Tumor Microenvironment CD14+ Cells Correlate with Poor Overall Survival in Patients with Early-Stage Lung Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Clinical Data
2.3. Lung Cancer Cell Lines
2.4. CD14+ Cell Isolation
2.5. CD14+ Cell Migration
2.6. HLA-DR Expression
2.7. Coculture Experiments
2.8. Data Analysis
3. Results
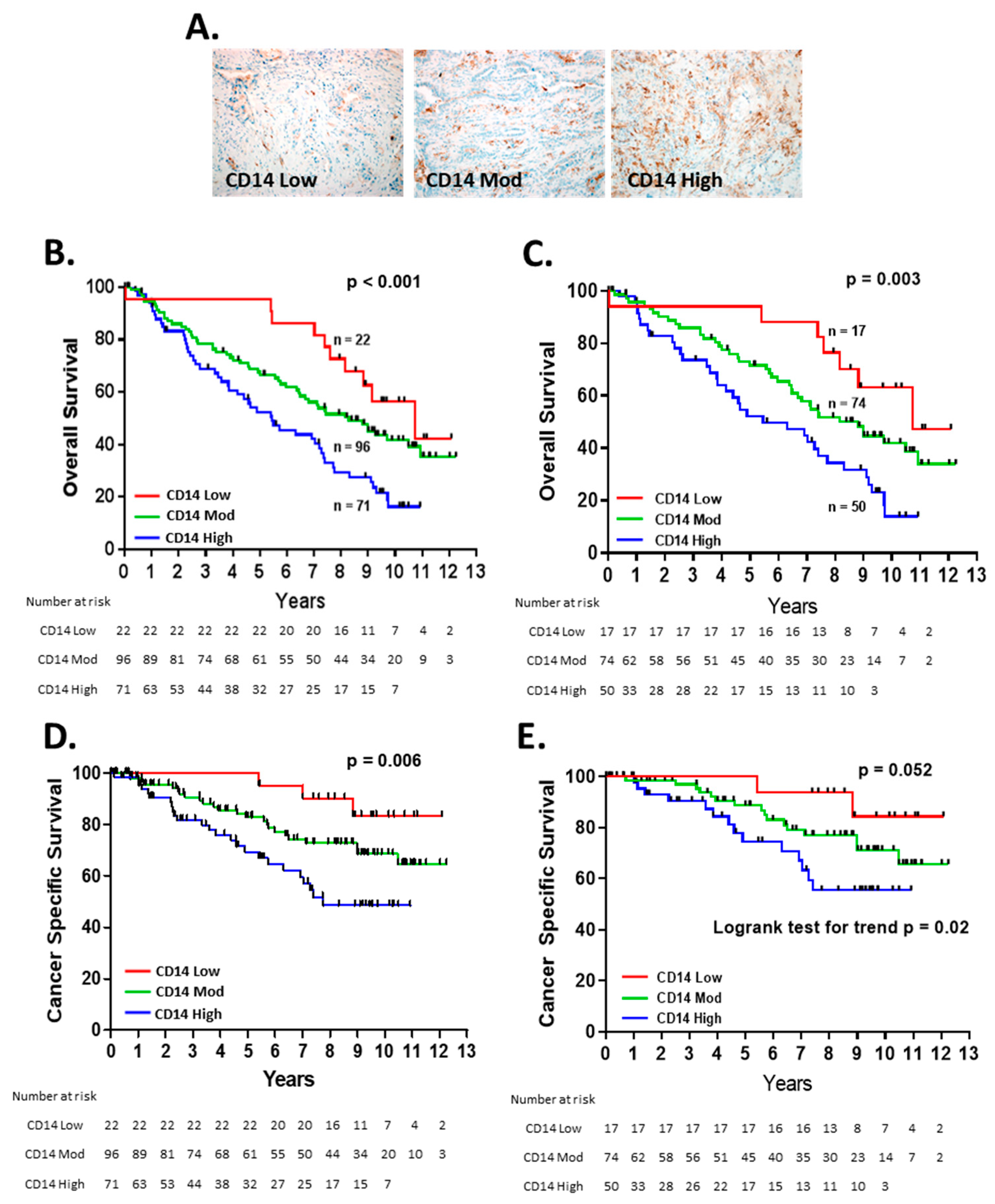
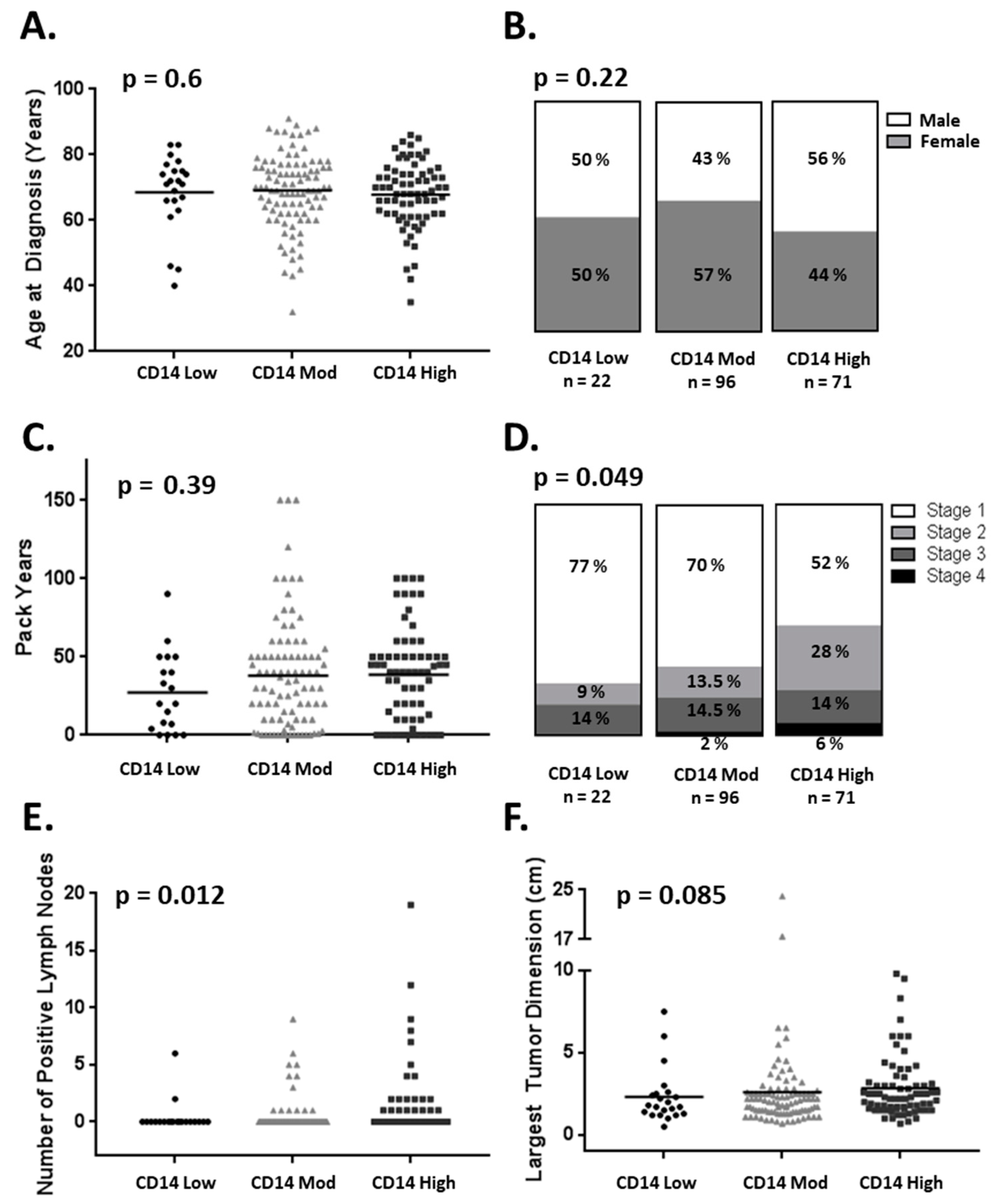
3.1. CD14+ Cell Tumor Infiltration Associates with Shorter Overall Survival in Patients with Early-Stage Lung Adenocarcinoma
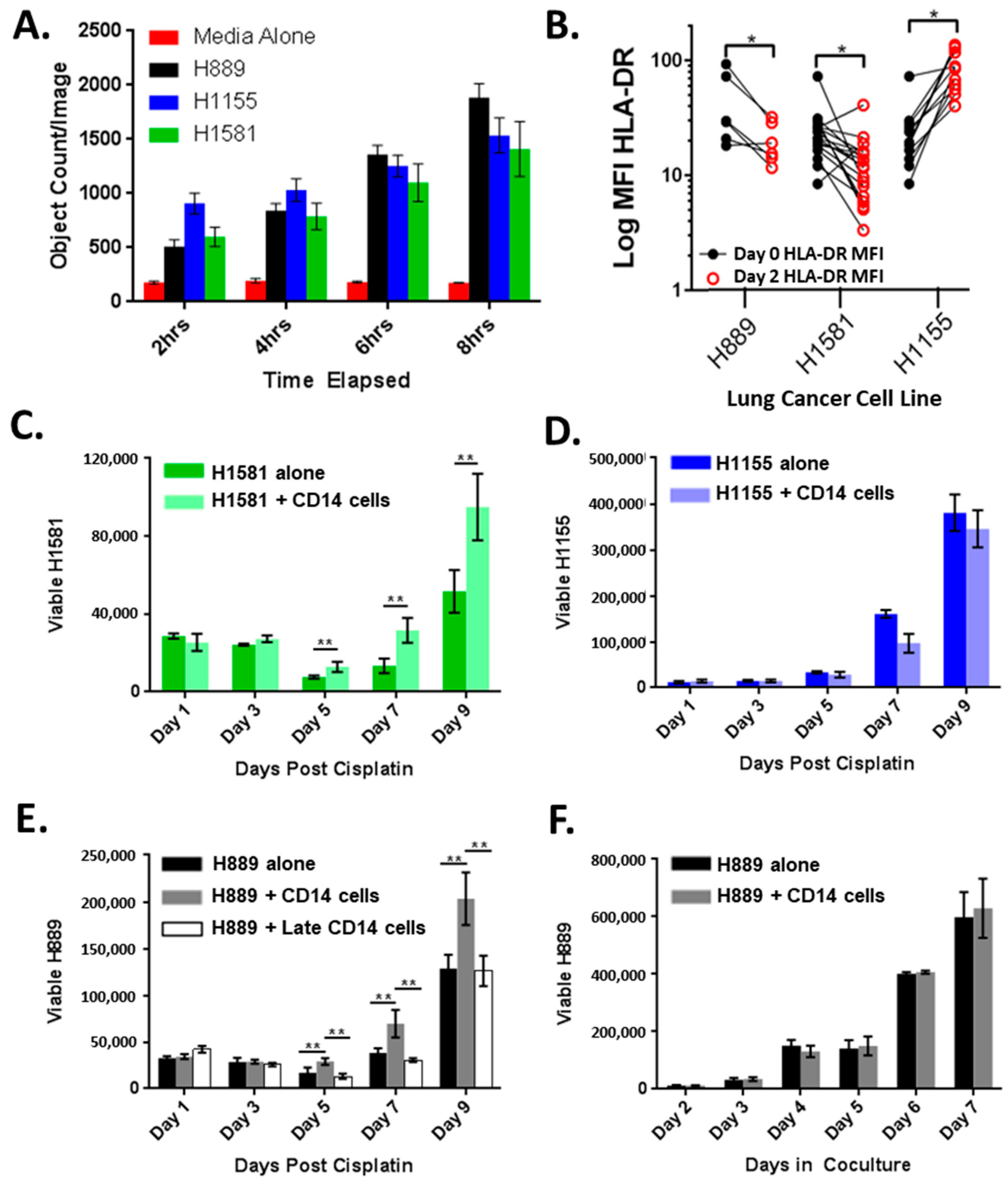
3.2. CD14+ Cells Are Recruited and Altered by Lung Cancer Cell Lines
3.3. CD14+ Cells Reduce Chemotherapy-Induced Tumor Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mahvi, D.A.; Liu, R.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA Cancer J. Clin. 2018, 68, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Tuminello, S.; Veluswamy, R.; Lieberman-Cribbin, W.; Gnjatic, S.; Petralia, F.; Wang, P.; Flores, R.; Taioli, E. Prognostic value of immune cells in the tumor microenvironment of early-stage lung cancer: A meta-analysis. Oncotarget 2019, 10, 7142–7155. [Google Scholar] [CrossRef]
- Remark, R.; Becker, C.; Gomez, J.E.; Damotte, D.; Dieu-Nosjean, M.-C.; Sautès-Fridman, C.; Fridman, W.-H.; Powell, C.A.; Altorki, N.K.; Merad, M.; et al. The Non–Small Cell Lung Cancer Immune Contexture. A Major Determinant of Tumor Characteristics and Patient Outcome. Am. J. Respir. Crit. Care Med. 2015, 191, 377–390. [Google Scholar] [CrossRef]
- Celus, W.; Di Conza, G.; Oliveira, A.I.; Ehling, M.; Costa, B.M.; Wenes, M.; Mazzone, M. Loss of Caveolin-1 in Metastasis-Associated Macrophages Drives Lung Metastatic Growth through Increased Angiogenesis. Cell Rep. 2017, 21, 2842–2854. [Google Scholar] [CrossRef]
- Park, M.S.; Yang, A.Y.; Lee, J.E.; Kim, S.K.; Roe, J.S.; Park, M.S.; Oh, M.J.; An, H.J.; Kim, M.Y. GALNT3 suppresses lung cancer by inhibiting myeloid-derived suppressor cell infiltration and angiogenesis in a TNFR and c-MET pathway-dependent manner. Cancer Lett. 2021, 521, 294–307. [Google Scholar] [CrossRef]
- Zhou, J.; Qu, Z.; Sun, F.; Han, L.; Li, L.; Yan, S.; Stabile, L.P.; Chen, L.F.; Siegfried, J.M.; Xiao, G. Myeloid STAT3 Promotes Lung Tumorigenesis by Transforming Tumor Immunosurveillance into Tumor-Promoting Inflammation. Cancer Immunol. Res. 2017, 5, 257–268. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Wilcox, R.A.; Wada, D.A.; Ziesmer, S.C.; Elsawa, S.F.; Comfere, N.I.; Dietz, A.B.; Novak, A.J.; Witzig, T.E.; Feldman, A.L.; Pittelkow, M.R.; et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood 2009, 114, 2936–2944. [Google Scholar] [CrossRef]
- Lin, Y.; Gustafson, M.P.; Bulur, P.A.; Gastineau, D.A.; Witzig, T.E.; Dietz, A.B. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood 2011, 117, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Lin, Y.; Bleeker, J.S.; Warad, D.; Tollefson, M.K.; Crispen, P.L.; Bulur, P.A.; Harrington, S.M.; Laborde, R.R.; Gastineau, D.A.; et al. Intratumoral CD14+ Cells and Circulating CD14+HLA-DRlo/neg Monocytes Correlate with Decreased Survival in Patients with Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4224–4233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Bulur, P.A.; Dogan, A.; Gastineau, D.A.; Dietz, A.B.; Lin, Y. Immune independent crosstalk between lymphoma and myeloid suppressor CD14+HLA-DRlow/neg monocytes mediates chemotherapy resistance. Oncoimmunology 2015, 4, e996470. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Lin, Y.; New, K.C.; Bulur, P.A.; O’Neill, B.P.; Gastineau, D.A.; Dietz, A.B. Systemic immune suppression in glioblastoma: The interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-oncology 2010, 12, 631–644. [Google Scholar] [CrossRef]
- Vuk-Pavlović, S.; Bulur, P.A.; Lin, Y.; Qin, R.; Szumlanski, C.L.; Zhao, X.; Dietz, A.B. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate 2010, 70, 443–455. [Google Scholar] [CrossRef]
- Gustafson, M.P.; Abraham, R.S.; Lin, Y.; Wu, W.; Gastineau, D.A.; Zent, C.S.; Dietz, A.B. Association of an increased frequency of CD14+ HLA-DRlo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br. J. Haematol. 2012, 156, 674–676. [Google Scholar] [CrossRef]
- Gustafson, M.P.; Lin, Y.; LaPlant, B.; Liwski, C.J.; Maas, M.L.; League, S.C.; Bauer, P.R.; Abraham, R.S.; Tollefson, M.K.; Kwon, E.D.; et al. Immune monitoring using the predictive power of immune profiles. J. Immunother. Cancer 2013, 1, 7. [Google Scholar] [CrossRef]
- Huang, A.; Zhang, B.; Wang, B.; Zhang, F.; Fan, K.-X.; Guo, Y.-J. Increased CD14+HLA-DR−/low myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol. Immunother. 2013, 62, 1439–1451. [Google Scholar] [CrossRef]
- Boral, B.; Balli, H.T.; Sozutok, S.; Pehlivan, U.A.; Aikimbaev, K. Clinical and prognostic significance of CD14+ HLA-DR−/low myeloid-derived suppressor cells in patients with hepatocellular carcinoma received transarterial radioembolization with Yttrium-90. Scand. J. Immunol. 2022, 95, e13132. [Google Scholar] [CrossRef]
- Porrello, A.; Leslie, P.L.; Harrison, E.B.; Gorentla, B.K.; Kattula, S.; Ghosh, S.K.; Azam, S.H.; Holtzhausen, A.; Chao, Y.L.; Hayward, M.C.; et al. Factor XIIIA—expressing inflammatory monocytes promote lung squamous cancer through fibrin cross-linking. Nat. Commun. 2018, 9, 1988. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Eguchi, T.; Bains, S.; Lee, M.C.; Tan, K.S.; Hristov, B.; Buitrago, D.H.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J. Clin. Oncol. 2017, 35, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Jhala, H.; Harling, L.; Rodrigo, A.; Nonaka, D.; McLean, E.; Ng, W.; Okiror, L.; Bille, A. Clinicopathological predictors of survival in resected primary lung adenocarcinoma. J. Clin. Pathol. 2022, 75, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Gazdar, A.F.; Chen, H.-C.; Johnson, B.E. Antitumor Activity of Magainin Analogues against Human Lung Cancer Cell Lines1. Cancer Res. 1992, 52, 3534–3538. [Google Scholar] [PubMed]
- Garon, E.B.; Christofk, H.R.; Hosmer, W.; Britten, C.D.; Bahng, A.; Crabtree, M.J.; Hong, C.S.; Kamranpour, N.; Pitts, S.; Kabbinavar, F.; et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 443–452. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 3.2022). Available online:https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 14 July 2022).
- Mengos, A.E.; Gastineau, D.A.; Gustafson, M.P. The CD14+HLA-DRlo/neg Monocyte: An Immunosuppressive Phenotype that Restrains Responses to Cancer Immunotherapy. Front. Immunol. 2019, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, H.; Zhu, Y.; Yu, G.; Gao, X.; Xu, Y.; Liu, C.; Hou, J.; Zhang, X. A novel subset of B7-H3+CD14+HLA-DR−/low myeloid-derived suppressor cells are associated with progression of human NSCLC. OncoImmunology 2015, 4, e977164. [Google Scholar] [CrossRef]
- Speigl, L.; Burow, H.; Bailur, J.K.; Janssen, N.; Walter, C.B.; Pawelec, G.; Shipp, C. CD14+ HLA-DR−/low MDSCs are elevated in the periphery of early-stage breast cancer patients and suppress autologous T cell proliferation. Breast Cancer Res. Treat. 2018, 168, 401–411. [Google Scholar] [CrossRef]
- Gneo, L.; Rizkalla, N.; Hejmadi, R.; Mussai, F.; de Santo, C.; Middleton, G. TGF-beta orchestrates the phenotype and function of monocytic myeloid-derived suppressor cells in colorectal cancer. Cancer Immunol. Immunother. 2022, 71, 1583–1596. [Google Scholar] [CrossRef]
- Singhal, S.; Stadanlick, J.; Annunziata, M.J.; Rao, A.S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Cantu, E.; Danet-Desnoyers, G.; Ra, H.-J.; et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci. Transl. Med. 2019, 11, eaat1500. [Google Scholar] [CrossRef]
- Feng, P.-H.; Lee, K.-Y.; Chang, Y.-L.; Chan, Y.-F.; Kuo, L.-W.; Lin, T.-Y.; Chung, F.-T.; Kuo, C.-S.; Yu, C.-T.; Lin, S.-M.; et al. CD14+S100A9+ Monocytic Myeloid-derived Suppressor Cells and Their Clinical Relevance in Non–Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2012, 186, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, K.A.; Postel-Vinay, S. Predictors of chemotherapy efficacy in non-small-cell lung cancer: A challenging landscape. Ann. Oncol. 2016, 27, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.-H.; Yu, C.-T.; Chen, K.-Y.; Luo, C.-S.; Wu, S.M.; Liu, C.-Y.; Kuo, L.W.; Chan, Y.-F.; Chen, T.-T.; Chang, C.-C.; et al. S100A9+ MDSC and TAM-mediated EGFR-TKI resistance in lung adenocarcinoma: The role of RELB. Oncotarget 2018, 9, 7631–7643. [Google Scholar] [CrossRef]
- De Henau, O.; Rausch, M.; Winkler, D.; Campesato, L.F.; Liu, C.; Cymerman, D.H.; Budhu, S.; Ghosh, A.; Pink, M.; Tchaicha, J.; et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2016, 539, 443. [Google Scholar] [CrossRef] [PubMed]
- Duruisseaux, M.; Martinez-Cardus, A.; Calleja-Cervantes, M.E.; Moran, S.; Castro de Moura, M.; Davalos, V.; Pineyro, D.; Sanchez-Cespedes, M.; Girard, N.; Brevet, M.; et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: A multicentre, retrospective analysis. Lancet Respir. Med. 2018, 6, 771–781. [Google Scholar] [CrossRef]
| Age (years) | |
| Median | 70 |
| Range | 32–91 |
| Gender | |
| F | 97 |
| Smoking Status | |
| Never | 32 |
| Current/former | 157 |
| Median pack/years | 35 |
| Range | 1–150 |
| Surgical Procedure | |
| Lobectomy | 122 |
| Lobectomy + wedge | 12 |
| Segmentectomy | 6 |
| Segmentectomy + wedge | 1 |
| Wedge alone | 42 |
| Pneumonectomy | 5 |
| Biopsy | 1 |
| Lymph Nodes Resected | |
| Median | 19 |
| Range | 0–52 |
| Pathological Stage | |
| IA | 81 |
| IB | 40 |
| IIA | 20 |
| IIB | 16 |
| IIIA | 24 |
| IIIB | 2 |
| IV | 6 |
| Systemic Therapy | |
| Chemotherapy | 28 |
| Neoadjuvant chemotherapy | 3 |
| Univariate Analysis All Patients | Multivariate Analysis All Patients | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Gender | ||||
| F | 1 | 1 | ||
| M | 1.67 (1.15–2.44) | 0.007 | 1.89 (1.19–2.78) | 0.006 |
| Age at Diagnosis | ||||
| <70 years | 1 | 1 | ||
| ≥70 years | 1.70 (1.23–2.38) | 0.0011 | 1.98 (1.31–3.00) | 0.001 |
| CD14 IHC Score | ||||
| 1 | 1 | 1 | ||
| 2 | 1.89 (1.01–4.01) | 0.045 | 2.74 (1.21–6.23) | 0.016 |
| 3 | 3.07 (1.66–6.5) | <0.0001 | 3.71 (1.59–8.68) | <0.003 |
| Stage at Diagnosis | ||||
| 1 | 1 | 1 | ||
| 2 | 1.15 (0.78–1.68) | 0.46 | 0.80 (0.40–1.59) | 0.52 |
| 3 | 1.65 (1.16–2.36) | 0.006 | 0.63 (0.24–1.66) | 0.35 |
| 4 | 2.15 (1.2–3.62) | 0.012 | 3.31 (0.90–12.14) | 0.071 |
| Surgical Procedure | ||||
| Lobectomy | 1 | 1 | ||
| Wedge | 1.02 (0.65–1.61) | 0.92 | 1.84 (1.06–3.19) | 0.029 |
| Segmentectomy | 1.37 (0.56–3.39) | 0.49 | 3.11 (1.17–8.21) | 0.023 |
| Pneumonectomy | 4.23 (1.30–13.72) | 0.016 | 4.20 (1.12–15.75) | 0.036 |
| Largest T Dimension | ||||
| ≤3 cm | 1 | 1 | ||
| >3cm–≤7 cm | 1.43 (0.97–2.07) | 0.067 | 1.6 (0.92–2.79) | 0.1 |
| >7 cm | 5.47 (3.13–9.07) | <0.0001 | 11.39 (3.68–35.25) | <0.001 |
| Number Positive Lymph Nodes | ||||
| 0 | 1 | 1 | ||
| ≥1 | 1.79 (1.26–2.53) | 0.001 | 1.99 (0.91–4.40) | 0.09 |
| Smoking Status | ||||
| <35 pack years | 1 | 1 | ||
| ≥35 pack years | 1.47 (1.15–1.91) | 0.002 | 1.41 (0.91–2.19) | 0.12 |
| Univariate Analysis Early-Stage Patients | Multivariate Analysis Early-Stage Patients | |||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Gender | ||||
| F | 1 | 1 | ||
| M | 1.65 (1.06–2.56) | 0.027 | 1.73 (1.07–2.80) | 0.024 |
| Age at Diagnosis | ||||
| <70 years | 1 | 1 | ||
| ≥70 years | 2.05 (1.31–3.20) | 0.002 | 2.38 (1.47–3.86) | <0.001 |
| CD14 IHC Score | ||||
| 1 | 1 | 1 | ||
| 2 | 1.80 (0.81–4.02) | 0.15 | 3.04 (1.16–7.94) | 0.023 |
| 3 | 3.27 (1.44–7.41) | 0.005 | 5.08 (1.86–13.91) | 0.002 |
| Surgical Procedure | ||||
| Lobectomy | 1 | 1 | ||
| Wedge | 1.13 (0.67–1.90) | 0.65 | 1.45 (0.81–2.89) | 0.21 |
| Segmentectomy | 0.91 (0.29–2.92) | 0.88 | 2.23 (0.66–7.50) | 0.19 |
| Pneumonectomy | 13.28 (1.69–104.28) | 0.014 | 23.44 (2.78–197.88) | 0.004 |
| Largest T Dimension | ||||
| ≤3 cm | 1 | 1 | ||
| >3 cm–≤4 cm | 1.46 (0.70–3.03) | 0.32 | 1.27 (0.58–2.76) | 0.55 |
| >5 cm | 1.19 (0.48–2.96) | 0.70 | 0.70 (0.21–2.36) | 0.56 |
| Stage at Diagnosis | ||||
| 1 | 1 | 1 | ||
| 2 | 1.50 (0.84–2.66) | 0.17 | 1.53 (0.71–3.29) | 0.42 |
| Smoking Status | ||||
| <35 pack years | 1 | 1 | ||
| ≥35 pack years | 1.27 (0.80–2.0) | 0.30 | 1.22 (0.75–1.98) | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schenk, E.L.; Boland, J.M.; Withers, S.G.; Bulur, P.A.; Dietz, A.B. Tumor Microenvironment CD14+ Cells Correlate with Poor Overall Survival in Patients with Early-Stage Lung Adenocarcinoma. Cancers 2022, 14, 4501. https://doi.org/10.3390/cancers14184501
Schenk EL, Boland JM, Withers SG, Bulur PA, Dietz AB. Tumor Microenvironment CD14+ Cells Correlate with Poor Overall Survival in Patients with Early-Stage Lung Adenocarcinoma. Cancers. 2022; 14(18):4501. https://doi.org/10.3390/cancers14184501
Chicago/Turabian StyleSchenk, Erin L., Jennifer M. Boland, Sarah G. Withers, Peggy A. Bulur, and Allan B. Dietz. 2022. "Tumor Microenvironment CD14+ Cells Correlate with Poor Overall Survival in Patients with Early-Stage Lung Adenocarcinoma" Cancers 14, no. 18: 4501. https://doi.org/10.3390/cancers14184501
APA StyleSchenk, E. L., Boland, J. M., Withers, S. G., Bulur, P. A., & Dietz, A. B. (2022). Tumor Microenvironment CD14+ Cells Correlate with Poor Overall Survival in Patients with Early-Stage Lung Adenocarcinoma. Cancers, 14(18), 4501. https://doi.org/10.3390/cancers14184501





