Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis
Abstract
Simple Summary
Abstract
1. Introduction
2. Immune Tolerance
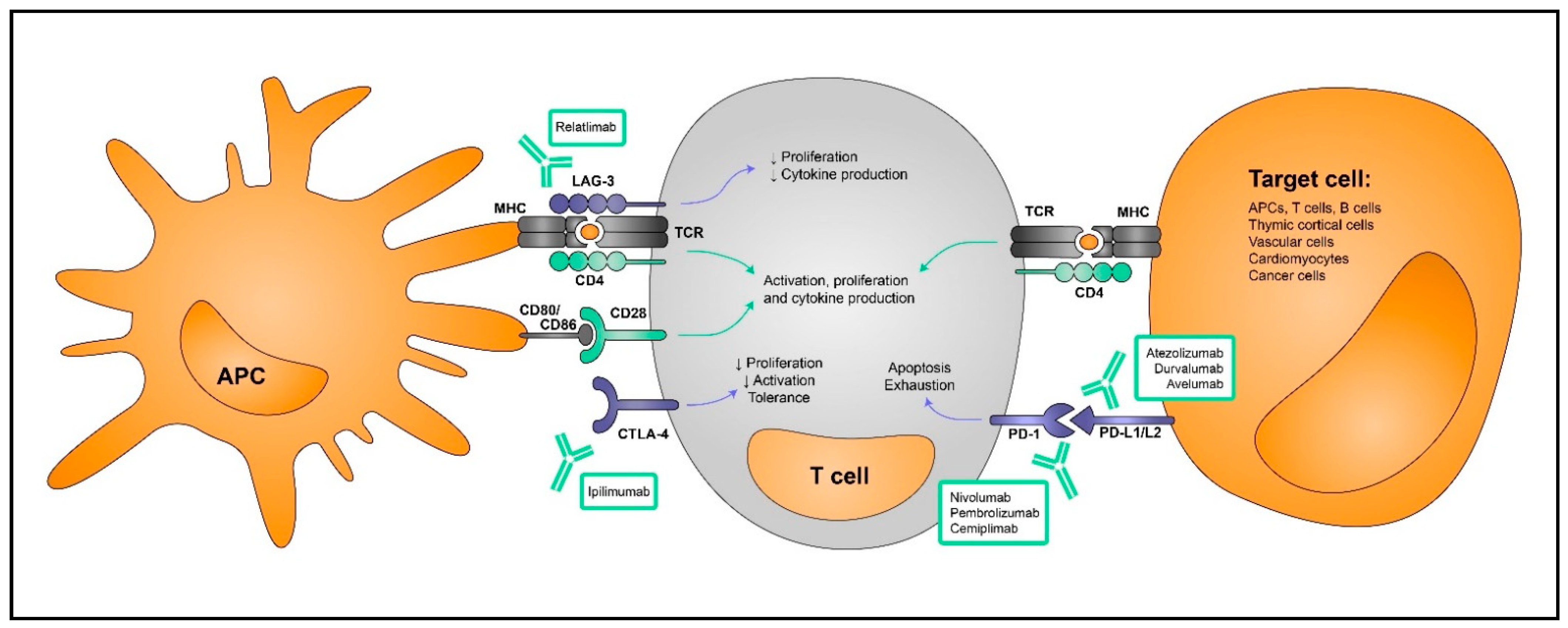
3. Immune Checkpoint Inhibitors in Cancer
4. Myocarditis Induced by Immune Checkpoint Blockade
| Type of Study | ICI Treatment Used | ICI-Associated Myocarditis Reported Cases | Total Number of ICI-Treated Patients Studied | Reference |
|---|---|---|---|---|
| Retrospective (VigiBase database) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Ipilimumab (anti-CTLA-4) Atezolizumab (anti-PD-L1) Durvalumab (anti-PD-L1) Avelumab (anti-PD-L1) Combination anti-PD-1/PD-L1 + anti-CTLA-4 | 101 | 101 | [11] |
| Retrospective (VigiBase database) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Ipilimumab (anti-CTLA-4) Atezolizumab (anti-PD-L1) Durvalumab (anti-PD-L1) Avelumab (anti-PD-L1) Combination anti-PD-1 (Nivolumab or Pembrolizumab) + anti-CTLA-4 | 122 | 31,321 | [49] |
| Both retrospective and prospective (8 center American registry) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Ipilimumab (anti-CTLA-4) Tremelimumab (anti-CTLA4) Atezolizumab (anti-PD-L1) Durvalumab (anti-PD-L1) Avelumab (anti-PD-L1) Combination anti-PD-1 (Nivolumab or Pembrolizumab) + anti-CTLA-4 (Ipilimumab) Combination anti-PDL1PD-L1 (avelumab or durvalumab) + anti-CTLA-4 Tremelimumab (anti-CTLA-4) | 35 | 964 | [7] |
| Prospective (19 center international registry) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Ipilimumab (anti-CTLA-4) Tremelimumab (anti-CTLA4) Atezolizumab (anti-PD-L1) Durvalumab (anti-PD-L1) Avelumab (anti-PD-L1) Combination anti-PD-1 (Nivolumab or Pembrolizumab) + anti-CTLA-4 (Ipilimumab) Combination anti-PDL1PD-L1 (avelumab or durvalumab) + anti-CTLA-4 Tremelimumab (anti-CTLA-4) | 113 | 3637 | [52] |
| Prospective (23 center international registry) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Ipilimumab (anti-CTLA-4) Tremelimumab (anti-CTLA-4) Atezolizumab (anti-PD-L1) Avelumab (anti-PD-L1) Combination anti-PD-1/PD-L1 + anti-CTLA-4 | 103 | 103 | [10] |
| Retrospective (Massachusetts General Hospital database) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Combination Ipilimumab (anti-CTLA-4) + Nivolumab (anti-PD-1) | 10 | 10 | [53] |
| Prospective (Danish Registry) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Ipilimumab (anti-CTLA-4) | 11 | 1103 | [54] |
| Retrospective (RPCCC medical records) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Atezolizumab (anti-PD-L1) | 23 | 23 | [55] |
| Retrospective (IBM MarketScan research databases) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Ipilimumab (anti-CTLA-4) Atezolizumab (anti-PD-L1) Avelumab (anti-PD-L1) Durvalumab (anti-PD-L1) Combination anti-PD-1 + anti-CTLA-4 | 6 | 12,187 | [56] |
| Retrospective (University of Tsukuba Hospital records) | Nivolumab (anti-PD-1) Pembrolizumab (anti-PD-1) Durvalumab (anti-PD-L1) | 4 | 625 | [57] |
| Clinical trial | Combination nivolumab (anti-PD-1) + relatlimab (anti-LAG-3) | 6 | 355 | [58] |
5. Preclinical Models of ICI-Myocarditis
5.1. Genetic Deletion of Immune Checkpoint Molecules Causes Myocarditis in Preclinical Models
5.1.1. Pdcd1 Knockout Mice
5.1.2. Pdcd1 Ligand 1 Knockout Mice
5.1.3. Ctla4 Knockout Mouse
5.1.4. ICI Combination Knockout Models
5.2. Antibody Blockade of Immune Checkpoint Molecules Causes Myocarditis in Preclinical Models
5.2.1. PD-1 Blockade
5.2.2. CTLA-4 Blockade
5.2.3. ICI Combination Blockade
6. Recent Insights into Human ICI-Associated Myocarditis Development
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, D.L. Mechanisms Maintaining Peripheral Tolerance. Nat. Immunol. 2009, 11, 21–27. [Google Scholar] [CrossRef]
- Nüssing, S.; Trapani, J.A.; Parish, I.A. Revisiting T Cell Tolerance as a Checkpoint Target for Cancer Immunotherapy. Front. Immunol. 2020, 11, 589641. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (IrAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. A Systematic Review of the Mechanisms Involved in Immune Checkpoint Inhibitors Cardiotoxicity and Challenges to Improve Clinical Safety. Front. Cell Dev. Biol. 2022, 10, 851032. [Google Scholar] [CrossRef]
- Makunts, T.; Saunders, I.M.; Cohen, I.V.; Li, M.; Moumedjian, T.; Issa, M.A.; Burkhart, K.; Lee, P.; Patel, S.P.; Abagyan, R. Myocarditis Occurrence with Cancer Immunotherapy across Indications in Clinical Trial and Post-Marketing Data. Sci. Rep. 2021, 11, 17324. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Moslehi, J.; Lichtman, A.H.; Sharpe, A.H.; Galluzzi, L.; Kitsis, R.N. Immune Checkpoint Inhibitor-Associated Myocarditis: Manifestations and Mechanisms. J. Clin. Investig. 2021, 131, e145186. [Google Scholar] [CrossRef]
- Dong, H.; Qi, Y.; Kong, X.; Wang, Z.; Fang, Y.; Wang, J. PD-1/PD-L1 Inhibitor-Associated Myocarditis: Epidemiology, Characteristics, Diagnosis, Treatment, and Potential Mechanism. Front. Pharmacol. 2022, 13, 835510. [Google Scholar] [CrossRef]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular Magnetic Resonance in Immune Checkpoint Inhibitor-Associated Myocarditis. Eur. Heart J. 2020, 41, 1733. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased Reporting of Fatal Immune Checkpoint Inhibitor- Associated Myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Lafferty, K.J.; Cunningham, A.J. A New Analysis of Allogeneic Interactions. Aust. J. Exp. Biol. Med. Sci. 1975, 53, 27–42. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Anderson, M. Tolerance in the Age of Immunotherapy. N. Engl. J. Med. 2020, 383, 1156. [Google Scholar] [CrossRef]
- Bouneaud, C.; Kourilsky, P.; Bousso, P. Impact of Negative Selection on the T Cell Repertoire Reactive to a Self-Peptide: A Large Fraction of T Cell Clones Escapes Clonal Deletion. Immunity 2000, 13, 829–840. [Google Scholar] [CrossRef]
- Parish, I.A.; Heath, W.R. Too Dangerous to Ignore: Self-Tolerance and the Control of Ignorant Autoreactive T Cells. Immunol. Cell Biol. 2008, 86, 146–152. [Google Scholar] [CrossRef]
- Macián, F.; García-Cózar, F.; Im, S.H.; Horton, H.F.; Byrne, M.C.; Rao, A. Transcriptional Mechanisms Underlying Lymphocyte Tolerance. Cell 2002, 109, 719–731. [Google Scholar] [CrossRef]
- Rocha, B.; Tanchot, C.; Von Boehmer, H. Clonal Anergy Blocks in Vivo Growth of Mature T Cells and Can Be Reversed in the Absence of Antigen. J. Exp. Med. 1993, 177, 1517–1521. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Abbas, A.K. Natural versus Adaptive Regulatory T Cells. Nat. Rev. Immunol. 2003, 3, 253–257. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Shevach, E.M.; Thornton, A.M. TTregs, PTregs, and ITregs: Similarities and Differences. Immunol. Rev. 2014, 259, 88. [Google Scholar] [CrossRef] [PubMed]
- Kalekar, L.A.; Schmiel, S.E.; Nandiwada, S.L.; Lam, W.Y.; Barsness, L.O.; Zhang, N.; Stritesky, G.L.; Malhotra, D.; Pauken, K.E.; Linehan, J.L.; et al. CD4+ T Cell Anergy Prevents Autoimmunity and Generates Regulatory T Cell Precursors. Nat. Immunol. 2016, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.A.L.; Leytze, G.M.; Emswiler, J.; Peach, R.; Bajorath, J.; Cosand, W.; Linsley, P.S. Covalent Dimerization of CD28/CTLA-4 and Oligomerization of CD80/CD86 Regulate T Cell Costimulatory Interactions. J. Biol. Chem. 1996, 271, 26762–26771. [Google Scholar] [CrossRef]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 Control over Foxp3+ Regulatory T Cell Function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef]
- Chuang, E.; Fisher, T.S.; Morgan, R.W.; Robbins, M.D.; Duerr, J.M.; Vander Heiden, M.G.; Gardner, J.P.; Hambor, J.E.; Neveu, M.J.; Thompson, C.B. The CD28 and CTLA-4 Receptors Associate with the Serine/Threonine Phosphatase PP2A. Immunity 2000, 13, 313–322. [Google Scholar] [CrossRef]
- Marengère, L.E.M.; Waterhouse, P.; Duncan, G.S.; Mittrücker, H.W.; Feng, G.S.; Mak, T.W. Regulation of T Cell Receptor Signaling by Tyrosine Phosphatase SYP Association with CTLA-4. Science 1996, 272, 1170–1173. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X. Study and Analysis of Antitumor Resistance Mechanism of PD1/PD-L1 Immune Checkpoint Blocker. Cancer Med. 2020, 9, 8086–8121. [Google Scholar] [CrossRef]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Activation Gene-3 Lymphocyte Negative Regulatory Function of. J. Immunol. Ref. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel Immune Checkpoint Targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Martín, P.; Blanco-Domínguez, R.; Sánchez-Díaz, R. Novel Human Immunomodulatory T Cell Receptors and Their Double-Edged Potential in Autoimmunity, Cardiovascular Disease and Cancer. Cell. Mol. Immunol. 2021, 18, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Martín, P.; Sánchez-Madrid, F. CD69: An Unexpected Regulator of TH17 Cell-Driven Inflammatory Responses. Sci. Signal. 2011, 4, pe14. [Google Scholar] [CrossRef] [PubMed]
- Kottschade, L.A. Incidence and Management of Immune-Related Adverse Events in Patients Undergoing Treatment with Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2018, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 38. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749. [Google Scholar] [CrossRef]
- Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J. Clin. Med. 2021, 10, 603. [Google Scholar] [CrossRef]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Wintersperger, B.J.; Calvillo-Argüelles, O.; Lheureux, S.; Houbois, C.P.; Spreafico, A.; Bedard, P.L.; Neilan, T.G.; Thavendiranathan, P. Immune Checkpoint Inhibitor-Related Myocarditis: An Illustrative Case Series of Applying the Updated Cardiovascular Magnetic Resonance Lake Louise Criteria. Eur. Hear. J. Case Rep. 2022, 6, ytab478. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 171. [Google Scholar] [CrossRef] [PubMed]
- Delombaerde, D.; Vervloet, D.; Franssen, C.; Croes, L.; Gremonprez, F.; Prenen, H.; Peeters, M.; Vulsteke, C. Clinical Implications of Isolated Troponinemia Following Immune Checkpoint Inhibitor Therapy. ESMO Open 2021, 6, 100216. [Google Scholar] [CrossRef]
- Spallarossa, P.; Tini, G.; Sarocchi, M.; Arboscello, E.; Grossi, F.; Queirolo, P.; Zoppoli, G.; Ameri, P. Identification and Management of Immune Checkpoint Inhibitor–Related Myocarditis: Use Troponin Wisely. J. Clin. Oncol. 2019, 37, 2201–2205. [Google Scholar] [CrossRef]
- Waliany, S.; Neal, J.W.; Reddy, S.; Wakelee, H.; Shah, S.A.; Srinivas, S.; Padda, S.K.; Fan, A.C.; Colevas, A.D.; Wu, S.M.; et al. Myocarditis Surveillance With High-Sensitivity Troponin I During Cancer Treatment With Immune Checkpoint Inhibitors. JACC Cardio Oncol. 2021, 3, 137. [Google Scholar] [CrossRef]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H.; Jiménez-Borreguero, L.J.; Matesanz-Marín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, M.L.; et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2021, 384, 2014. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. Cardiotoxicity Associated with Immune Checkpoint Inhibitor Therapy: A Meta-Analysis. Eur. J. Heart Fail. 2021, 23, 1739–1747. [Google Scholar] [CrossRef]
- Dolladille, C.; Akroun, J.; Morice, P.M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.F.; Legallois, D.; L’Orphelin, J.M.; et al. Cardiovascular Immunotoxicities Associated with Immune Checkpoint Inhibitors: A Safety Meta-Analysis. Eur. Heart J. 2021, 42, 4964–4977. [Google Scholar] [CrossRef]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.O.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Champion, S.N.; Stone, J.R. Immune Checkpoint Inhibitor Associated Myocarditis Occurs in Both High-Grade and Low-Grade Forms. Mod. Pathol. 2020, 33, 99–108. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Nielsen, D.; Svane, I.M.; Iversen, K.; Rasmussen, P.V.; Madelaire, C.; Fosbøl, E.; Køber, L.; Gustafsson, F.; Andersson, C.; et al. The Risk of Cardiac Events in Patients Receiving Immune Checkpoint Inhibitors: A Nationwide Danish Study. Eur. Heart J. 2021, 42, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Subramanian, P.; Yatsynovich, Y.V.; Jacobs, D.M.; Chilbert, M.R.; Sharma, U.C.; Ito, F.; Feuerstein, S.G.; Stefanovic, F.; Switzer, B.; et al. Original Research: Clinical Characteristics, Time Course, Treatment and Outcomes of Patients with Immune Checkpoint Inhibitor-Associated Myocarditis. J. Immunother. Cancer 2021, 9, 2553. [Google Scholar] [CrossRef]
- Jain, P.; Bugarin, J.G.; Guha, A.; Jain, C.; Patil, N.; Shen, T.; Stanevich, I.; Nikore, V.; Margolin, K.; Ernstoff, M.; et al. Cardiovascular Adverse Events Are Associated with Usage of Immune Checkpoint Inhibitors in Real-World Clinical Data across the United States. ESMO Open 2021, 6, 100286. [Google Scholar] [CrossRef]
- Nakagomi, Y.; Tajiri, K.; Shimada, S.; Li, S.; Inoue, K.; Murakata, Y.; Murata, M.; Sakai, S.; Sato, K.; Ieda, M. Immune Checkpoint Inhibitor-Related Myositis Overlapping With Myocarditis: An Institutional Case Series and a Systematic Review of Literature. Front. Pharmacol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Barão, V.A.; Costa, R.C.; Shibli, J.A.; Bertolini, M.; Souza, J.G.S. OPDUALAGTM (Nivolumab and Relatlimab-Rmbw) I. Braz. Dent. J. 2022, 33, 1–12. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Wang, J.; Yoshida, T.; Nakaki, F.; Hiai, H.; Okazaki, T.; Honjo, T. Establishment of NOD-Pdcd1-/- Mice as an Efficient Animal Model of Type I Diabetes. Proc. Natl. Acad. Sci. USA 2005, 102, 11823–11828. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor–Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against Cardiac Troponin I Are Responsible for Dilated Cardiomyopathy in PD-1-Deficient Mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Okazaki, I.M.; Yoshida, T.; Chikuma, S.; Kato, Y.; Nakaki, F.; Hiai, H.; Honjo, T.; Okazaki, T. PD-1 Deficiency Results in the Development of Fatal Myocarditis in MRL Mice. Int. Immunol. 2010, 22, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Tarrio, M.L.; Grabie, N.; Bu, D.-X.; Sharpe, A.H.; Lichtman, A.H. PD-1 Protects against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J. Immunol. 2012, 188, 4876–4884. [Google Scholar] [CrossRef]
- Grabie, N.; Gotsman, I.; Dacosta, R.; Pang, H.; Stavrakis, G.; Butte, M.J.; Keir, M.E.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Endothelial Programmed Death-1 Ligand 1 (PD-L1) Regulates CD8 T-Cell-Mediated Injury in the Heart. Circulation 2007, 116, 2062–2071. [Google Scholar] [CrossRef]
- Lucas, J.A.; Menke, J.; Rabacal, W.A.; Schoen, F.J.; Sharpe, A.H.; Kelley, V.R. Programmed Death Ligand 1 Regulates a Critical Checkpoint for Autoimmune Myocarditis and Pneumonitis in MRL Mice. J. Immunol. Ref. 2008, 181, 2513–2521. [Google Scholar] [CrossRef]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 Leads to Massive Lymphoproliferation and Fatal Multiorgan Tissue Destruction, Revealing a Critical Negative Regulatory Role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef]
- Love, V.A.; Grabie, N.; Duramad, P.; Stavrakis, G.; Sharpe, A.; Lichtman, A. CTLA-4 Ablation and Interleukin-12-Driven Differentiation Synergistically Augment Cardiac Pathogenicity of Cytotoxic T Lymphocytes. Circ. Res. 2007, 101, 248–257. [Google Scholar] [CrossRef]
- Wei, S.C.; Meijers, W.C.; Axelrod, M.L.; Anang, N.A.S.; Screever, E.M.; Wescott, E.C.; Johnson, D.B.; Whitley, E.; Lehmann, L.; Courand, P.Y.; et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021, 11, 614–639. [Google Scholar] [CrossRef]
- Okazaki, T.; Okazaki, I.M.; Wang, J.; Sugiura, D.; Nakaki, F.; Yoshida, T.; Kato, Y.; Fagarasan, S.; Muramatsu, M.; Eto, T.; et al. PD-1 and LAG-3 Inhibitory Co-Receptors Act Synergistically to Prevent Autoimmunity in Mice. J. Exp. Med. 2011, 208, 395–407. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Microenvironment and Immunology Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-Cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Fang, Y.H.; Beh, S.T.; Liu, Y.W.; Hsu, L.W.; Yen, C.J.; Liu, P.Y. Programmed Cell Death-1: Programmed Cell Death-Ligand 1 Interaction Protects Human Cardiomyocytes against T-Cell Mediated Inflammation and Apoptosis Response in Vitro. Int. J. Mol. Sci. 2020, 21, 2399. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Helfrich, I.; Hendgen-Cotta, U.B.; Mincu, R.I.; Korste, S.; Mrotzek, S.M.; Spomer, A.; Odersky, A.; Rischpler, C.; Herrmann, K.; et al. Targeting Early Stages of Cardiotoxicity from Anti-PD1 Immune Checkpoint Inhibitor Therapy. Eur. Heart J. 2022, 43, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Hu, G.; Hu, Q.; Chang, Y.; Hu, Y.; Gao, L.; Chen, X.; Yang, P.-C.; Zhang, Y.; Li, M.; et al. Single-Cell RNA Sequencing to Dissect the Immunological Network of Autoimmune Myocarditis. Circulation 2020, 142, 348–400. [Google Scholar] [CrossRef] [PubMed]
- Baldeviano, G.C.; Barin, J.G.; Talor, M.V.; Srinivasan, S.; Bedja, D.; Zheng, D.; Gabrielson, K.; Iwakura, Y.; Rose, N.R.; Cihakova, D. Interleukin-17A Is Dispensable for Myocarditis but Essential for the Progression to Dilated Cardiomyopathy. Circ. Res. 2010, 106, 1646–1655. [Google Scholar] [CrossRef]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac Myosin-Th17 Responses Promote Heart Failure in Human Myocarditis. JCI Insight 2016, 1, e85851. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, D.; Xie, J.; Zhang, H.; Ying, L.; Yang, G.; Qiao, Z.; Li, L. Cutting Edge: CTLA-4 Suppresses Th17 Cell Differentiation B7 Interaction. J. Immunol. 2010, 185, 1375–1378. [Google Scholar] [CrossRef]
- Von Euw, E.; Chodon, T.; Attar, N.; Jalil, J.; Koya, R.C.; Comin-Anduix, B.; Ribas, A. CTLA4 Blockade Increases Th17 Cells in Patients with Metastatic Melanoma. J. Transl. Med. 2009, 7, 35. [Google Scholar] [CrossRef]
- Liu, S.Y.; Huang, W.C.; Yeh, H.I.; Ko, C.C.; Shieh, H.R.; Hung, C.L.; Chen, T.Y.; Chen, Y.-J. Sequential Blockade of PD-1 and PD-L1 Causes Fulminant Cardiotoxicity—From Case Report to Mouse Model Validation. Cancers 2019, 11, 580. [Google Scholar] [CrossRef]
- Du, X.; Liu, M.; Su, J.; Zhang, P.; Tang, F.; Ye, P.; Devenport, M.; Wang, X.; Zhang, Y.; Liu, Y.; et al. Uncoupling Therapeutic from Immunotherapy-Related Adverse Effects for Safer and Effective Anti-CTLA-4 Antibodies in CTLA4 Humanized Mice. Cell Res. 2018, 28, 433–447. [Google Scholar] [CrossRef]
- Ji, C.; Roy, M.D.; Golas, J.; Vitsky, A.; Ram, S.; Kumpf, S.W.; Martin, M.; Barletta, F.; Meier, W.A.; Hooper, A.T.; et al. Myocarditis in Cynomolgus Monkeys Following Treatment with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2019, 25, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Havari, E.; Pinto, S.; Gottumukkala, R.V.S.R.K.; Cornivelli, L.; Raddassi, K.; Matsui, T.; Rosenzweig, A.; Bronson, R.T.; Smith, R.; et al. Impaired Thymic Tolerance to α-Myosin Directs Autoimmunity to the Heart in Mice and Humans. J. Clin. Investig. 2011, 121, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Cham, J.; Zhang, L.; Fong, G.; Kwek, S.S.; Klinger, M.; Faham, M.; Fong, L. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T Cell Repertoire. Cancer Res. 2017, 77, 1322. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.X.; Chaudhuri, A.A.; Nene, A.; Bacchiocchi, A.; Earland, N.; Vesely, M.D.; Usmani, A.; Turner, B.E.; Steen, C.B.; Luca, B.A.; et al. T Cell Characteristics Associated with Toxicity to Immune Checkpoint Blockade in Patients with Melanoma. Nat. Med. 2022, 28, 353–362. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, J.H.; Gide, T.N.; Menzies, A.M.; Guminski, A.; Carlino, M.S.; Breen, E.J.; Yang, J.Y.H.; Ghazanfar, S.; Kefford, R.F.; et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. 2019, 25, 1557–1563. [Google Scholar] [CrossRef]
- Kim, K.H.; Hur, J.Y.; Cho, J.; Ku, B.M.; Koh, J.; Koh, J.Y.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; et al. Immune-Related Adverse Events Are Clustered into Distinct Subtypes by T-Cell Profiling before and Early after Anti-PD-1 Treatment. Oncoimmunology 2020, 9, e1722023. [Google Scholar] [CrossRef]
- Grabie, N.; Lichtman, A.H.; Padera, R. T Cell Checkpoint Regulators in the Heart. Cardiovasc. Res. 2019, 115, 869–877. [Google Scholar] [CrossRef]
- van der Vegt, S.A.; Polonchuk, L.; Wang, K.; Waters, S.L.; Baker, R.E. Mathematical Modelling of Autoimmune Myocarditis and the Effects of Immune Checkpoint Inhibitors. J. Theor. Biol. 2022, 537, 111002. [Google Scholar] [CrossRef]
| Immune Checkpoint | Immune Checkpoint Inhibitor | FDA Approval Date | Approved Target Cancers |
|---|---|---|---|
| CTLA-4 | Ipilimumab | 2011 | Unresectable and metastatic melanoma (alone, with nivolumab or as an adjuvant) Advanced renal cell carcinoma (in combination with nivolumab) Microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer (in combination with nivolumab) Hepatocellular carcinoma (alone or in combination with nivolumab) Metastatic non-small cell lung cancer (in combination with nivolumab) Unresectable malignant pleural mesothelioma (in combination with nivolumab) Unresectable and metastatic esophageal cancer (in combination with nivolumab) |
| PD-1 | Nivolumab | 2014 | Unresectable and metastatic melanoma (alone, with ipilimumab or with relatlimab) Resectable non-small cell lung cancer (as a neoadjuvant) Metastatic non-small cell lung cancer (alone or in combination with ipilimumab) Malignant pleural mesothelioma (in combination with ipilimumab) Advanced renal cell carcinoma (alone or with ipilimumab) Refractory classical Hodgkin lymphoma Recurrent and metastatic squamous cell carcinoma of the head and neck Advanced and metastatic urothelial carcinoma Microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer (in combination with ipilimumab) Hepatocellular carcinoma (alone or in combination with ipilimumab) Unresectable and metastatic esophageal cancer (alone or in combination with ipilimumab) Resected esophageal cancer (as an adjuvant) Advanced and metastatic gastric cancer, gastresophageal junction cancer, and esophageal adenocarcinoma |
| Pembrolizumab | 2014 | Unresectable and metastatic melanoma Stage III and metastatic non-small cell lung cancer Unresectable and metastatic head and neck squamous cell cancer Refractory classical Hodgkin lymphoma Refractory primary mediastinal large B cell lymphoma Advanced and metastatic urothelial carcinoma Microsatellite instability-high or mismatch repair deficient solid tumors Microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer Advanced unresectable and metastatic gastric cancer Unresectable and metastatic esophageal cancer Recurrent and metastatic cervical cancer Hepatocellular carcinoma Advanced and metastatic Merkel cell carcinoma Renal cell carcinoma Endometrial carcinoma Unresectable and metastatic tumor mutational burden-high cancer Recurrent and metastatic cutaneous squamous cell carcinoma Recurrent unresectable and metastatic triple-negative breast cancer | |
| Cemiplimab | 2019 | Metastatic cutaneous squamous cell carcinoma Advanced and metastatic basal cell carcinoma Advanced and metastatic non-small cell lung cancer | |
| PD-L1 | Atezolizumab | 2016 | Advanced and metastatic urothelial Metastatic non-small cell lung cancer Small cell lung cancer Unresectable and metastatic hepatocellular carcinoma Unresectable and metastatic melanoma |
| Durvalumab | 2017 | Advanced and metastatic urothelial carcinoma Stage III non-small cell lung cancer Advanced small cell lung cancer | |
| Avelumab | 2017 | Metastatic Merkel cell carcinoma Advanced and metastatic urothelial carcinoma Advanced renal cell carcinoma | |
| LAG-3 | Relatlimab | 2022 | Unresectable or metastatic melanoma (in combination with nivolumab) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Alejandre, R.; Ruiz-Fernández, I.; Martín, P. Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis. Cancers 2022, 14, 4494. https://doi.org/10.3390/cancers14184494
Jiménez-Alejandre R, Ruiz-Fernández I, Martín P. Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis. Cancers. 2022; 14(18):4494. https://doi.org/10.3390/cancers14184494
Chicago/Turabian StyleJiménez-Alejandre, Rosa, Ignacio Ruiz-Fernández, and Pilar Martín. 2022. "Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis" Cancers 14, no. 18: 4494. https://doi.org/10.3390/cancers14184494
APA StyleJiménez-Alejandre, R., Ruiz-Fernández, I., & Martín, P. (2022). Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis. Cancers, 14(18), 4494. https://doi.org/10.3390/cancers14184494





