Real-World Outcomes and Treatments Patterns Prior and after the Introduction of First-Line Immunotherapy for the Treatment of Metastatic Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Source
2.2. Study Population
2.3. Cohort Description
2.4. Assessments and Study Endpoints
2.5. Statistical Analysis
3. Results
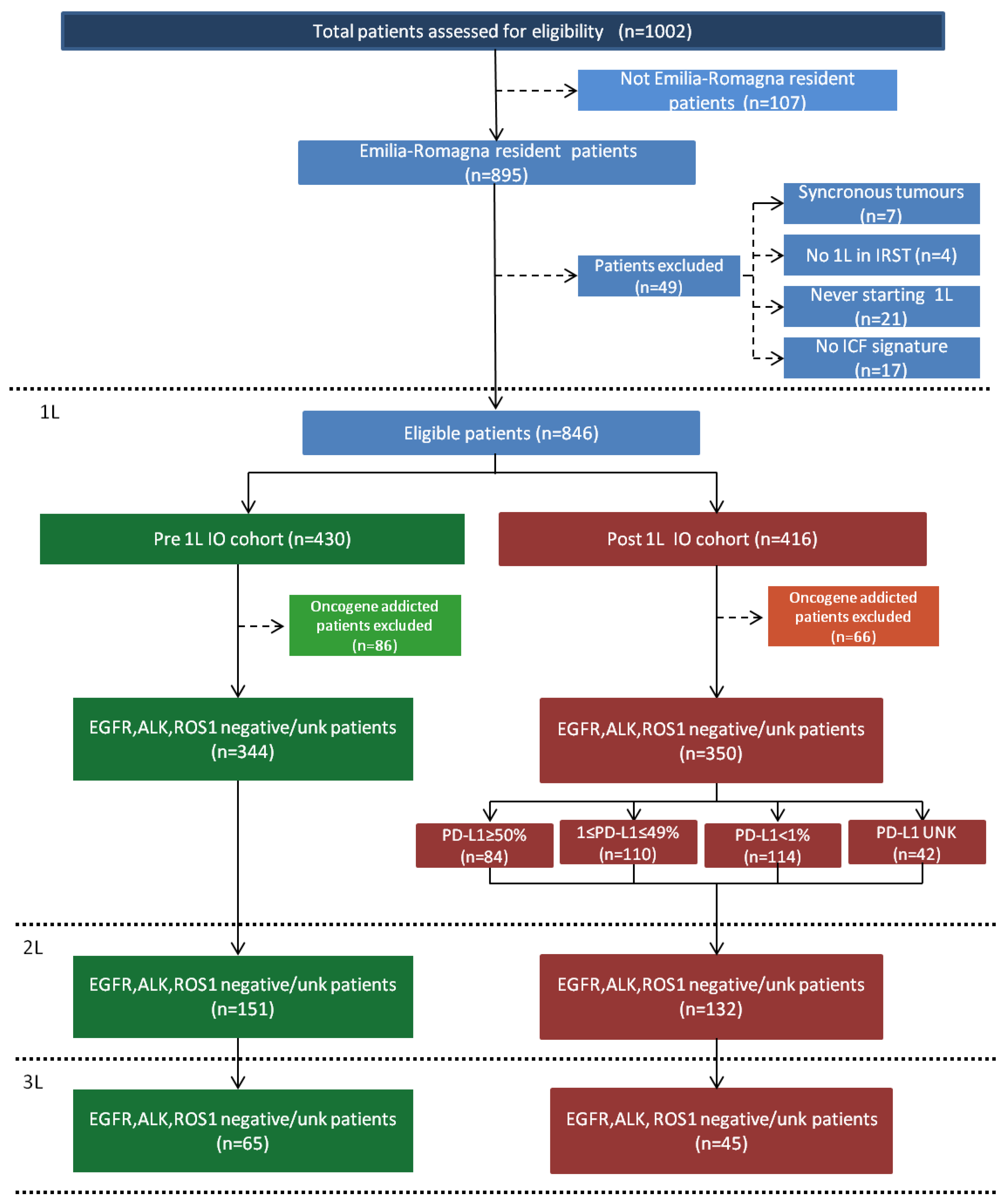
3.1. Patients
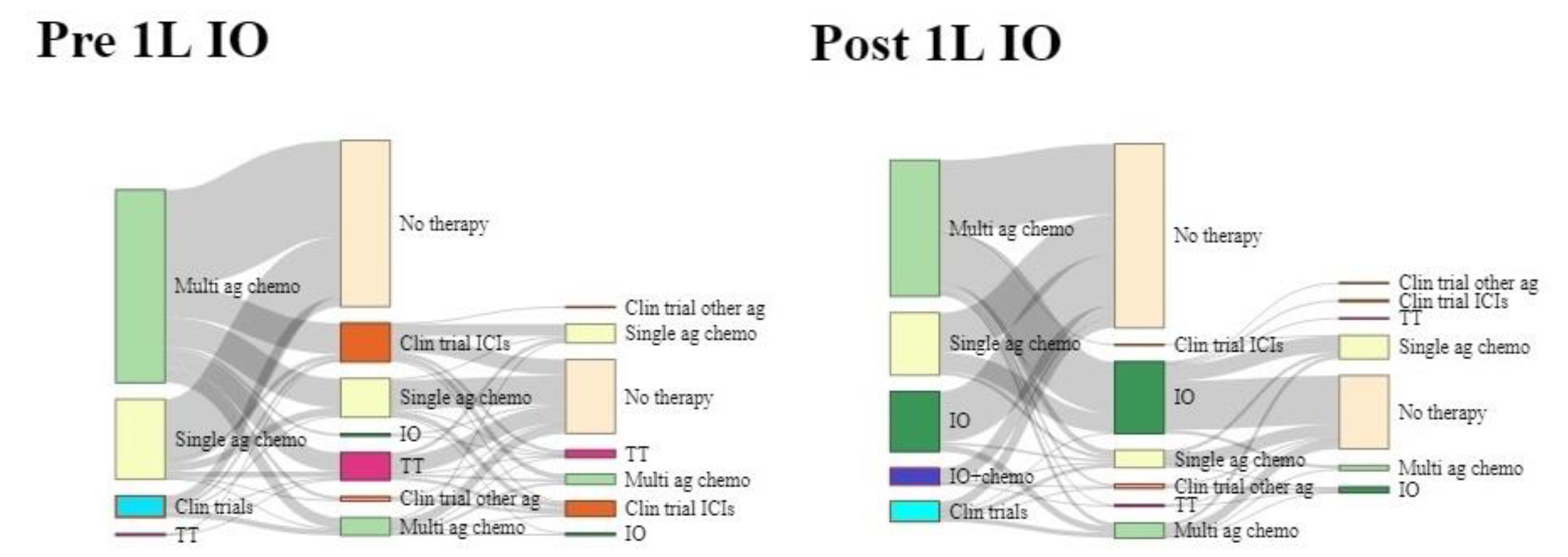
3.2. First-Line Treatment Patterns
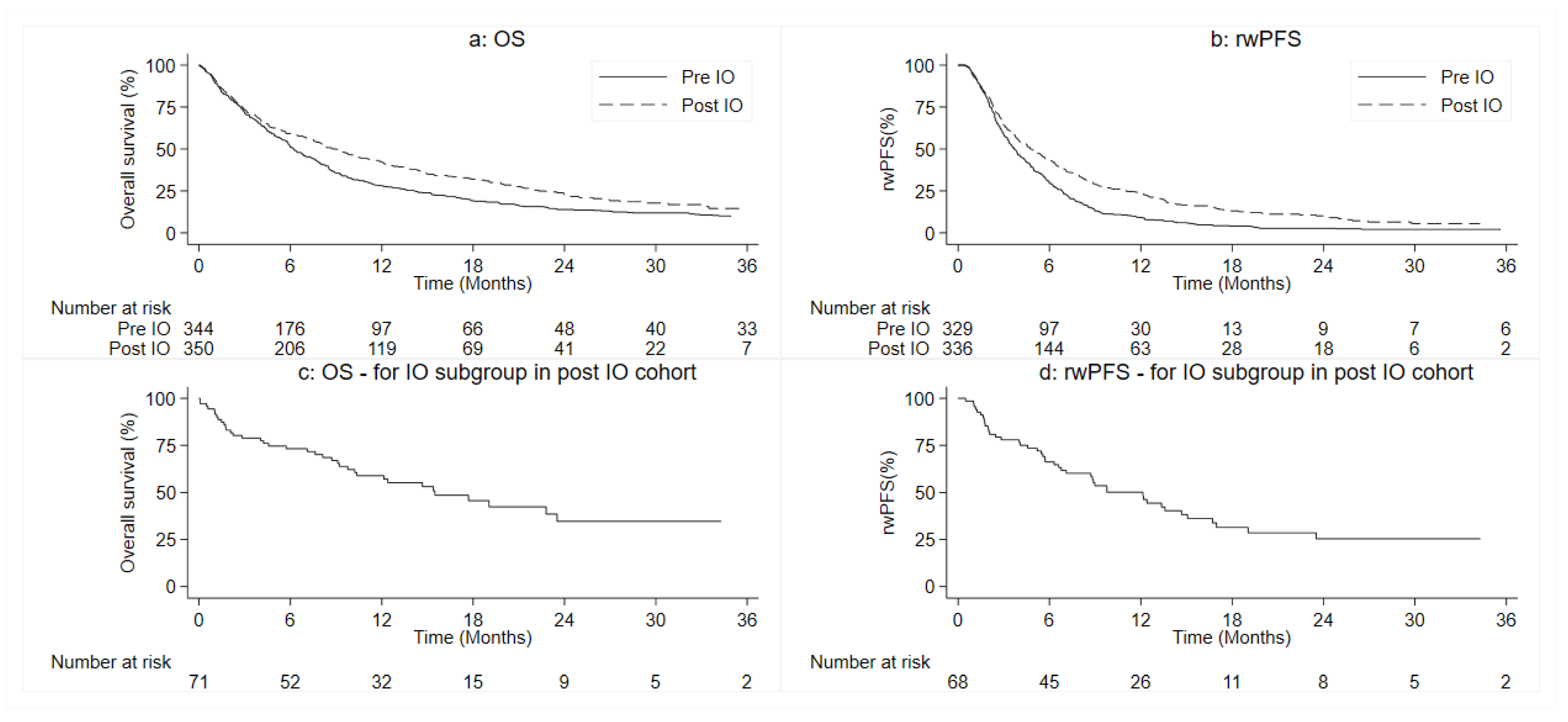
3.3. Patients’ Outcomes
3.4. Response to the First-Line Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bittoni, M.A.; Arunachalam, A.; Li, H.; Camacho, R.; He, J.; Zhong, Y.; Lubiniecki, G.M.; Carbone, D.P. Real-World Treatment Patterns, Overall Survival, and Occurrence and Costs of Adverse Events Associated With First-line Therapies for Medicare Patients 65 Years and Older With Advanced Non-small-cell Lung Cancer: A Retrospective Study. Clin. Lung Cancer 2018, 19, e629–e645. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Parikh, P.; Von Pawel, J.; Biesma, B.; Vansteenkiste, J.; Manegold, C.; Serwatowski, P.; Gatzemeier, U.; Digumarti, R.; Zukin, M.; et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008, 26, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Available online: https://www.aifa.gov.it/en/-/pubblicazione-schede-di-monitoraggio-registro-opdivo-22-04-2016- (accessed on 8 September 2022).
- Available online: https://www.aifa.gov.it/en/-/cda-aifa-rimborsabilita-per-pembrolizumab (accessed on 8 September 2022).
- Il Prontuario Terapeutico Regionale-Elenco dei Farmaci. Available online: https://salute.regione.emilia-romagna.it/ssr/strumenti-e-informazioni/ptr (accessed on 8 September 2022).
- Pasello, G.; Pavan, A.; Attili, I.; Bortolami, A.; Bonanno, L.; Menis, J.; Conte, P.F.; Guarneri, V. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat. Rev. 2020, 87, 102031. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Fan, Y. Progression Patterns, Treatment, and Prognosis Beyond Resistance of Responders to Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 642883. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Perez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 tria. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A.P.; Arunachalam, A.; Burke, T.; McKay, C.; Cao, X.; Sorg, R.; Carbone, D.P. Real-world first-line treatment and overall survival in non-small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting. PLoS ONE 2017, 12, e0178420. [Google Scholar] [CrossRef]
- Isobe, H.; Mori, K.; Minato, K.; Katsura, H.; Taniguchi, K.; Arunachalam, A.; Kothari, S.; Cao, X.; Kato, T. Real-world practice patterns for patients with advanced non-small cell lung cancer: Multicenter retrospective cohort study in Japan. Lung Cancer Targets Ther. 2017, 8, 191–206. [Google Scholar] [CrossRef]
- Lee, D.H.; Tsao, M.-S.; Kambartel, K.-O.; Isobe, H.; Huang, M.-S.; Barrios, C.H.; Khattak, A.; de Marinis, F.; Kothari, S.; Arunachalam, A.; et al. Molecular testing and treatment patterns for patients with advanced non-small cell lung cancer: PIvOTAL observational study. PLoS ONE 2018, 13, e0202865. [Google Scholar] [CrossRef]
- Luciani, A.; Ghidini, A.; Dottorini, L.; Petrelli, F. Safety and Effectiveness of Immune Checkpoint Inhibitors in Older Patients with Cancer: A Systematic Review of 48 Real-World Studies. Drugs Aging 2021, 38, 1055–1065. [Google Scholar] [CrossRef]
- Zhang, S.; Pease, D.F.; Kulkarni, A.A.; Kumar, M.; Shanley, R.M.; Xu, B.; Joshi, S.P.; Patel, M.R. Real-World Outcomes and Clinical Predictors of Immune Checkpoint Inhibitor Monotherapy in Advanced Lung Cancer. Clin. Med. Insights Oncol. 2021, 15, 11795549211004489. [Google Scholar] [CrossRef] [PubMed]
- Mouritzen, M.T.; Carus, A.; Ladekarl, M.; Meldgaard, P.; Nielsen, A.W.M.; Livbjerg, A.; Larsen, J.W.; Skuladottir, H.; Kristiansen, C.; Wedervang, K.; et al. Nationwide survival benefit after implementation of first-line immunotherapy for patients with advanced nsclc—Real world efficacy. Cancers 2021, 13, 4846. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.M.; Silverstein, S.C.; Quinn, M.; Waterston, L.B.; Thomas, C.A.; Benneyan, J.C.; Han, P.K.J. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017, 112, 156–164. [Google Scholar] [CrossRef]
- Gregg, J.P.; Li, T.; Yoneda, K.Y. Molecular testing strategies in non-small cell lung cancer: Optimizing the diagnostic journey. Transl. Lung Cancer Res. 2019, 8, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Pérol, M.; Girard, N.; Durand-Zaleski, I.; Zacharias, S.; Bosquet, L.; Jänicke, M.; Quantin, X.; Groth, A.; Fleitz, A.; et al. Impact of immune checkpoint inhibitors on the management of locally advanced or metastatic non-small cell lung cancer in real-life practice in patients initiating treatment between 2015 and 2018 in France and Germany. Lung Cancer 2022, 172, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pezzuto, A.; Sini, C.; Tuzi, A.; Citarella, F.; McCusker, M.G.; Nigro, O.; Tanda, E.; Russo, A. Concomitant medications during immune checkpoint blockage in cancer patients: Novel insights in this emerging clinical scenario. Crit. Rev. Oncol. Hematol. 2019, 142, 26–34. [Google Scholar] [CrossRef]
- von Itzstein, M.S.; Gonugunta, A.S.; Mayo, H.G.; Minna, J.D.; Gerber, D.E. Immunotherapy Use in Patients With Lung Cancer and Comorbidities. Cancer J. 2020, 26, 525–536. [Google Scholar] [CrossRef]
| Characteristics | Pre-1L IO n = 344 (%) | Post-1L IO n = 350 (%) |
|---|---|---|
| IIIBrp */IV stage | ||
| IIIBrp | 14(4.1) | 6(1.7) |
| IV | 330(95.9) | 346(98.3) |
| Age at IIIBrp /IV stage diagnosis | ||
| <70 years | 190 (55.2) | 152 (43.4) |
| 70–74 years | 78 (22.7) | 81 (23.1) |
| 75–79 years | 46 (13.4) | 82 (23.5) |
| 80–84 years | 26 (7.6) | 25 (7.1) |
| ≥85 years | 4 (1.1) | 10 (2.9) |
| Gender | ||
| Female | 118 (34.3) | 112 (32.0) |
| Male | 226 (65.7) | 238 (68.0) |
| Race | ||
| White | 341 (99.1) | 350 (100.0) |
| Others | 3 (0.9) | 0 (0.0) |
| Smoking history | ||
| Never | 13 (4.7) | 23 (8.2) |
| Ever | 266 (95.3) | 258 (91.8) |
| Unknown | 65 | 69 |
| Year smoked | ||
| ≤20 years | 19 (8.8) | 18 (11.2) |
| >20 years | 196 (91.2) | 143 (88.8) |
| Unknown | 129 | 189 |
| Packs/year | ||
| ≤20 packs/years | 26(13.0) | 24(15.4) |
| ˃20 pack/years | 174(87.0) | 132(84.6) |
| Unknown | 144 | 194 |
| ECOG PS at IIIBrp/IV stage diagnosis | ||
| 0 | 62(18.9) | 55(16.9) |
| 1 | 217 (63.1) | 212(65.0) |
| ≥2 | 49 (14.9) | 59 (18.1) |
| Unknown | 16 | 24 |
| Histology | ||
| Squamous cell | 57 (16.8) | 70 (20.5) |
| Non-squamous cell | 263 (77.4) | 267 (77.8) |
| Adenocarcinoma | 259 (76.2) | 267 (77.8) |
| Large cell carcinoma | 4 (1.2) | 0 (0.0) |
| Other | 20 (5.8) | 6 (1.7) |
| Unknown | 4 | 7 |
| Unknown biomarker status | ||
| EGFR unknown | 85 (24.7) | 99 (28.3) |
| ALK unknown | 142 (41.3) | 109 (31.1) |
| ROS-1 unknown | 275 (79.9) | 122 (34.9) |
| Location of metastasis | ||
| Bone | 114 (33.1) | 99 (28.3) |
| Lymph nodes | 73 (21.2) | 95 (27.1) |
| Brain | 44 (12.8) | 55 (15.7) |
| Liver | 33 (9.6) | 32 (9.1) |
| Pleura | 48 (14.0) | 56 (16.0) |
| Contralateral lung | 121 (35.2) | 122 (34.9) |
| Other | 92 (26.7) | 55 (15.7) |
| Missing/Unknown | 3 (0.9) | 6 (1.7) |
| Pre-1L IO | Post-1L IO | Post-1L IO | ||
|---|---|---|---|---|
| First-Line Therapies | n = 344 (%) | n = 350 (%) | TPS ≥50% n = 84 (%) | TPS <50% n = 266 (%) |
| Multi-agents chemotherapy | 224 (65.1) | 161 (46.0) | 6 (7.1) | 155 (58.2) |
| Carboplatin + Gemcitabine | 92 (26.7) | 99 (28.3) | 1 (1.1) | 98 (36.8) |
| Pemetrexed +/− Platin | 123 (35.8) | 51(14.6) | 2 (2.4) | 49 (18.4) |
| Other combinations | 9 (2.6) | 11 (3.1) | 3 (3.6) | 8 (3.0) |
| Single-agent chemotherapy | 93 (27.0) | 74 (21.1) | 3 (3.6) | 71 (26.7) |
| Gemcitabine | 51 (14.8) | 35 (10.0) | 2 (2.4) | 33 (12.4) |
| Vinorelbine | 36 (10.5) | 38 (10.9) | 1 (1.2) | 37 (13.9) |
| Other agents | 6 (1.7) | 1 (0.3) | - | 1 (0.4) |
| Targeted therapy | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PD-1/PD-L1 inhibitor single agent | - | 71 (20.3) | 71 (84.5) | 0 (0.0) |
| Pembrolizumab | - | 71(20.3) | 71 (84.5) | 0 (0.0) |
| PD-1/PDL1 inhibitor + chemotherapy | - | 20 (5.7) | 0 (0.0) | 20 (7.5) |
| Clinical Trials | 25 (7.3) | 24 (6.9) | 4 (4.8) | 20 (7.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danesi, V.; Massa, I.; Foca, F.; Delmonte, A.; Crinò, L.; Bronte, G.; Ragonesi, M.; Maltoni, R.; Manunta, S.; Cravero, P.; et al. Real-World Outcomes and Treatments Patterns Prior and after the Introduction of First-Line Immunotherapy for the Treatment of Metastatic Non-Small Cell Lung Cancer. Cancers 2022, 14, 4481. https://doi.org/10.3390/cancers14184481
Danesi V, Massa I, Foca F, Delmonte A, Crinò L, Bronte G, Ragonesi M, Maltoni R, Manunta S, Cravero P, et al. Real-World Outcomes and Treatments Patterns Prior and after the Introduction of First-Line Immunotherapy for the Treatment of Metastatic Non-Small Cell Lung Cancer. Cancers. 2022; 14(18):4481. https://doi.org/10.3390/cancers14184481
Chicago/Turabian StyleDanesi, Valentina, Ilaria Massa, Flavia Foca, Angelo Delmonte, Lucio Crinò, Giuseppe Bronte, Maria Ragonesi, Roberta Maltoni, Silvia Manunta, Paola Cravero, and et al. 2022. "Real-World Outcomes and Treatments Patterns Prior and after the Introduction of First-Line Immunotherapy for the Treatment of Metastatic Non-Small Cell Lung Cancer" Cancers 14, no. 18: 4481. https://doi.org/10.3390/cancers14184481
APA StyleDanesi, V., Massa, I., Foca, F., Delmonte, A., Crinò, L., Bronte, G., Ragonesi, M., Maltoni, R., Manunta, S., Cravero, P., Andrikou, K., Priano, I., Balzi, W., Gentili, N., Burke, T., & Altini, M. (2022). Real-World Outcomes and Treatments Patterns Prior and after the Introduction of First-Line Immunotherapy for the Treatment of Metastatic Non-Small Cell Lung Cancer. Cancers, 14(18), 4481. https://doi.org/10.3390/cancers14184481







