Contralesional Cortical and Network Features Associated with Preoperative Language Deficit in Glioma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Acquisition
2.3. Awake Craniotomy Protocol
2.4. Language Function Assessment
2.5. Tumor Segmentation and Voxel-Based Morphometry
2.6. DTI Preprocessing
2.7. White Matter Connectome Construction
2.8. Graph Theory Analysis
2.9. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. VBM Analysis
3.3. Global Topological Properties
3.4. Nodal Topological Properties
3.5. Causal Mediation Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ammanuel, S.G.; Almeida, N.C.; Kurteff, G.; Kakaizada, S.; Molinaro, A.M.; Berger, M.S.; Chang, E.F.; Hervey-Jumper, S.L. Correlation of natural language assessment results with health-related quality of life in adult glioma patients. J. Neurosurg. 2021, 136, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Gallucci, M.; Fava, M.; Carrabba, G.; Giussani, C.; Acerbi, F.; Baratta, P.; Songa, V.; Conte, V.; Branca, V.; et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 2007, 60, 67–80. [Google Scholar] [CrossRef]
- Satoer, D.; Vork, J.; Visch-Brink, E.; Smits, M.; Dirven, C.; Vincent, A. Cognitive functioning early after surgery of gliomas in eloquent areas. J. Neurosurg. 2012, 117, 831–838. [Google Scholar] [CrossRef]
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Moritz-Gasser, S.; Lemaitre, A.L.; Almairac, F.; Duffau, H. Functional compensation of the left inferior longitudinal fasciculus for picture naming. Cognit. Neuropsychol. 2019, 36, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Satoer, D.; Vincent, A.; Smits, M.; Dirven, C.; Visch-Brink, E. Spontaneous speech of patients with gliomas in eloquent areas before and early after surgery. Acta Neurochir. 2013, 155, 685–692. [Google Scholar] [CrossRef]
- Fang, S.; Liang, Y.; Li, L.; Wang, L.; Fan, X.; Wang, Y.; Jiang, T. Tumor location-based classification of surgery-related language impairments in patients with glioma. J. Neurooncol. 2021, 155, 143–152, Erratum in J. Neurooncol. 2021, 155, 153. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, N.; Yan, J.; Cheng, J.; Lu, J.; Wu, J. Tumor grade-related language and control network reorganization in patients with left cerebral glioma. Cortex 2020, 129, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Berger, M.S. Introduction: Surgical Management of Eloquent Area Tumors. Neurosurgery 2020, 87, 1076–1077. [Google Scholar] [CrossRef]
- Gunal, V.; Savardekar, A.R.; Devi, B.I.; Bharath, R.D. Preoperative functional magnetic resonance imaging in patients undergoing surgery for tumors around left (dominant) inferior frontal gyrus region. Surg. Neurol. Int. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Cargnelutti, E.; Ius, T.; Skrap, M.; Tomasino, B. What do we know about pre- and postoperative plasticity in patients with glioma? A review of neuroimaging and intraoperative mapping studies. Neuroimage Clin. 2020, 28, 102435. [Google Scholar] [CrossRef] [PubMed]
- Bryszewski, B.; Tybor, K.; Ormezowska, E.A.; Jaskolski, D.J.; Majos, A. Rearrangement of motor centers and its relationship to the neurological status of low-grade glioma examined on pre- and postoperative fMRI. Clin. Neurol. Neurosurg. 2013, 115, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Majos, A.; Bryszewski, B.; Kosla, K.N.; Pfaifer, L.; Jaskolski, D.; Stefanczyk, L. Process of the Functional Reorganization of the Cortical Centers for Movement in GBM Patients: fMRI Study. Clin. Neuroradiol. 2017, 27, 71–79. [Google Scholar] [CrossRef]
- Huang, Z.; Li, G.; Li, Z.; Sun, S.; Zhang, Y.; Hou, Z.; Xie, J. Contralesional Structural Plasticity in Different Molecular Pathologic Subtypes of Insular Glioma. Front. Neurol. 2021, 12, 636573. [Google Scholar] [CrossRef]
- Briganti, C.; Sestieri, C.; Mattei, P.A.; Esposito, R.; Galzio, R.J.; Tartaro, A.; Romani, G.L.; Caulo, M. Reorganization of functional connectivity of the language network in patients with brain gliomas. AJNR Am. J. Neuroradiol. 2012, 33, 1983–1990. [Google Scholar] [CrossRef]
- Chivukula, S.; Pikul, B.K.; Black, K.L.; Pouratian, N.; Bookheimer, S.Y. Contralateral functional reorganization of the speech supplementary motor area following neurosurgical tumor resection. Brain Lang. 2018, 183, 41–46. [Google Scholar] [CrossRef]
- Pinson, H.; Van Lerbeirghe, J.; Vanhauwaert, D.; Van Damme, O.; Hallaert, G.; Kalala, J.P. The supplementary motor area syndrome: A neurosurgical review. Neurosurg. Rev. 2022, 45, 81–90. [Google Scholar] [CrossRef]
- Almairac, F.; Duffau, H.; Herbet, G. Contralesional macrostructural plasticity of the insular cortex in patients with glioma: A VBM study. Neurology 2018, 91, e1902–e1908. [Google Scholar] [CrossRef]
- Yuan, T.; Zuo, Z.; Ying, J.; Jin, L.; Kang, J.; Gui, S.; Wang, R.; Li, C. Structural and Functional Alterations in the Contralesional Medial Temporal Lobe in Glioma Patients. Front. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—the methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Audrain, S.; Barnett, A.J.; McAndrews, M.P. Language network measures at rest indicate individual differences in naming decline after anterior temporal lobe resection. Hum. Brain Mapp. 2018, 39, 4404–4419. [Google Scholar] [CrossRef] [PubMed]
- Shewan, C.M.; Kertesz, A. Reliability and validity characteristics of the Western Aphasia Battery (WAB). J. Speech Hear Disord. 1980, 45, 308–324. [Google Scholar] [CrossRef]
- Cui, Z.; Zhong, S.; Xu, P.; He, Y.; Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Kahn, R.S.; Goni, J.; Sporns, O. High-cost, high-capacity backbone for global brain communication. Proc. Natl. Acad. Sci. USA 2012, 109, 11372–11377. [Google Scholar] [CrossRef]
- Duffau, H. Lessons from brain mapping in surgery for low-grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol. 2005, 4, 476–486. [Google Scholar] [CrossRef]
- Herbet, G.; Maheu, M.; Costi, E.; Lafargue, G.; Duffau, H. Mapping neuroplastic potential in brain-damaged patients. Brain 2016, 139, 829–844. [Google Scholar] [CrossRef]
- Desmurget, M.; Bonnetblanc, F.; Duffau, H. Contrasting acute and slow-growing lesions: A new door to brain plasticity. Brain 2007, 130, 898–914. [Google Scholar] [CrossRef]
- Cotman, C.W.; Nieto-Sampedro, M. Brain function, synapse renewal, and plasticity. Annu. Rev. Psychol. 1982, 33, 371–401. [Google Scholar] [CrossRef]
- Andoh, M.; Koyama, R. Microglia regulate synaptic development and plasticity. Dev. Neurobiol. 2021, 81, 568–590. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Hu, X.; Hu, G.; Liu, Y.; Yang, K.; Xiao, C.; Zou, Y.; Liu, H. Contralesional homotopic functional plasticity in patients with temporal glioma. J. Neurosurg. 2020, 134, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Krainik, A.; Duffau, H.; Capelle, L.; Cornu, P.; Boch, A.L.; Mangin, J.F.; Le Bihan, D.; Marsault, C.; Chiras, J.; Lehericy, S. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology 2004, 62, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Butz, M.; Steenbuck, I.D.; van Ooyen, A. Homeostatic structural plasticity increases the efficiency of small-world networks. Front. Synaptic Neurosci. 2014, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia 2019, 128, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef]
- Du, J.; Rolls, E.T.; Cheng, W.; Li, Y.; Gong, W.; Qiu, J.; Feng, J. Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex 2020, 123, 185–199. [Google Scholar] [CrossRef]
- Martino, J.; Vergani, F.; Robles, S.G.; Duffau, H. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: Implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery 2010, 66, 4–12. [Google Scholar] [CrossRef] [Green Version]
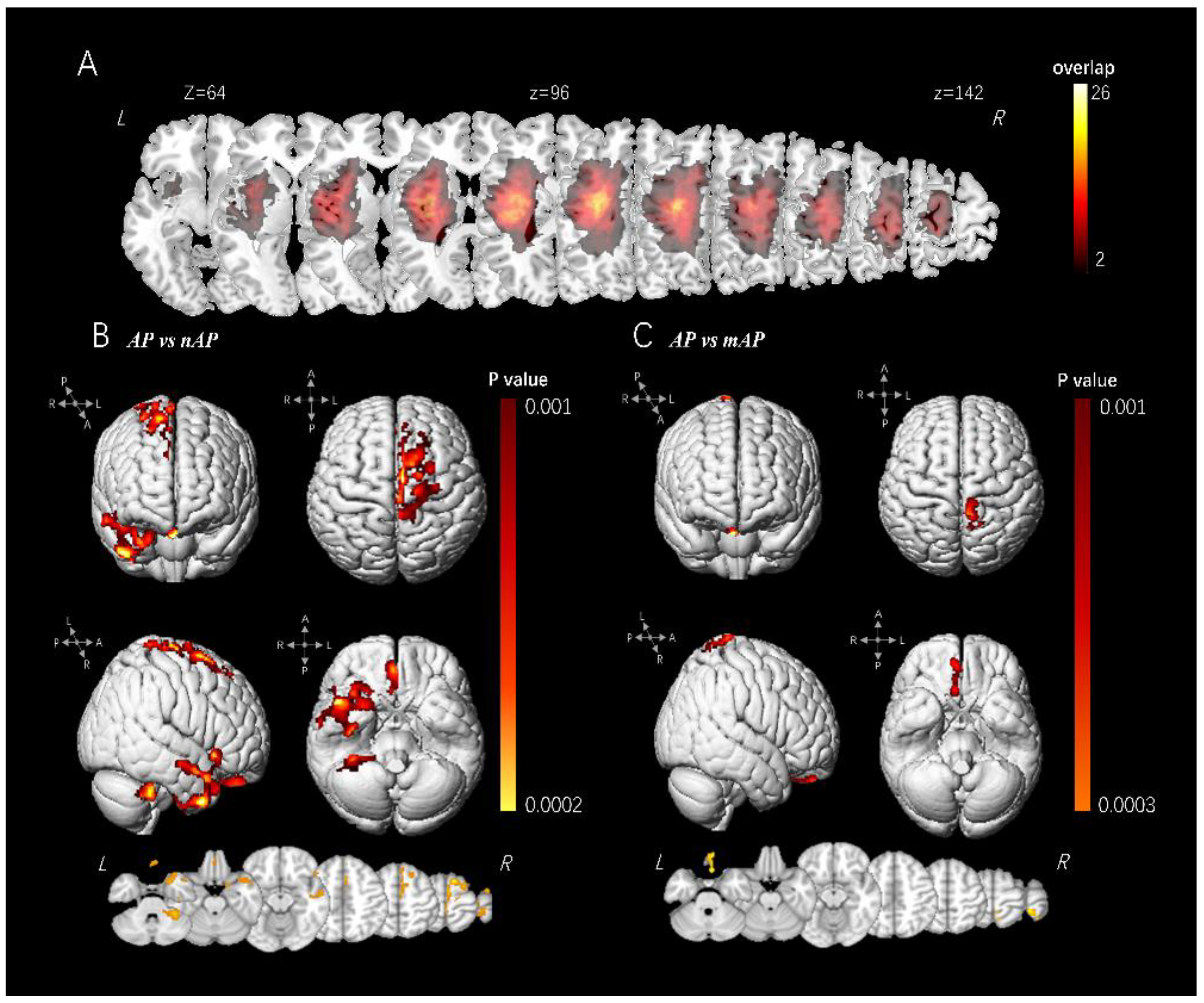
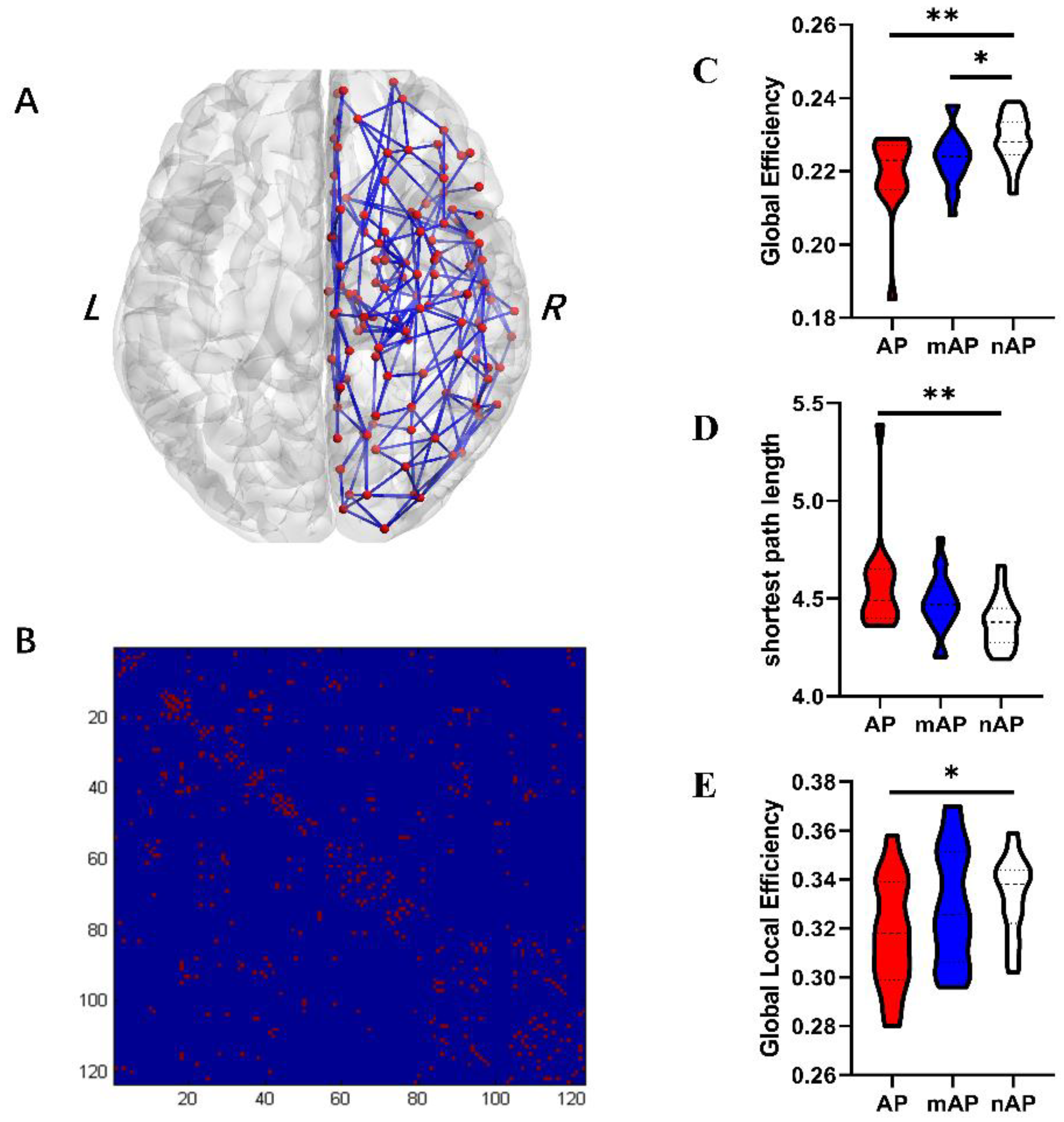

| Group | AP | mAP | nAP | p Value |
|---|---|---|---|---|
| Sex | 0.39 | |||
| Male | 7 | 11 | 17 | |
| Female | 8 | 9 | 8 | |
| Age (yrs.) | 47.8 ± 11.5 | 43.9 ±11.0 | 41.5 ± 7.3 | 0.15 |
| Education Level (yrs.) | 11.7 ± 2.9 | 12.7 ± 2.9 | 13.6 ± 2.9 | 0.12 |
| Pathology | 0.65 | |||
| Astrocytoma | 8 | 13 | 13 | |
| Oligodendroglioma | 7 | 7 | 12 | |
| Tumor Volume (CC) | 33.34 ± 23.91 | 31.74 ± 19.90 | 26.22 ± 19.38 | 0.51 |
| SDTN (mm) | 5.66 ± 1.39 | 11.92 ± 6.00 | 15.40 ± 8.86 | <0.01 |
| AQ Score | 8.65 ± 0.48 | 9.36 ± 1.67 | 10.00 ± 0.00 | <0.01 |
| Naming Score | 87.05 ± 8.92 | 98.68 ± 0.52 | 99.91 ± 0.25 | <0.01 |
| Property | Value (Mean ± Standard Deviation) | One-Way ANOVA (p Value) | Post-Hoc Analysis with LSD (p Value) | ||||
|---|---|---|---|---|---|---|---|
| AP | mAP | nAP | AP vs. mAP | AP vs. nAP | mAP vs. nAP | ||
| Global efficiency | 0.219 ± 0.011 | 0.224 ± 0.007 | 0.225 ± 0.009 | 0.003 | 0.124 | 0.001 | 0.039 |
| Global local efficiency | 0.318 ± 0.022 | 0.329 ± 0.237 | 0.333 ± 0.016 | 0.075 | 0.110 | 0.025 | 0.508 |
| Shortest path length | 4.570 ± 0.254 | 4.479 ± 0.148 | 4.374 ± 0.125 | 0.003 | 0.123 | 0.001 | 0.049 |
| Assortativity | 1.601 ± 0.716 | 1.449 ± 0.520 | 1.469 ± 0.596 | 0.715 | 0.448 | 0.488 | 0.913 |
| Hierarchy | 3.587 ± 0.686 | 3.644 ± 0.408 | 3.779 ± 0.576 | 0.532 | 0.767 | 0.424 | 0.077 |
| Synchronization | 0.034 ± 0.912 | 0.176 ± 1.151 | 0.328 ± 0.920 | 0.246 | 0.540 | 0.323 | 0.099 |
| Node | Value (Mean ± Standard Deviation) | One-Way ANOVA (p Value) | Post-Hoc Analysis with LSD (p Value) | ||||
|---|---|---|---|---|---|---|---|
| AP | mAP | nAP | AP vs. mAP | AP vs. nAP | mAP vs. nAP | ||
| A12_47l_r | 0.221 ± 0.024 | 0.233 ± 0.015 | 0.241 ± 0.012 | 0.002 | 0.041 | <0.001 | 0.098 |
| A1_2_3tru_r | 0.238 ± 0.023 | 0.243 ± 0.017 | 0.258 ± 0.012 | <0.001 | 0.445 | <0.001 | 0.003 |
| A1_2_3ulhf_r | 0.208 ± 0.008 | 0.209 ± 0.008 | 0.216 ± 0.004 | <0.001 | 0.580 | 0.001 | 0.002 |
| A28_34_r | 0.208 ± 0.012 | 0.220 ± 0.002 | 0.220 ± 0.008 | 0.002 | 0.002 | 0.001 | 0.856 |
| A2_r | 0.244 ± 0.011 | 0.245 ± 0.012 | 0.254 ± 0.010 | 0.003 | 0.865 | 0.004 | 0.003 |
| A44op_r | 0.232 ± 0.024 | 0.241 ± 0.019 | 0.255 ± 0.013 | 0.001 | 0.176 | <0.001 | 0.017 |
| A4ul_r | 0.234 ± 0.013 | 0.237 ± 0.014 | 0.248 ± 0.007 | <0.001 | 0.482 | 0.001 | 0.002 |
| A7ip_r | 0.233 ± 0.010 | 0.233 ± 0.008 | 0.240 ± 0.005 | 0.003 | 0.893 | 0.006 | 0.002 |
| dlPu_r | 0.242 ± 0.033 | 0.253 ± 0.020 | 0.270 ± 0.015 | <0.001 | 0.134 | <0.001 | 0.017 |
| dmPOS_r | 0.242 ± 0.009 | 0.247 ± 0.007 | 0.249 ± 0.005 | 0.003 | 0.030 | 0.001 | 0.198 |
| Effect | SE or Boot SE | t Value | p Value | Lower Limited 95% CI | Upper Limited 95% CI | Percentage of Effect | |
|---|---|---|---|---|---|---|---|
| Distance to language network | |||||||
| Constant | −28.68 | 18.43 | −1.56 | 0.1252 | −65.58 | 8.22 | - |
| Nodal efficiency A28_38 | 181.47 | 84.83 | 2.14 | 0.0366 | 11.66 | 351.28 | - |
| Indirect effect model | |||||||
| Constant | 47.57 | 15.78 | 3.02 | 0.0038 | 15.97 | 79.16 | - |
| Distance | 0.23 | 0.11 | 2.11 | 0.0395 | 0.01 | 0.45 | - |
| Nodal efficiency A28_38 | 213.02 | 73.88 | 2.88 | 0.0055 | 65.07 | 360.97 | - |
| Total effect model | |||||||
| Constant | 40.91 | 15.90 | 2.57 | 0.0127 | 9.07 | 72.76 | - |
| Nodal efficiency A28_38 | 255.13 | 73.21 | 3.48 | 0.0009 | 108.58 | 401.68 | - |
| Summary | |||||||
| Total effect | 255.13 | 73.21 | 3.48 | 0.0009 | 108.58 | 401.68 | - |
| Direct effect | 213.02 | 73.88 | 2.88 | 0.0055 | 65.07 | 360.97 | 83.49% |
| Indirect effect | 42.11 | 18.90 | - | <0.05 | 9.06 | 82.93 | 16.51% |
| Effect | SE or Boot SE | t Value | p Value | Lower Limited 95% CI | Upper Limited 95% CI | Percentage of Effect | |
|---|---|---|---|---|---|---|---|
| Distance to language network | |||||||
| Constant | −56.31 | 32.24 | −1.75 | 0.0860 | −120.85 | 8.23 | - |
| Nodal efficiency dmPOS | 272.01 | 130.81 | 2.08 | 0.0420 | 10.16 | 533.87 | - |
| Indirect effect model | |||||||
| Constant | −14.21 | 26.01 | −0.55 | 0.5870 | −66.28 | 37.87 | - |
| Distance | 0.21 | 0.10 | 1.99 | 0.0499 | 0.01 | 0.41 | - |
| Nodal efficiency dmPOS | 439.53 | 106.61 | 4.12 | 0.0001 | 226.05 | 653.01 | - |
| Total effect model | |||||||
| Constant | −25.79 | 25.99 | −0.99 | 0.3251 | −77.82 | 26.23 | - |
| Nodal efficiency dmPOS | 495.50 | 105.45 | 4.70 | <0.0001 | 284.43 | 706.57 | - |
| Summary | |||||||
| Total effect | 495.50 | 105.45 | 4.70 | <0.0001 | 284.43 | 706.57 | - |
| Direct effect | 439.53 | 106.61 | 4.12 | 0.0001 | 226.05 | 653.01 | 88.70% |
| Indirect effect | 55.97 | 19.90 | - | <0.05 | 16.50 | 94.87 | 11.30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Fang, S.; Weng, S.; Zhang, Z.; Jiang, T.; Wang, Y.; Wang, L.; Tang, K. Contralesional Cortical and Network Features Associated with Preoperative Language Deficit in Glioma Patients. Cancers 2022, 14, 4469. https://doi.org/10.3390/cancers14184469
Zhou C, Fang S, Weng S, Zhang Z, Jiang T, Wang Y, Wang L, Tang K. Contralesional Cortical and Network Features Associated with Preoperative Language Deficit in Glioma Patients. Cancers. 2022; 14(18):4469. https://doi.org/10.3390/cancers14184469
Chicago/Turabian StyleZhou, Chunyao, Shengyu Fang, Shimeng Weng, Zhong Zhang, Tao Jiang, Yinyan Wang, Lei Wang, and Kai Tang. 2022. "Contralesional Cortical and Network Features Associated with Preoperative Language Deficit in Glioma Patients" Cancers 14, no. 18: 4469. https://doi.org/10.3390/cancers14184469
APA StyleZhou, C., Fang, S., Weng, S., Zhang, Z., Jiang, T., Wang, Y., Wang, L., & Tang, K. (2022). Contralesional Cortical and Network Features Associated with Preoperative Language Deficit in Glioma Patients. Cancers, 14(18), 4469. https://doi.org/10.3390/cancers14184469





