A Novel, Quick, and Reliable Smartphone-Based Method for Serum PSA Quantification: Original Design of a Portable Microfluidic Immunosensor-Based System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Electrode Fabrication
2.3. PDMS Microfluidic Device Fabrication
2.4. Characterization of the Solid Support
2.5. Immobilization of the Anti-PSA in Magnetic Microbeads
2.6. Analytical Procedure for PSA Determination
2.7. Blood Sample Collection
3. Results
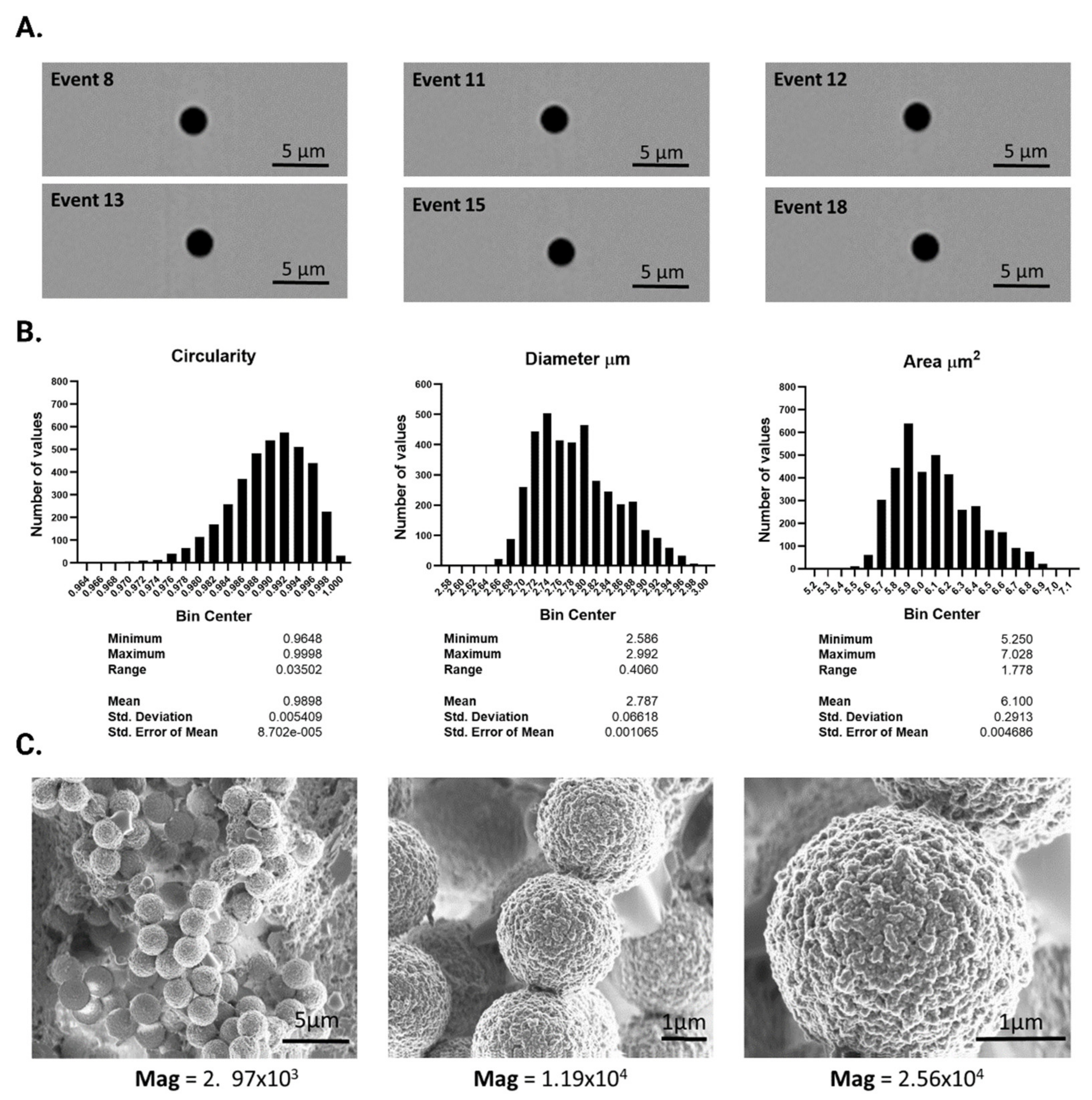
3.1. Characterization and Functionalization of the Solid Support
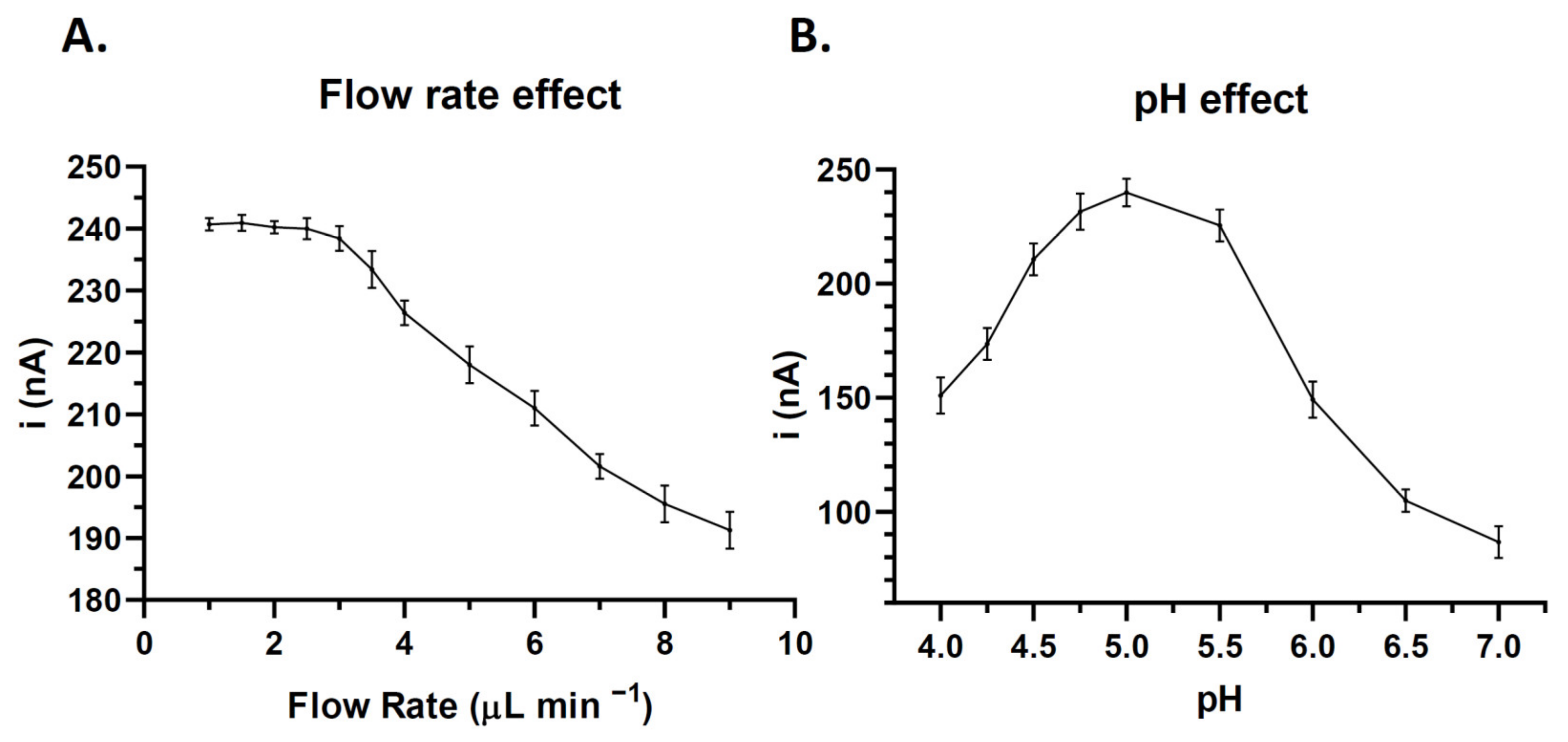
3.2. Optimization of Experimental Variables
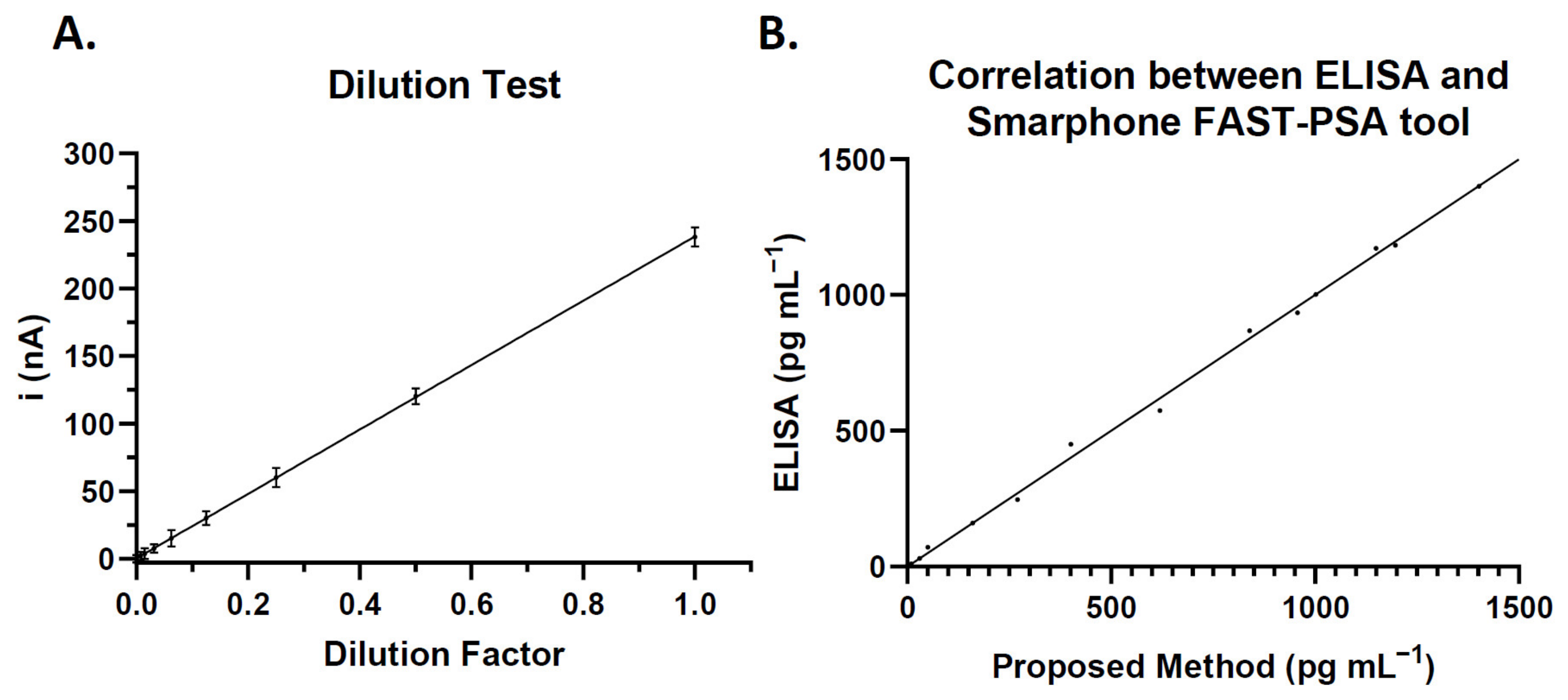
3.3. Quantitative Determination of PSA Biomarker in the Microfluidic Immunosensor
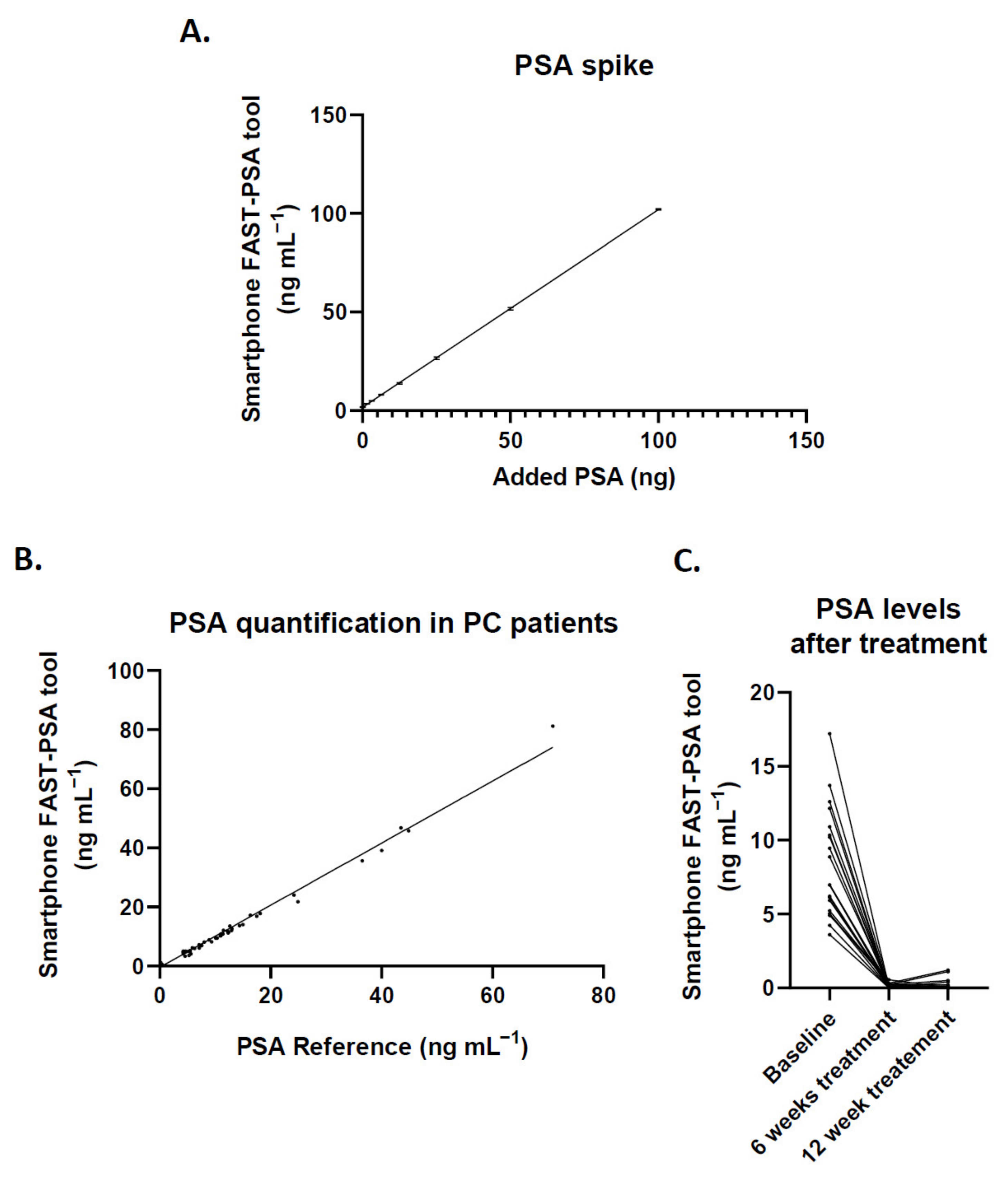
3.4. Clinical Performance of the Smartphone FAST-PSA Tool Platform for PSA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lianidou, E.; Pantel, K. Liquid biopsies. Genes Chromosom. Cancer 2019, 58, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 185, 297–312. [Google Scholar] [CrossRef]
- Jacobs, E.L.; Haskell, C.M. Clinical use of tumor markers in oncology. Curr. Probl. Cancer 1991, 15, 301–350. [Google Scholar] [CrossRef]
- Chatterjee, S.K.; Zetter, B.R. Cancer biomarkers: Knowing the present and predicting the future. Futur. Oncol. 2005, 1, 37–50. [Google Scholar] [CrossRef]
- Harris, R.; Lohr, K.N. Screening for prostate cancer: An update of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2002, 137, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Zazzara, M.; Zattoni, F. Stem cells, Biomarkers and Genetic Profiling: Approaching future challenges in Urology. Urologia J. 2016, 83, 4–13. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Bast, R.C.; Gold, P.; Chu, T.M.; Magnani, J.L. Reflection on the discovery of carcinoembryonic antigen, prostate-specific antigen and carbohydrate antigens ca125 and ca19.9. Clin. Chem. 2013, 59, 22. [Google Scholar] [CrossRef]
- Skrzipczyk, H.J.; Verdier, P. Bioanalytical Assays: RIA/EIA. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 869–886. [Google Scholar] [CrossRef]
- Li, W.; Shao, B.; Liu, C.; Wang, H.; Zheng, W.; Kong, W.; Liu, X.; Xu, G.; Wang, C.; Li, H.; et al. Noninvasive Diagnosis and Molecular Phenotyping of Breast Cancer through Microbead-Assisted Flow Cytometry Detection of Tumor-Derived Extracellular Vesicles. Small Methods 2018, 2, 1800122. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, C.; Chang, H.; Jiang, M.; Sun, X.; Jing, W.; Huang, H.; Huang, D.; Kong, L.; Chen, Z.; et al. A Label-Free Photoelectrochemical Immunosensor for Prostate Specific Antigen Detection Based on Ag2S Sensitized Ag/AgBr/BiOBr Heterojunction by in-Situ Growth Method. Bioelectrochemistry 2021, 142, 107928. [Google Scholar] [CrossRef]
- Darvishi, E.; Ehzari, H.; Shahlaei, M.; Behbood, L.; Arkan, E. The Electrochemical Immunosensor for Detection of Prostatic Specific Antigen Using Quince Seed Mucilage-GNPs-SNPs as a Green Composite. Bioelectrochemistry 2021, 139, 107744. [Google Scholar] [CrossRef] [PubMed]
- Assari, P.; Rafati, A.A.; Feizollahi, A.; Joghani, R.A. Fabrication of a Sensitive Label Free Electrochemical Immunosensor for Detection of Prostate Specific Antigen Using Functionalized Multi-Walled Carbon Nanotubes/Polyaniline/AuNPs. Mater. Sci. Eng. C 2020, 115, 111066. [Google Scholar] [CrossRef] [PubMed]
- Shamsazar, A.; Asadi, A.; Seifzadeh, D.; Mahdavi, M. A Novel and Highly Sensitive Sandwich-Type Immunosensor for Prostate-Specific Antigen Detection Based on MWCNTs-Fe3O4 Nanocomposite. Sens. Actuators B Chem. 2021, 346, 130459. [Google Scholar] [CrossRef]
- Chen, S.; Xu, L.; Sheng, K.; Zhou, Q.; Dong, B.; Bai, X.; Lu, G.; Song, H. A Label-Free Electrochemical Immunosensor Based on Facet-Controlled Au Nanorods/Reduced Graphene Oxide Composites for Prostate Specific Antigen Detection. Sens. Actuators B Chem. 2021, 336, 129748. [Google Scholar] [CrossRef]
- Yang, C.; Guo, Q.; Lu, Y.; Zhang, B.; Nie, G. Ultrasensitive “Signal-on” Electrochemiluminescence Immunosensor for Prostate-Specific Antigen Detection Based on Novel Nanoprobe and Poly(Indole-6-Carboxylic Acid)/Flower-like Au Nanocomposite. Sens. Actuators B Chem. 2020, 303, 127246. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Yan, L.; Petrenko, V.A.; Liu, A. Specific Phages-Based Electrochemical Impedimetric Immunosensors for Label-Free and Ultrasensitive Detection of Dual Prostate-Specific Antigens. Sens. Actuators B Chem. 2019, 297, 126727. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Fu, Y.Z.; Ren, S.W.; Cao, J.T.; Liu, Y.M. A Novel Chemiluminescence Imaging Immunosensor for Prostate Specific Antigen Detection Based on a Multiple Signal Amplification Strategy. Biosens. Bioelectron. 2021, 171, 112729. [Google Scholar] [CrossRef]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A Novel and Ultrasensitive Sandwich-Type Electrochemical Immunosensor Based on Delaminated MXene@AuNPs as Signal Amplification for Prostate Specific Antigen (PSA) Detection and Immunosensor Validation. Talanta 2020, 220, 121403. [Google Scholar] [CrossRef]
- Ehzari, H.; Amiri, M.; Safari, M. Enzyme-Free Sandwich-Type Electrochemical Immunosensor for Highly Sensitive Prostate Specific Antigen Based on Conjugation of Quantum Dots and Antibody on Surface of Modified Glassy Carbon Electrode with Core–Shell Magnetic Metal-Organic Frameworks. Talanta 2020, 210, 120641. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.H.; Saianand, G.; Gopalan, A.I.; Jakmunee, J. Highly Sensitive Voltammetric Immunosensor for the Detection of Prostate Specific Antigen Based on Silver Nanoprobe Assisted Graphene Oxide Modified Screen Printed Carbon Electrode. Talanta 2020, 208, 120389. [Google Scholar] [CrossRef]
- Choosang, J.; Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. An Ultrasensitive Label-Free Electrochemical Immunosensor Based on 3D Porous Chitosan–Graphene–Ionic Liquid–Ferrocene Nanocomposite Cryogel Decorated with Gold Nanoparticles for Prostate-Specific Antigen. Talanta 2021, 224, 121787. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A. Dual-Modality Impedimetric Immunosensor for Early Detection of Prostate-Specific Antigen and Myoglobin Markers Based on Antibody-Molecularly Imprinted Polymer. Talanta 2019, 202, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Lin, Z.; Lu, F.; Chen, Y.; Huang, X.; Gao, W. A Sensitive Electrochemiluminescence Immunosensor for the Detection of PSA Based on CdWS Nanocrystals and Ag + @UIO-66-NH 2 as a Novel Coreaction Accelerator. Electrochim. Acta 2019, 302, 207–215. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Mei, L.; Zhang, L.; Wang, X.; Liao, X.; Qiao, X.; Hong, C. PSA Detection Electrochemical Immunosensor Based on MOF-235 Nanomaterial Adsorption Aggregation Signal Amplification Strategy. Microchem. J. 2021, 171, 106870. [Google Scholar] [CrossRef]
- Liu, X.P.; Chang, N.; Chen, J.S.; Mao, C.J.; Jin, B.K. Ultrasensitive Photoelectrochemical Immunosensor Based on a G-C3N4/SnS2 Nanocomposite for Prostate-Specific Antigen Detection. Microchem. J. 2021, 168, 106337. [Google Scholar] [CrossRef]
- Feng, J.; Li, Y.; Li, M.; Li, F.; Han, J.; Dong, Y.; Chen, Z.; Wang, P.; Liu, H.; Wei, Q. A Novel Sandwich-Type Electrochemical Immunosensor for PSA Detection Based on PtCu Bimetallic Hybrid (2D/2D) RGO/g-C3N4. Biosens. Bioelectron. 2017, 91, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, B.; Salimi, A.; Hallaj, R.; Moradi, F. Ultrasensitive Electrochemical Immunosensor for PSA Biomarker Detection in Prostate Cancer Cells Using Gold Nanoparticles/PAMAM Dendrimer Loaded with Enzyme Linked Aptamer as Integrated Triple Signal Amplification Strategy. Biosens. Bioelectron. 2015, 74, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, N.E.; Park, J.S.; Park, I.J.; Kim, J.G.; Cho, H.J. Organic Electrochemical Transistor Based Immunosensor for Prostate Specific Antigen (PSA) Detection Using Gold Nanoparticles for Signal Amplification. Biosens. Bioelectron. 2010, 25, 2477–2482. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Sun, X.; Ma, H.; Zhang, Y.; Wei, Q. Dual-Responsive Electrochemical Immunosensor for Prostate Specific Antigen Detection Based on Au-CoS/Graphene and CeO2/Ionic Liquids Doped with Carboxymethyl Chitosan Complex. Biosens. Bioelectron. 2017, 94, 141–147. [Google Scholar] [CrossRef]
- Gutiérrez-Zúñiga, G.G.; Hernández-López, J.L. Sandwich-Type ELISA Impedimetric Immunosensor for Early Detection of Prostate-Specific Antigen (PSA) in Human Serum. Procedia Chem. 2014, 12, 47–54. [Google Scholar] [CrossRef]
- Suresh, L.; Brahman, P.K.; Reddy, K.R.; Bondili, J.S. Development of an Electrochemical Immunosensor Based on Gold Nanoparticles Incorporated Chitosan Biopolymer Nanocomposite Film for the Detection of Prostate Cancer Using PSA as Biomarker. Enzyme Microb. Technol. 2018, 112, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Avan, A.A. Nanostructures for Nonlabeled and Labeled Electrochemical Immunosensors: Simultaneous Electrochemical Detection of Cancer Markers: A Review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Espro, C.; Celesti, C.; Ferlazzo, A.; Neri, G. Smart Biosensors for Cancer Diagnosis Based on Graphene Quantum Dots. Cancers 2021, 13, 3194. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, Y.; Zhang, S.; Chen, X.; Shao, T.; Feng, D. Electrochemical Immunosensor Based on Metal Ions Functionalized CNSs@Au NPs Nanocomposites as Signal Amplifier for Simultaneous Detection of Triple Tumor Markers. J. Electroanal. Chem. 2021, 880, 114882. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Zhao, X.; Chen, H.; Xu, H.; Bai, L.; Wang, W.; Yang, H.; Wei, D.; Yuan, B. Highly Sensitive Electrochemical Immunosensor for the Simultaneous Detection of Multiple Tumor Markers for Signal Amplification. Talanta 2021, 226, 122133. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Zhou, B.; Ian, H.; Shao, H. Advanced Sensitivity Amplification Strategies for Voltammetric Immunosensors of Tumor Marker: State of the Art. Biosens. Bioelectron. 2021, 178, 113021. [Google Scholar] [CrossRef]
- Cho, H.Y.; Choi, J.H.; Lim, J.; Lee, S.N.; Choi, J.W. Microfluidic Chip-Based Cancer Diagnosis and Prediction of Relapse by Detecting Circulating Tumor Cells and Circulating Cancer Stem Cells. Cancers 2021, 13, 1385. [Google Scholar] [CrossRef]
- Regiart, M.; Fernández-Baldo, M.A.; Spotorno, V.G.; Bertolino, F.A.; Raba, J. Ultra Sensitive Microfluidic Immunosensor for Determination of Clenbuterol in Bovine Hair Samples Using Electrodeposited Gold Nanoparticles and Magnetic Micro Particles as Bio-Affinity Platform. Biosens. Bioelectron. 2013, 41, 211–217. [Google Scholar] [CrossRef]
- Regiart, M.; Fernández-Baldo, M.A.; Villarroel-Rocha, J.; Messina, G.A.; Bertolino, F.A.; Sapag, K.; Timperman, A.T.; Raba, J. Microfluidic Immunosensor Based on Mesoporous Silica Platform and CMK-3/Poly-Acrylamide-Co-Methacrylate of Dihydrolipoic Acid Modified Gold Electrode for Cancer Biomarker Detection. Anal. Chim. Acta 2017, 963, 83–92. [Google Scholar] [CrossRef]
- Zhang, J.; Chua, S.L.; Khoo, B.L. Worm-Based Microfluidic Biosensor for Real-Time Assessment of the Metastatic Status. Cancers 2021, 13, 873. [Google Scholar] [CrossRef]
- Chan, K.M.; Gleadle, J.M.; Gregory, P.A.; Phillips, C.A.; Safizadeh Shirazi, H.; Whiteley, A.; Li, J.; Vasilev, K.; Macgregor, M. Selective Microfluidic Capture and Detection of Prostate Cancer Cells from Urine without Digital Rectal Examination. Cancers 2021, 13, 5544. [Google Scholar] [CrossRef] [PubMed]
- Panini, N.V.; Messina, G.A.; Salinas, E.; Fernández, H.; Raba, J. Integrated Microfluidic Systems with an Immunosensor Modified with Carbon Nanotubes for Detection of Prostate Specific Antigen (PSA) in Human Serum Samples. Biosens. Bioelectron. 2008, 23, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Huang, J.; Liu, S.; Lin, T.; Zhao, S. Magneto-Controlled Fluorescent Immunosensor for Sensitive Determination of Biomarker via Three-Dimensional AuNCs/Liposome Networks. Sens. Actuators B Chem. 2021, 342, 130075. [Google Scholar] [CrossRef]
- Barry, M.J. Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N. Engl. J. Med. 2001, 344, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Triroj, N.; Jaroenapibal, P.; Shi, H.; Yeh, J.; Beresford, R. Microfluidic chip-based nanoelectrode array as miniaturized biochemical sensing platform for prostate-specific antigen detection. Biosens. Bioelectron. 2011, 26, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Chikkaveeraiah, B.V.; Mani, V.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Microfluidic electrochemical immunoarray for ultrasensitive detection of two cancer biomarker proteins in serum. Biosens. Bioelectron. 2011, 26, 4477–4483. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Vaze, A.; Shen, M.; Rusling, J.F. High-Throughput Electrochemical Microfluidic Immunoarray for Multiplexed Detection of Cancer Biomarker Proteins. ACS Sensors 2016, 1, 1036–1043. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Am. Chem. Soc. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Saem, S.; Zhu, Y.; Luu, H.; Moran-Mirabal, J. Bench-Top Fabrication of an All-PDMS Microfluidic Electrochemical Cell Sensor Integrating Micro/Nanostructured Electrodes. Sensors 2017, 17, 732. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Gehlot, P.; Sidapra, K.; Edwards, A.D.; Reis, N.M. Portable smartphone quantitation of prostate specific antigen (PSA) in a fluoropolymer microfluidic device. Biosens. Bioelectron. 2015, 70, 5–14. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Zafeirakis, A.; Kalogeropoulou, K.; Giannakos, G.; Skevis, A.; Kintzios, S. An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum. Sensors 2018, 18, 3834. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Nanus, D.M.; Erickson, D.; Mehta, S. Highly Portable Quantitative Screening Test for Prostate-Specific Antigen at Point of Care. Curr. Res. Biotechnol. 2021, 3, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Traynor, S.M.; Pandey, R.; Maclachlan, R.; Hosseini, A.; Didar, T.F.; Li, F.; Soleymani, L. Review—Recent Advances in Electrochemical Detection of Prostate Specific Antigen (PSA) in Clinically-Relevant Samples. J. Electrochem. Soc. 2020, 167, 037551. [Google Scholar] [CrossRef]

| a Control Sample | Intra-Assay | Inter-Assay | ||
|---|---|---|---|---|
| Mean | CV% | Mean | CV% | |
| 10 | 9.98 | 2.71 | 10.04 | 4.46 |
| 800 | 799.95 | 3.82 | 800.02 | 5.42 |
| 1200 | 1199.98 | 3.50 | 1200.03 | 6.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, F.G.; Gómez, G.E.; González-Martinez, C.; Valero, T.; Expósito-Hernández, J.; Puche, I.; Rodriguez-Martinez, A.; Serrano, M.J.; Lorente, J.A.; Fernández-Baldo, M.A. A Novel, Quick, and Reliable Smartphone-Based Method for Serum PSA Quantification: Original Design of a Portable Microfluidic Immunosensor-Based System. Cancers 2022, 14, 4483. https://doi.org/10.3390/cancers14184483
Ortega FG, Gómez GE, González-Martinez C, Valero T, Expósito-Hernández J, Puche I, Rodriguez-Martinez A, Serrano MJ, Lorente JA, Fernández-Baldo MA. A Novel, Quick, and Reliable Smartphone-Based Method for Serum PSA Quantification: Original Design of a Portable Microfluidic Immunosensor-Based System. Cancers. 2022; 14(18):4483. https://doi.org/10.3390/cancers14184483
Chicago/Turabian StyleOrtega, Francisco Gabriel, Germán E. Gómez, Coral González-Martinez, Teresa Valero, José Expósito-Hernández, Ignacio Puche, Alba Rodriguez-Martinez, María José Serrano, José Antonio Lorente, and Martín A. Fernández-Baldo. 2022. "A Novel, Quick, and Reliable Smartphone-Based Method for Serum PSA Quantification: Original Design of a Portable Microfluidic Immunosensor-Based System" Cancers 14, no. 18: 4483. https://doi.org/10.3390/cancers14184483
APA StyleOrtega, F. G., Gómez, G. E., González-Martinez, C., Valero, T., Expósito-Hernández, J., Puche, I., Rodriguez-Martinez, A., Serrano, M. J., Lorente, J. A., & Fernández-Baldo, M. A. (2022). A Novel, Quick, and Reliable Smartphone-Based Method for Serum PSA Quantification: Original Design of a Portable Microfluidic Immunosensor-Based System. Cancers, 14(18), 4483. https://doi.org/10.3390/cancers14184483








