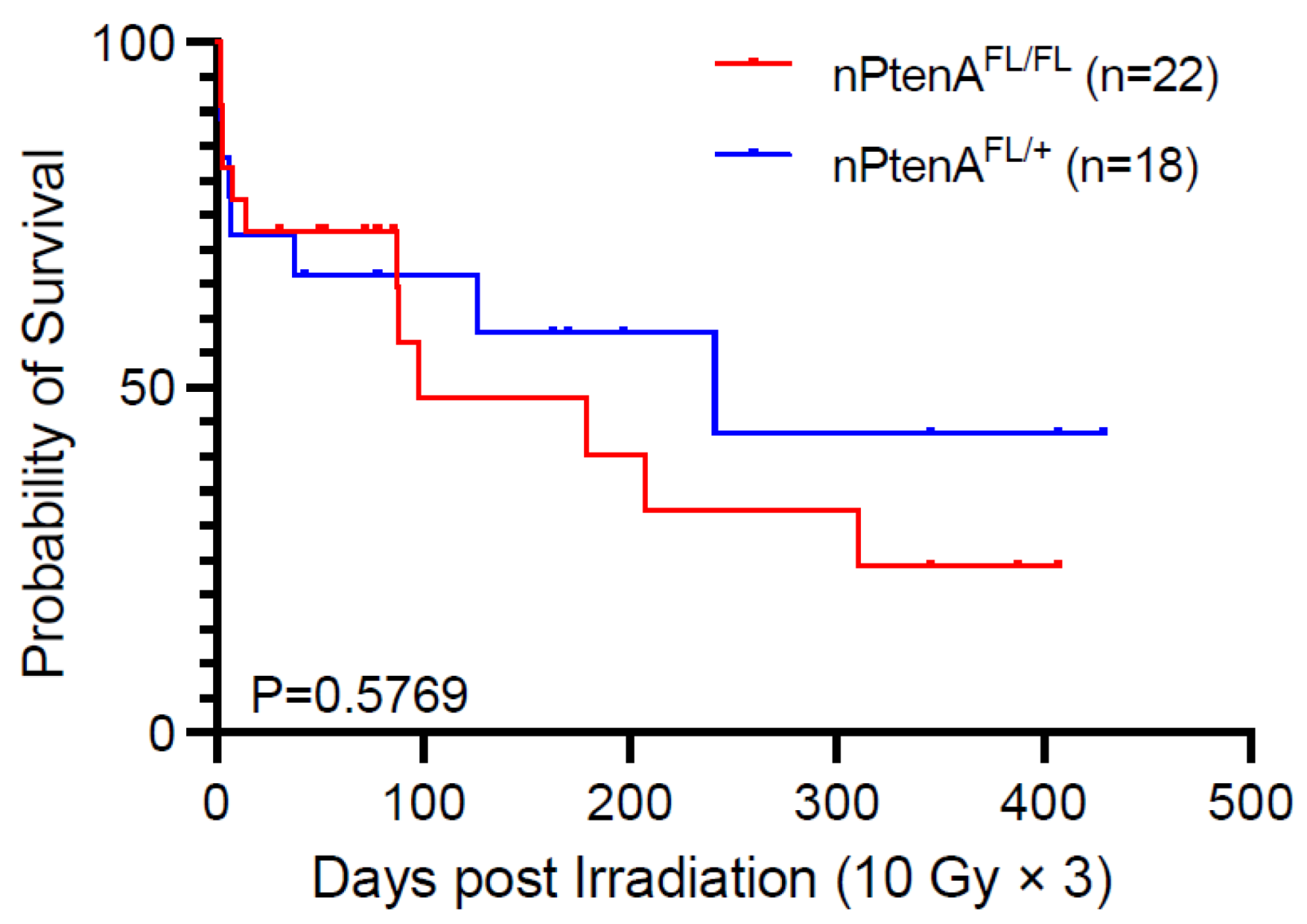
| nPtenA-FL/+ | Survival after 10 Gy × 3 delivered following tumor detection * | 18 | Figure 4 |
| nPtenA-FL/FL | Survival after 10 Gy × 3 delivered following tumor detection | 22 | Figure 4 |

 ) as biologically similar to the standard-of-care for DMG of 1.8 Gy × 30 = 54 Gy (
) as biologically similar to the standard-of-care for DMG of 1.8 Gy × 30 = 54 Gy (  ) compared to other fractionation schemes (
) compared to other fractionation schemes (  ). BED10 is shown on the y-axis to model effective dose to rapidly dividing tissues, such as tumors. BED2 is shown on the x-axis to model effective doses for slowly dividing normal tissues, such as normal brain tissue, estimated to have a low α/β ratio of two. (F) Survival of nPtenAFL/+ mice with tumors lacking PTEN but retaining ATM treated with or without 10 Gy × 3. p < 0.001, log-rank test.
). BED10 is shown on the y-axis to model effective dose to rapidly dividing tissues, such as tumors. BED2 is shown on the x-axis to model effective doses for slowly dividing normal tissues, such as normal brain tissue, estimated to have a low α/β ratio of two. (F) Survival of nPtenAFL/+ mice with tumors lacking PTEN but retaining ATM treated with or without 10 Gy × 3. p < 0.001, log-rank test.
 ) as biologically similar to the standard-of-care for DMG of 1.8 Gy × 30 = 54 Gy (
) as biologically similar to the standard-of-care for DMG of 1.8 Gy × 30 = 54 Gy (  ) compared to other fractionation schemes (
) compared to other fractionation schemes (  ). BED10 is shown on the y-axis to model effective dose to rapidly dividing tissues, such as tumors. BED2 is shown on the x-axis to model effective doses for slowly dividing normal tissues, such as normal brain tissue, estimated to have a low α/β ratio of two. (F) Survival of nPtenAFL/+ mice with tumors lacking PTEN but retaining ATM treated with or without 10 Gy × 3. p < 0.001, log-rank test.
). BED10 is shown on the y-axis to model effective dose to rapidly dividing tissues, such as tumors. BED2 is shown on the x-axis to model effective doses for slowly dividing normal tissues, such as normal brain tissue, estimated to have a low α/β ratio of two. (F) Survival of nPtenAFL/+ mice with tumors lacking PTEN but retaining ATM treated with or without 10 Gy × 3. p < 0.001, log-rank test.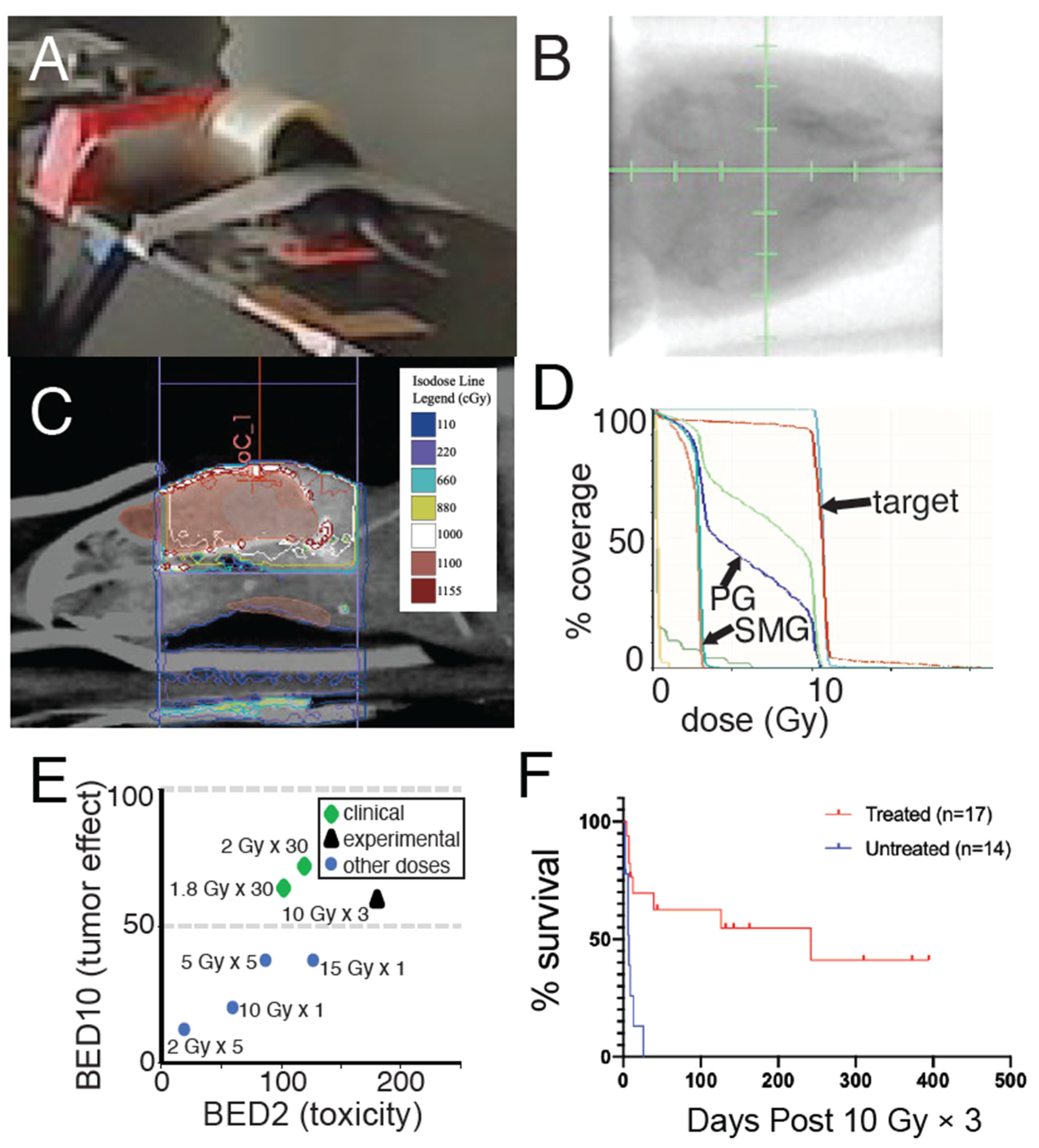
3. Results
3.1. Establishment of Brainstem Gliomas Lacking Pten and ATM
3.2. ATM Loss Does Not Affect Tumor Latency of Brainstem Gliomas Driven by Pten Loss
3.3. Focal Radiation Therapy Extends Survival of Mice Bearing Pten-Null Brainstem Gliomas
3.4. ATM Loss Does Not Improve Survival of Pten-Null Brainstem Gliomas following Radiation Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e5. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.A.; Wood, M.D.; Tihan, T.; Bollen, A.W.; Gupta, N.; Phillips, J.J.J.; Perry, A. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol. 2015, 26, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.H.; Wan, H.; Yang, R.; Wang, Z.; Feng, J.; Yang, S.; Jones, S.; Wang, S.; Zhou, W.; et al. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat. Genet. 2014, 46, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Diaz, A.K.; Paugh, B.S.; Rankin, S.L.; Ju, B.; Li, Y.; Zhu, X.; Qu, C.; Chen, X.; Zhang, J.; et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014, 46, 444–450. [Google Scholar] [CrossRef]
- Buczkowicz, P.; Hoeman, C.; Rakopoulos, P.; Pajovic, S.; Letourneau, L.; Dzamba, M.; Morrison, A.; Lewis, P.; Bouffet, E.; Bartels, U.; et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014, 46, 451–456. [Google Scholar] [CrossRef]
- Khadka, P.; Reitman, Z.J.; Lu, S.; Buchan, G.; Gionet, G.; Dubois, F.; Carvalho, D.M.; Shih, J.; Zhang, S.; Greenwald, N.F.; et al. PPM1D mutations are oncogenic drivers of de novo diffuse midline glioma formation. Nat. Commun. 2022, 13, 604. [Google Scholar] [CrossRef]
- Carvalho, D.; Taylor, K.R.; Olaciregui, N.G.; Molinari, V.; Clarke, M.; Mackay, A.; Ruddle, R.; Henley, A.; Valenti, M.; Hayes, A.; et al. ALK2 inhibitors display beneficial effects in preclinical models of ACVR1 mutant diffuse intrinsic pontine glioma. Commun. Biol. 2019, 2, 156. [Google Scholar] [CrossRef]
- Kastan, M.B.; Lim, D.-S. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 2000, 1, 179–186. [Google Scholar] [CrossRef]
- Rainey, M.D.; Charlton, M.E.; Stanton, R.V.; Kastan, M.B. Transient Inhibition of ATM Kinase Is Sufficient to Enhance Cellular Sensitivity to Ionizing Radiation. Cancer Res. 2008, 68, 7466–7474. [Google Scholar] [CrossRef]
- Westphal, C.H.; Hoyes, K.P.; Canman, C.E.; Huang, X.; Kastan, M.B.; Hendry, J.H.; Leder, P. Loss of atm radiosensitizes multiple p53 null tissues. Cancer Res. 1998, 58, 5637–5639. [Google Scholar]
- Herzog, K.-H.; Chong, M.J.; Kapsetaki, M.; Morgan, J.I.; McKinnon, P.J.; Steffen, W.; Noble, I.; Canadell, J.; Apps, M.; Schulze, E.-D.; et al. Requirement for Atm in Ionizing Radiation-Induced Cell Death in the Developing Central Nervous System. Science 1998, 280, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Rios, V.; Dumitrache, L.C.; Downing, S.M.; Li, Y.; Brown, E.J.; Russell, H.R.; McKinnon, P.J. DNA-PKcs, ATM, and ATR Interplay Maintains Genome Integrity during Neurogenesis. J. Neurosci. 2016, 37, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Deland, K.; Starr, B.F.; Mercer, J.S.; Byemerwa, J.; Crabtree, D.M.; Williams, N.T.; Luo, L.; Ma, Y.; Chen, M.; Becher, O.J.; et al. Tumor genotype dictates radiosensitization after Atm deletion in primary brainstem glioma models. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.L.; et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. 2018, 4, eaat1719. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Amankulor, N.M.; Helmy, K.Y.; Becher, O.J.; Holland, E.C. Modeling Adult Gliomas Using RCAS/t-va Technology. Transl. Oncol. 2009, 2, 89-IN6. [Google Scholar] [CrossRef]
- Hoeman, C.M.; Cordero, F.J.; Hu, G.; Misuraca, K.; Romero, M.M.; Cardona, H.J.; Nazarian, J.; Hashizume, R.; McLendon, R.; Yu, P.; et al. ACVR1 R206H cooperates with H3.1K27M in promoting diffuse intrinsic pontine glioma pathogenesis. Nat. Commun. 2019, 10, 1023. [Google Scholar] [CrossRef]
- Zha, S.; Guo, C.; Boboila, C.; Oksenych, V.; Cheng, H.-L.; Zhang, Y.; Wesemann, D.R.; Yuen, G.; Patel, H.; Goff, P.H.; et al. ATM damage response and XLF repair factor are functionally redundant in joining DNA breaks. Nat. 2010, 469, 250–254. [Google Scholar] [CrossRef]
- Lesche, R.; Groszer, M.; Gao, J.; Wang, Y.; Messing, A.; Sun, H.; Wu, H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 2002, 32, 148–149. [Google Scholar] [CrossRef]
- Barton, K.L.; Misuraca, K.; Cordero, F.; Dobrikova, E.; Min, H.D.; Gromeier, M.; Kirsch, D.G.; Becher, O.J. PD-0332991, a CDK4/6 Inhibitor, Significantly Prolongs Survival in a Genetically Engineered Mouse Model of Brainstem Glioma. PLoS ONE 2013, 8, e77639. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Mitchell, G.C.; Limesand, K.H. Sensitivity of Salivary Glands to Radiation: From Animal Models to Therapies. J. Dent. Res. 2009, 88, 894–903. [Google Scholar] [CrossRef]
- Fowler, J.F. 21 years of Biologically Effective Dose. Br. J. Radiol. 2010, 83, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Douglas, B.G.; Fowler, J.F. The Effect of Multiple Small Doses of X Rays on Skin Reactions in the Mouse and a Basic Interpretation. Radiat. Res. 1976, 66, 401. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Dale, R.; Deehan, C.; Hopkins, K.; Morgan, D. The Role of Biologically Effective Dose (BED) in Clinical Oncology. Clin. Oncol. 2001, 13, 71–81. [Google Scholar] [CrossRef]
- Kuperman, V.Y.; Spradlin, G.S. Use of radiation protraction to escalate biologically effective dose to the treatment target. Med. Phys. 2011, 38, 6553. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Leu, M.Y.; Smathers, J.B.; McBride, W.H.; Parker, R.G.; Withers, H. Biologically effective dose distribution based on the linear quadratic model and its clinical relevance. Int. J. Radiat. Oncol. 1995, 33, 375–389. [Google Scholar] [CrossRef]
- Otsuka, S.; Shibamoto, Y.; Iwata, H.; Murata, R.; Sugie, C.; Ito, M.; Ogino, H. Compatibility of the Linear-Quadratic Formalism and Biologically Effective Dose Concept to High-Dose-Per-Fraction Irradiation in a Murine Tumor. Int. J. Radiat. Oncol. 2011, 81, 1538–1543. [Google Scholar] [CrossRef]
- Cordero, F.J.; Huang, Z.; Grenier, C.; He, X.; Hu, G.; McLendon, R.E.; Murphy, S.K.; Hashizume, R.; Becher, O.J. Histone H3.3K27M Represses p16 to Accelerate Gliomagenesis in a Murine Model of DIPG. Mol. Cancer Res. 2017, 15, 1243–1254. [Google Scholar] [CrossRef]
- Lewis, P.W.; Müller, M.M.; Koletsky, M.S.; Cordero, F.; Lin, S.; Banaszynski, L.A.; Garcia, B.A.; Muir, T.W.; Becher, O.J.; Allis, C.D. Inhibition of PRC2 Activity by a Gain-of-Function H3 Mutation Found in Pediatric Glioblastoma. Science 2013, 340, 857–861. [Google Scholar] [CrossRef]
- Larson, J.D.; Kasper, L.H.; Paugh, B.S.; Jin, H.; Wu, G.; Kwon, C.-H.; Fan, Y.; Shaw, T.I.; Silveira, A.B.; Qu, C.; et al. Histone H3.3 K27M Accelerates Spontaneous Brainstem Glioma and Drives Restricted Changes in Bivalent Gene Expression. Cancer Cell 2018, 35, 140–155.e7. [Google Scholar] [CrossRef] [PubMed]
- Krug, B.; De Jay, N.; Harutyunyan, A.S.; Deshmukh, S.; Marchione, D.M.; Guilhamon, P.; Bertrand, K.C.; Mikael, L.G.; McConechy, M.K.; Chen, C.C.; et al. Pervasive H3K27 Acetylation Leads to ERV Expression and a Therapeutic Vulnerability in H3K27M Gliomas. Cancer Cell 2019, 36, 338–339. [Google Scholar] [CrossRef]
- Tomita, Y.; Shimazu, Y.; Somasundaram, A.; Tanaka, Y.; Takata, N.; Ishi, Y.; Gadd, S.; Hashizume, R.; Angione, A.; Pinero, G.; et al. A novel mouse model of diffuse midline glioma initiated in neonatal oligodendrocyte progenitor cells highlights cell-of-origin dependent effects of H3K27M. Glia 2022, 70, 1681–1698. [Google Scholar] [CrossRef] [PubMed]
- Deland, K.; Mercer, J.S.; Crabtree, D.M.; Garcia, M.E.G.; Reinsvold, M.; Campos, L.D.S.; Williams, N.T.; Luo, L.; Ma, Y.; Reitman, Z.J.; et al. Radiosensitizing the Vasculature of Primary Brainstem Gliomas Fails to Improve Tumor Response to Radiation Therapy. Int. J. Radiat. Oncol. 2021, 112, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Hopewell, J.W. Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: Derivation of “protective” dose modification factors. Br. J. Radiol. 2018, 92, 20180111. [Google Scholar] [CrossRef] [PubMed]
| Mouse Strain | p53 | Ink4A/Arf | Pten | Baseline Radiosensitivity (AtmFL/+) | Effect of ATM Loss on Radiosensitivity (AtmFL/FL) | Ref. |
|---|---|---|---|---|---|---|
| nPA | - | + | + | Resistant | Radiosensitization | [13] |
| nIA | + | - | + | Sensitive | None | [13] |
| nPIA | - | - | + | Very resistant | Radiosensitization | [13] |
| nPtenA | + | + | - | Sensitive | None | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, C.E.; Guerra-García, M.E.; Luo, L.; Williams, N.T.; Ma, Y.; Regal, J.A.; Ghosh, D.; Sansone, P.; Oldham, M.; Deland, K.; et al. The Effect of Atm Loss on Radiosensitivity of a Primary Mouse Model of Pten-Deleted Brainstem Glioma. Cancers 2022, 14, 4506. https://doi.org/10.3390/cancers14184506
Stewart CE, Guerra-García ME, Luo L, Williams NT, Ma Y, Regal JA, Ghosh D, Sansone P, Oldham M, Deland K, et al. The Effect of Atm Loss on Radiosensitivity of a Primary Mouse Model of Pten-Deleted Brainstem Glioma. Cancers. 2022; 14(18):4506. https://doi.org/10.3390/cancers14184506
Chicago/Turabian StyleStewart, Connor E., María E. Guerra-García, Lixia Luo, Nerissa T. Williams, Yan Ma, Joshua A. Regal, Debosir Ghosh, Patrick Sansone, Mark Oldham, Katherine Deland, and et al. 2022. "The Effect of Atm Loss on Radiosensitivity of a Primary Mouse Model of Pten-Deleted Brainstem Glioma" Cancers 14, no. 18: 4506. https://doi.org/10.3390/cancers14184506
APA StyleStewart, C. E., Guerra-García, M. E., Luo, L., Williams, N. T., Ma, Y., Regal, J. A., Ghosh, D., Sansone, P., Oldham, M., Deland, K., Becher, O. J., Kirsch, D. G., & Reitman, Z. J. (2022). The Effect of Atm Loss on Radiosensitivity of a Primary Mouse Model of Pten-Deleted Brainstem Glioma. Cancers, 14(18), 4506. https://doi.org/10.3390/cancers14184506







