Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Risk of Bias Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
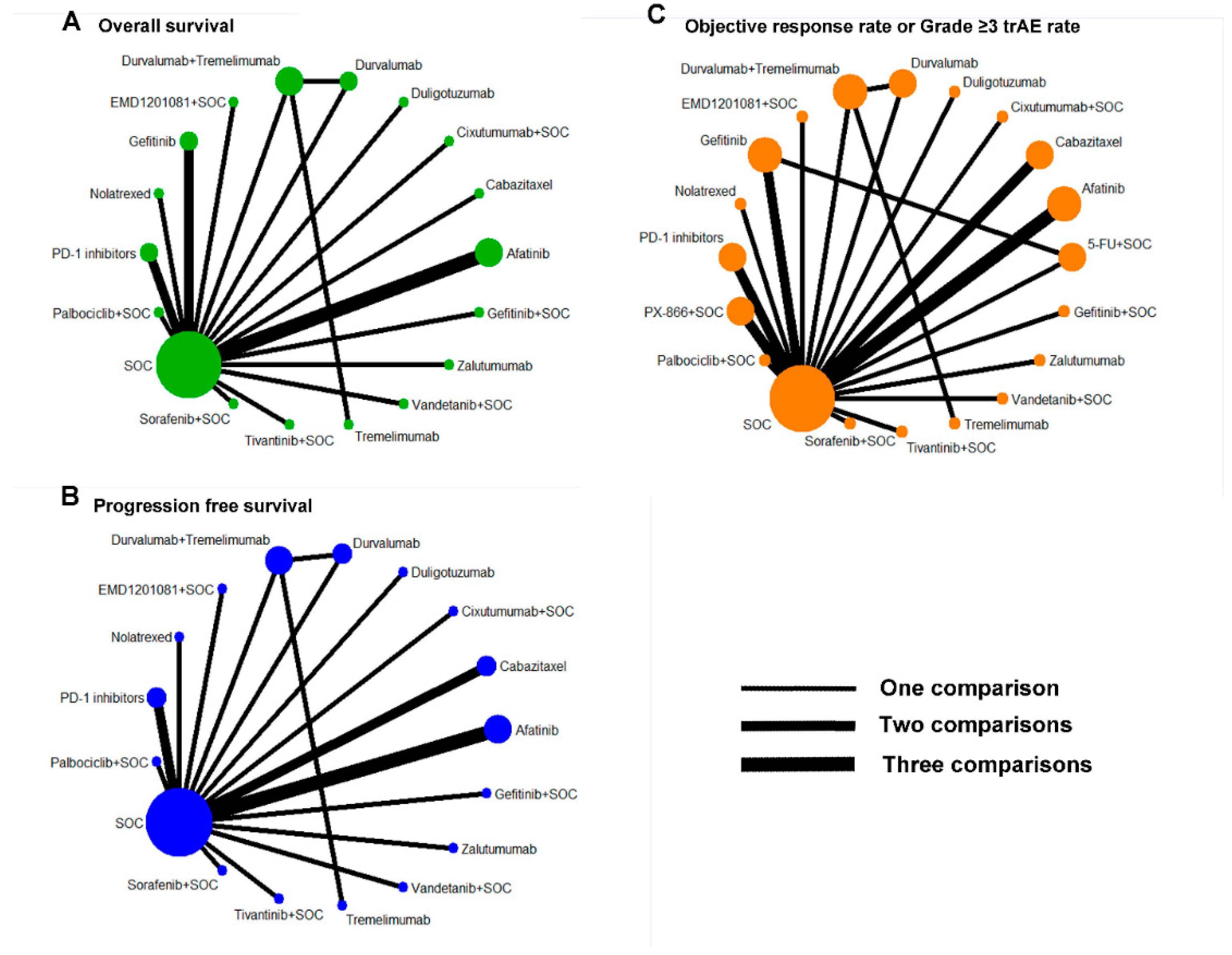
3.3. Network Meta-Analysis
3.4. Rank Probabilities
3.5. Heterogeneity and Inconsistency Assessment
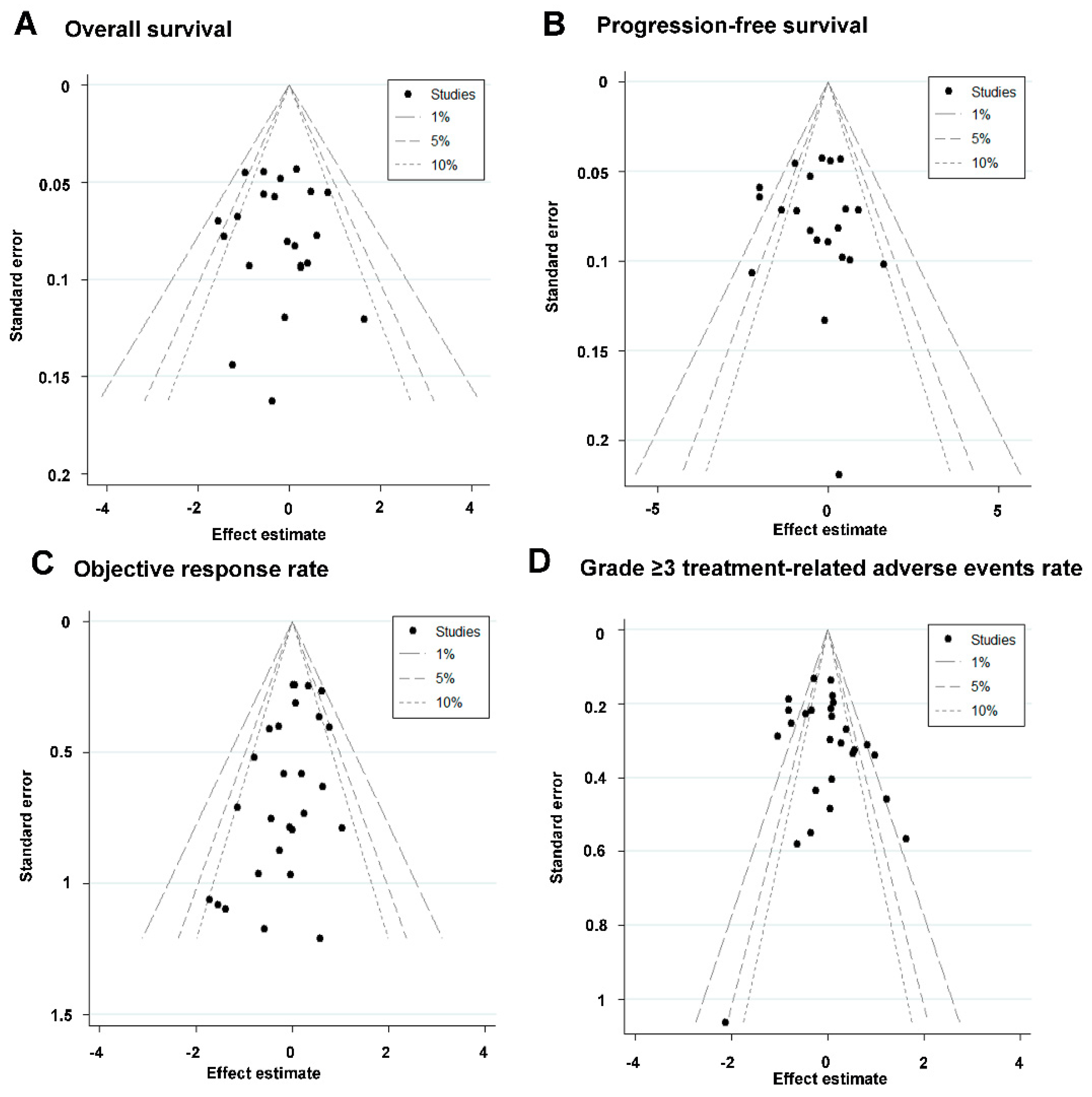
3.6. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients with Squamous Cell Carcinoma of the Head and Neck. JAMA Oncol. 2018, 4, 1583–1588. [Google Scholar] [CrossRef]
- Ionna, F.; Bossi, P.; Guida, A.; Alberti, A.; Muto, P.; Salzano, G.; Ottaiano, A.; Maglitto, F.; Leopardo, D.; De Felice, M.; et al. Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: A Big and Intriguing Challenge Which May Be Resolved by Integrated Treatments Combining Locoregional and Systemic Therapies. Cancers 2021, 13, 2371. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Stewart, J.S.W.; Cohen, E.E.; Licitra, L.; Van Herpen, C.M.; Khorprasert, C.; Soulieres, D.; Vodvarka, P.; Rischin, D.; Garin, A.M.; Hirsch, F.R.; et al. Phase III Study of Gefitinib Compared with Intravenous Methotrexate for Recurrent Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2009, 27, 1864–1871. [Google Scholar] [CrossRef]
- Ruzsa, A.; Sen, M.; Evans, M.; Lee, L.W.; Hideghety, K.; Rottey, S.; Klimak, P.; Holeckova, P.; Fayette, J.; Csoszi, T.; et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naïve patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Investig. New Drugs 2014, 32, 1278–1284. [Google Scholar] [CrossRef]
- Ferrarotto, R.; William, W.N.; Tseng, J.E.; Marur, S.; Shin, D.M.; Murphy, B.; Cohen, E.E.; Thomas, C.Y.; Willey, R.; Cosaert, J.; et al. Randomized phase II trial of cixutumumab alone or with cetuximab for refractory recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2018, 82, 83–90. [Google Scholar] [CrossRef]
- Kochanny, S.E.; Worden, F.P.; Adkins, D.R.; Lim, D.W.; Bauman, J.E.; Wagner, S.A.; Brisson, R.J.; Karrison, T.G.; Stadler, W.M.; Vokes, E.E.; et al. A randomized phase 2 network trial of tivantinib plus cetuximab versus cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Cancer 2020, 126, 2146–2152. [Google Scholar] [CrossRef]
- Limaye, S.; Riley, S.; Zhao, S.; O’Neill, A.; Posner, M.; Adkins, D.; Jaffa, Z.; Clark, J.; Haddad, R. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol. 2013, 49, 835–841. [Google Scholar] [CrossRef]
- Gilbert, J.; Schell, M.J.; Zhao, X.; Murphy, B.; Tanvetyanon, T.; Leon, M.E.; Hayes, D.N.; Haigentz, M.; Saba, N.; Nieva, J.; et al. A randomized phase II efficacy and correlative studies of cetuximab with or without sorafenib in recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2015, 51, 376–382. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.-J.; Yao, W.-Y.; Zhang, F.; Qiu, W.-Z.; Liao, K.; Feng, J.-H.; Tan, J.-Y.; Liu, H.; Yuan, T.-Z.; Zheng, R.-H.; et al. The Optimal Second-Line Systemic Treatment Model for Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: A Bayesian Network Meta-Analysis. Front. Immunol. 2021, 12, 719650. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Kochanny, S.; Wood, K.; Worden, F.P.; Adkins, D.; Wade, J.L.; Sleckman, B.G.; Anderson, D.; Brisson, R.J.; Karrison, T.; et al. A randomized phase 2 study of temsirolimus and cetuximab versus temsirolimus alone in recurrent/metastatic, cetuximab-resistant head and neck cancer: The MAESTRO study. Cancer 2020, 126, 3237–3243. [Google Scholar] [CrossRef]
- Gilbert, J.; Lee, J.W.; Argiris, A.; Haigentz, M.; Feldman, L.E.; Jang, M.; Arun, P.; Van Waes, C.; Forastiere, A.A. Phase II 2-arm trial of the proteasome inhibitor, PS-341 (bortezomib) in combination with irinotecan or PS-341 alone followed by the addition of irinotecan at time of progression in patients with locally recurrent or metastatic squamous cell carcinoma of. Head Neck 2013, 35, 942–948. [Google Scholar] [CrossRef]
- Soulières, D.; Faivre, S.; Mesía, R.; Remenár, É.; Li, S.H.; Karpenko, A.; Dechaphunkul, A.; Ochsenreither, S.; Kiss, L.A.; Lin, J.C.; et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017, 18, 323–335. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Fayette, J.; Cupissol, D.; del Campo, J.M.; Clement, P.M.; Hitt, R.; Degardin, M.; Zhang, W.; Blackman, A.; Ehrnrooth, E.; et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann. Oncol. 2014, 25, 1813–1820. [Google Scholar] [CrossRef]
- Pivot, X.; Wadler, S.; Kelly, C.; Ruxer, R.; Tortochaux, J.; Stern, J.; Belpomme, D.; Humblet, Y.; Domenge, C.; Clendeninn, N.; et al. Result of two randomized trials comparing nolatrexed (Thymitaq™) versus methotrexate in patients with recurrent head and neck cancer. Ann. Oncol. 2001, 12, 1595–1599. [Google Scholar] [CrossRef]
- Machiels, J.-P.H.; Haddad, R.I.; Fayette, J.; Licitra, L.F.; Tahara, M.; Vermorken, J.B.; Clement, P.M.; Gauler, T.; Cupissol, D.; Grau, J.J.; et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015, 16, 583–594. [Google Scholar] [CrossRef]
- Machiels, J.-P.H.; Van Maanen, A.; Vandenbulcke, J.-M.; Filleul, B.; Seront, E.; Henry, S.; D’Hondt, L.; Lonchay, C.; Holbrechts, S.; Boegner, P.; et al. Randomized Phase II Study of Cabazitaxel Versus Methotrexate in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Previously Treated with Platinum-Based Therapy. Oncologist 2016, 21, 1416-e17. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.-P.; Subramanian, S.; Ruzsa, A.; Repassy, G.; Lifirenko, I.; Flygare, A.; Sørensen, P.; Nielsen, T.; Lisby, S.; Clement, P.M. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: An open-label, randomised phase 3 trial. Lancet Oncol. 2011, 12, 333–343. [Google Scholar] [CrossRef]
- Guo, Y.; Ahn, M.-J.; Chan, A.; Wang, C.-H.; Kang, J.-H.; Kim, S.-B.; Bello, M.; Arora, R.; Zhang, Q.; He, X.; et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): An open-label, randomised phase III trial. Ann. Oncol. 2019, 30, 1831–1839. [Google Scholar] [CrossRef]
- Kushwaha, V.S.; Gupta, S.; Husain, N.; Khan, H.; Negi, M.; Jamal, N.; Ghatak, A. Gefitinib, Methotrexate and Methotrexate plus 5-Fluorouracil as palliative treatment in recurrent head and neck squamous cell carcinoma. Cancer Biol. Ther. 2015, 16, 346–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jimeno, A.; Shirai, K.; Choi, M.; Laskin, J.; Kochenderfer, M.; Spira, A.; Cline-Burkhardt, V.; Winquist, E.; Hausman, D.; Walker, L.; et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann. Oncol. 2015, 26, 556–561. [Google Scholar] [CrossRef]
- Jimeno, A.; Bauman, J.E.; Weissman, C.; Adkins, D.; Schnadig, I.; Beauregard, P.; Bowles, D.W.; Spira, A.; Levy, B.; Seetharamu, N.; et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Oral Oncol. 2015, 51, 383–388. [Google Scholar] [CrossRef]
- Fayette, J.; Wirth, L.; Oprean, C.; Udrea, A.; Jimeno, A.; Rischin, D.; Nutting, C.; Harari, P.M.; Csoszi, T.; Cernea, D.; et al. Randomized Phase II Study of Duligotuzumab (MEHD7945A) vs. Cetuximab in Squamous Cell Carcinoma of the Head and Neck (MEHGAN Study). Front. Oncol. 2016, 6, 232. [Google Scholar] [CrossRef]
- Argiris, A.; Ghebremichael, M.; Gilbert, J.; Lee, J.-W.; Sachidanandam, K.; Kolesar, J.M.; Burtness, B.; Forastiere, A.A. Phase III Randomized, Placebo-Controlled Trial of Docetaxel with or without Gefitinib in Recurrent or Metastatic Head and Neck Cancer: An Eastern Cooperative Oncology Group Trial. J. Clin. Oncol. 2013, 31, 1405–1414. [Google Scholar] [CrossRef]
- Adkins, D.R.; Lin, J.-C.; Sacco, A.; Ley, J.; Oppelt, P.; Vanchenko, V.; Komashko, N.; Yen, C.-J.; Wise-Draper, T.; Gonzalez, J.L.-P.; et al. Palbociclib and cetuximab compared with placebo and cetuximab in platinum-resistant, cetuximab-naïve, human papillomavirus-unrelated recurrent or metastatic head and neck squamous cell carcinoma: A double-blind, randomized, phase 2 trial. Oral Oncol. 2021, 115, 105192. [Google Scholar] [CrossRef]
- Joshi, A.; Patil, V.; Noronha, V.; Dhumal, S.; Pande, N.; Chandrasekharan, A.; Turkar, S.; Dsouza, H.; Shrirangwar, S.; Mahajan, A.; et al. Results of a phase II randomized controlled clinical trial comparing efficacy of Cabazitaxel versus Docetaxel as second line or above therapy in recurrent head and neck cancer. Oral Oncol. 2017, 75, 54–60. [Google Scholar] [CrossRef]
- Siu, L.L.; Even, C.; Mesía, R.; Remenar, E.; Daste, A.; Delord, J.-P.; Krauss, J.; Saba, N.F.; Nabell, L.; Ready, N.E.; et al. Safety and Efficacy of Durvalumab with or without Tremelimumab in Patients with PD-L1-Low/Negative Recurrent or Metastatic HNSCC. The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2019, 5, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.; Clement, P.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R.W.; Borson, S.; Tsagianni, A.; Zandberg, D.P. Immunotherapy in Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2021, 11, 705614. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Noronha, V.; Patil, V.; Joshi, A.; Menon, N.; Prabhash, K. Advances in pharmacotherapy for head and neck cancer. Expert Opin. Pharmacother. 2021, 22, 2007–2018. [Google Scholar] [CrossRef]
- Specenier, P.; Vermorken, J.B. Optimizing treatments for recurrent or metastatic head and neck squamous cell carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 901–915. [Google Scholar] [CrossRef]
- Uozumi, S.; Enokida, T.; Suzuki, S.; Nishizawa, A.; Kamata, H.; Okano, T.; Fujisawa, T.; Ueda, Y.; Okano, S.; Tahara, M.; et al. Predictive Value of Cetuximab-Induced Skin Toxicity in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and NECK. Front. Oncol. 2018, 8, 616. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Zhang, W.; Liu, X. Anti-PD1/PD-L1 monotherapy vs standard of care in patients with recurrent or metastatic head and neck squamous cell carcinoma. Medicine 2021, 100, e24339. [Google Scholar] [CrossRef]
- Wang, B.; Cao, R.; Li, P.; Fu, C. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: A systematic review and meta-analysis. Cancer Med. 2019, 8, 5969–5978. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, G.; Maroun, C.A.; Wu, I.X.; Huang, D.; Seiwert, T.Y.; Liu, Y.; Mandal, R.; Zhang, X. Programmed Death-1/Programmed Death-Ligand 1-Axis Blockade in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Stratified by Human Papillomavirus Status: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 645170. [Google Scholar] [CrossRef]
- Clarke, E.; Eriksen, J.G.; Barrett, S. The effects of PD-1/PD-L1 checkpoint inhibitors on recurrent/metastatic head and neck squamous cell carcinoma: A critical review of the literature and meta-analysis. Acta Oncol. 2021, 60, 1534–1542. [Google Scholar] [CrossRef]
- Lau, A.; Yang, W.; Li, K.-Y.; Su, Y. Systemic Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma—A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020, 153, 102984. [Google Scholar] [CrossRef] [PubMed]


| Trials | Phase | Multi- Center | Inclusion Period | Treatments | Number of Patients | Median Age (Years) | Median OS (Months) | Median PFS (Months) | ORR rates (%) | Grade ≥ 3 trAE Rates (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kochanny et al., 2020 [8] | II | yes | 2012 to 2014 | Tivantinib + SOC SOC | 40 38 | 60.5 63.6 | 7.4 8.6 | 3.5 3.5 | 7.5 7.9 | 67.50 26.32 |
| Douglas et al., 2021 [29] | II | yes | 2015 to 2017 | Palbociclib + SOC SOC | 65 60 | NA NA | 9.7 7.8 | 3.9 4.6 | 27.7 25 | 53.13 15.00 |
| Guo et al., 2019 [23] | III | yes | 2013 to 2018 | Afatinib SOC | 228 112 | 55.5 58 | 6.9 6.4 | 2.9 2.6 | 28.1 13.4 | 39.04 59.82 |
| Cohen et al., 2019 [4] | III | yes | 2014 to 2016 | PD-1 inhibitors SOC | 247 248 | 60 60 | 8.4 6.9 | 8.3 6.6 | 14.6 10.1 | 13.41 36.32 |
| Ferrarotto et al., 2018 [7] | II | yes | 2008 to 2010 | Cixutumumab + SOC SOC | 47 44 | NA NA | 5.3 5.5 | 1.9 2.0 | 2.1 9.1 | 10.64 15.91 |
| Joshi et al., 2017 [30] | II | no | 2015 to 2016 | Cabazitaxel SOC | 46 46 | 47.5 42.5 | 3.833 5.16 | 0.7 2.03 | 2.2 13.6 | 36.96 34.78 |
| Machiels et al., 2016 [21] | II | yes | 2012 to 2014 | Cabazitaxel SOC | 53 48 | 58 57.5 | 5 3.6 | 1.9 1.9 | 0 2.1 | 39.62 27.08 |
| Ferris et al., 2016 [11] | III | yes | 2014 to 2015 | PD-1 inhibitor SOC | 240 121 | 59 61 | 7.5 5.1 | 2.0 2.3 | 13.3 5.8 | 13.14 35.14 |
| Fayette et al., 2016 [27] | II | yes | 2012 to 2013 | Duligotuzumab SOC | 59 62 | 62 62 | 7.2 8.7 | 4.2 4 | 15.3 21.0 | 61.02 50.00 |
| Machiels et al., 2015 [20] | III | yes | 2012 to 2013 | Afatinib SOC | 322 161 | 60 59 | 6.8 6.0 | 2.6 1.7 | 10.2 5.6 | 39.69 35.63 |
| Jimeno et al., 2015 [25] | II | yes | 2012 to 2013 | PX-866 + SOC SOC | 42 41 | 59 63 | 7.03 8.53 | 2.67 2.67 | 9.5 7.3 | 62.50 53.85 |
| Jimeno et al., 2015 [26] | II | yes | 2011 to 2013 | PX-866 + SOC SOC | 42 43 | 62 60 | 8.76 6.5 | 3.07 2.73 | 14.3 4.7 | 61.90 34.88 |
| Gilbert et al., 2015 [10] | II | yes | 2009 to 2011 | SOC Sorafenib + SOC | 27 28 | 59 60 | 9 5.7 | 3 3.2 | 7.4 7.1 | 11.11 92.86 |
| Seiwert et al., 2014 [18] | II | yes | 2007 to 2011 | Afatinib SOC | 62 62 | 58 58 | 9 11.8 | 3.25 3.5 | 8.1 9.7 | 51.61 17.74 |
| Ruzsa et al., 2014 [6] | II | yes | 2009 to 2012 | EMD1201081 + SOC SOC | 53 53 | 58 57 | 6.3 NA | 1.5 1.9 | 5.7 5.7 | 56.60 50.94 |
| Limaye et al., 2013 [9] | II | yes | 2007 to 2009 | SOC Vandetanib + SOC | 14 15 | 56 60 | 6.7 6.03 | 0.8 2.25 | 7.1 13.3 | 42.86 40.00 |
| Argiris et al., 2013 [28] | III | yes | 2004 to 2008 | SOC Gefitinib + SOC | 117 122 | 60.8 61.4 | 6.0 7.3 | 2.1 3.5 | 4.3 9.8 | 41.03 35.25 |
| Machiels et al., 2011 [22] | III | yes | 2006 to 2009 | Zalutumumab SOC | 191 95 | 57 58 | 6.7 5.2 | 2.48 2.1 | 6.3 10.5 | 20.63 5.32 |
| Pivot et al., 2001 [19] | NA | yes | 1997 to 1998 | Nolatrexed SOC | 93 46 | 57.9 62 | 3.1 3.1 | 1.9 1.5 | 3.2 10.9 | 38.71 19.57 |
| Kushwaha et al., 2015 [24] | NA | no | 2010 to 2012 | Gefitinib SOC 5-Fu + SOC | 39 40 38 | NA NA NA | 8.8 7.8 8.1 | NA NA NA | 7.7 5.0 7.9 | 2.56 12.50 26.32 |
| Stewart et al., 2009 [5] | III | yes | 2003 to 2006 | Gefitinib SOC | 325 161 | NA NA | 6 6.7 | NA NA | 2.5–7.6 3.9 | 10.13–19.88 35.22 |
| Siu et al., 2019 [31] | II | yes | 2015 to 2016 | Durv Treme Durv + Treme | 67 67 133 | 62 61 62 | 6.0 5.5 7.6 | 1.9 1.9 2.0 | 9.2 1.6 7.8 | 12.31 16.92 15.79 |
| Ferris et al., 2020 [32] | III | yes | 2015 to 2017 | Durv Durv + Treme SOC | 240 247 249 | 60 | 7.6 6.5 8.3 | 2.1 2.0 3.7 | 17.9 18.2 17.3 | 10.1 16.3 24.2 |
| Sucra (Ranks) | OS | PFS | ORR Rates | Grade ≥ 3 trAE Rates |
|---|---|---|---|---|
| SOC | 49% | 48% | 47% | 43% |
| Cabazitaxel | 14% | 36% | 19% | 57% |
| PD-1 inhibitor | 86% | 60% | 75% | 4% |
| Cixutumumab + SOC | 42% | 91% | 11% | 27% |
| Duligotuzumab | 31% | 20% | 34% | 56% |
| Afatinib | 60% | 81% | 79% | 40% |
| PX-866 + SOC | NA | NA | 79% | 64% |
| EMD1201081 + SOC | 37% | 35% | 51% | 50% |
| Zalutumumab | 83% | 92% | 29% | 91% |
| Gefitinib + SOC | 38% | 20% | 84% | 33% |
| Vandetanib + SOC | 42% | 53% | 72% | 41% |
| Nolatrexed | 45% | 38% | 15% | 76% |
| 5-FU + SOC | NA | NA | 73% | 80% |
| Gefitinib | 25% | NA | 64% | 9% |
| Sorafenib + SOC | 23% | 45% | 50% | 99% |
| Tivantinib + SOC | 52% | 30% | 49% | 79% |
| Palbociclib + SOC | 74% | 47% | 54% | 89% |
| Durvalumab | 66% | 37% | 52% | 9% |
| Durva + Treme | 47% | 40% | 51% | 22% |
| Tremelimumab | 86% | 78% | 10% | 30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zeng, J.; Wei, Z.; Huang, Y.; Yang, L.; Hu, X.; Su, Y.; Peng, X. Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis. Cancers 2022, 14, 4472. https://doi.org/10.3390/cancers14184472
He Y, Zeng J, Wei Z, Huang Y, Yang L, Hu X, Su Y, Peng X. Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis. Cancers. 2022; 14(18):4472. https://doi.org/10.3390/cancers14184472
Chicago/Turabian StyleHe, Yan, Junsong Zeng, Zhigong Wei, Yan Huang, Lianlian Yang, Xiaolin Hu, Yonglin Su, and Xingchen Peng. 2022. "Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis" Cancers 14, no. 18: 4472. https://doi.org/10.3390/cancers14184472
APA StyleHe, Y., Zeng, J., Wei, Z., Huang, Y., Yang, L., Hu, X., Su, Y., & Peng, X. (2022). Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis. Cancers, 14(18), 4472. https://doi.org/10.3390/cancers14184472







