Scalloping of the Liver and Spleen on Preoperative CT-Scan of Pseudomyxoma Peritonei Patients: Impact on Prediction of Resectability, Grade, Morbidity and Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Framework
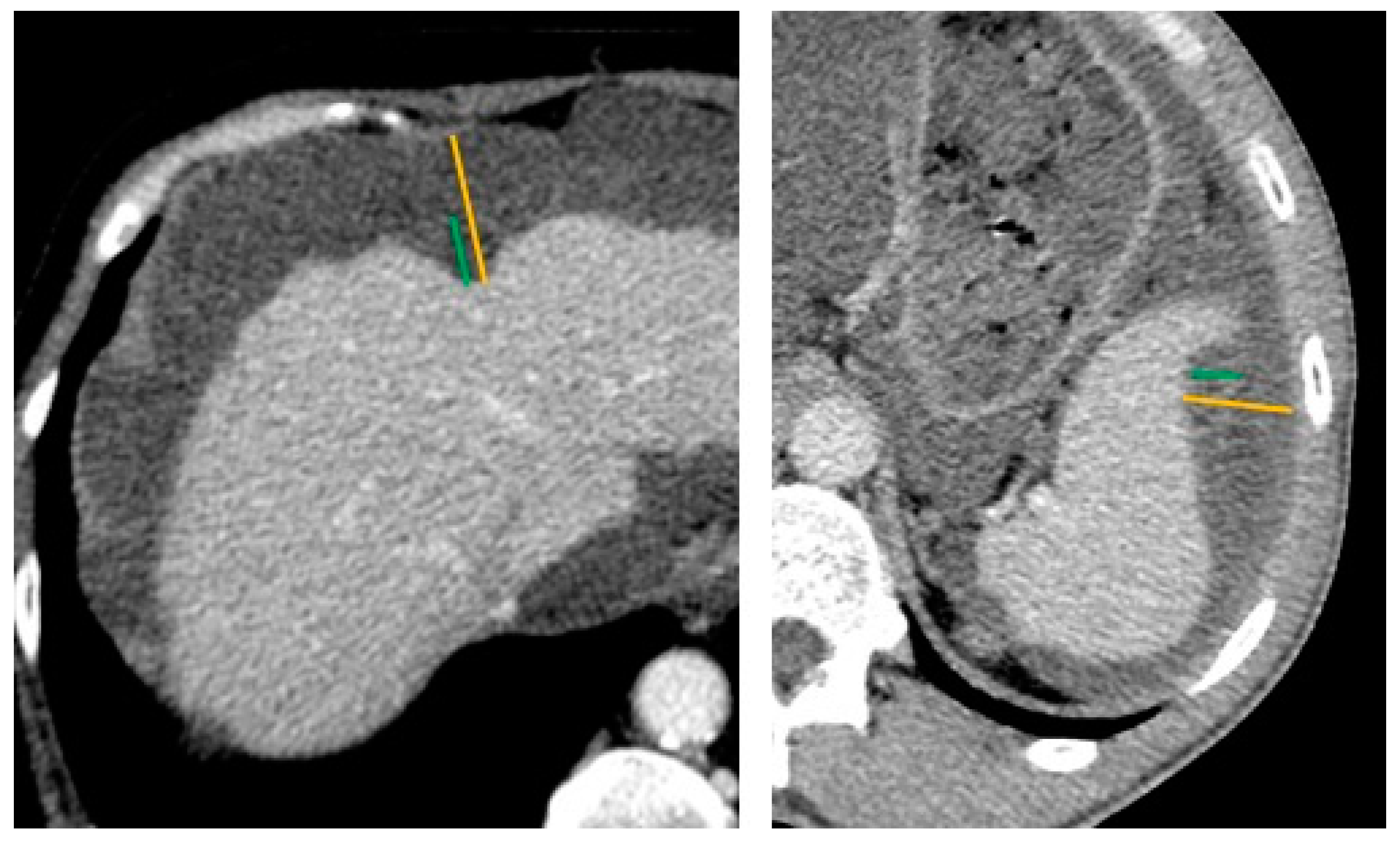
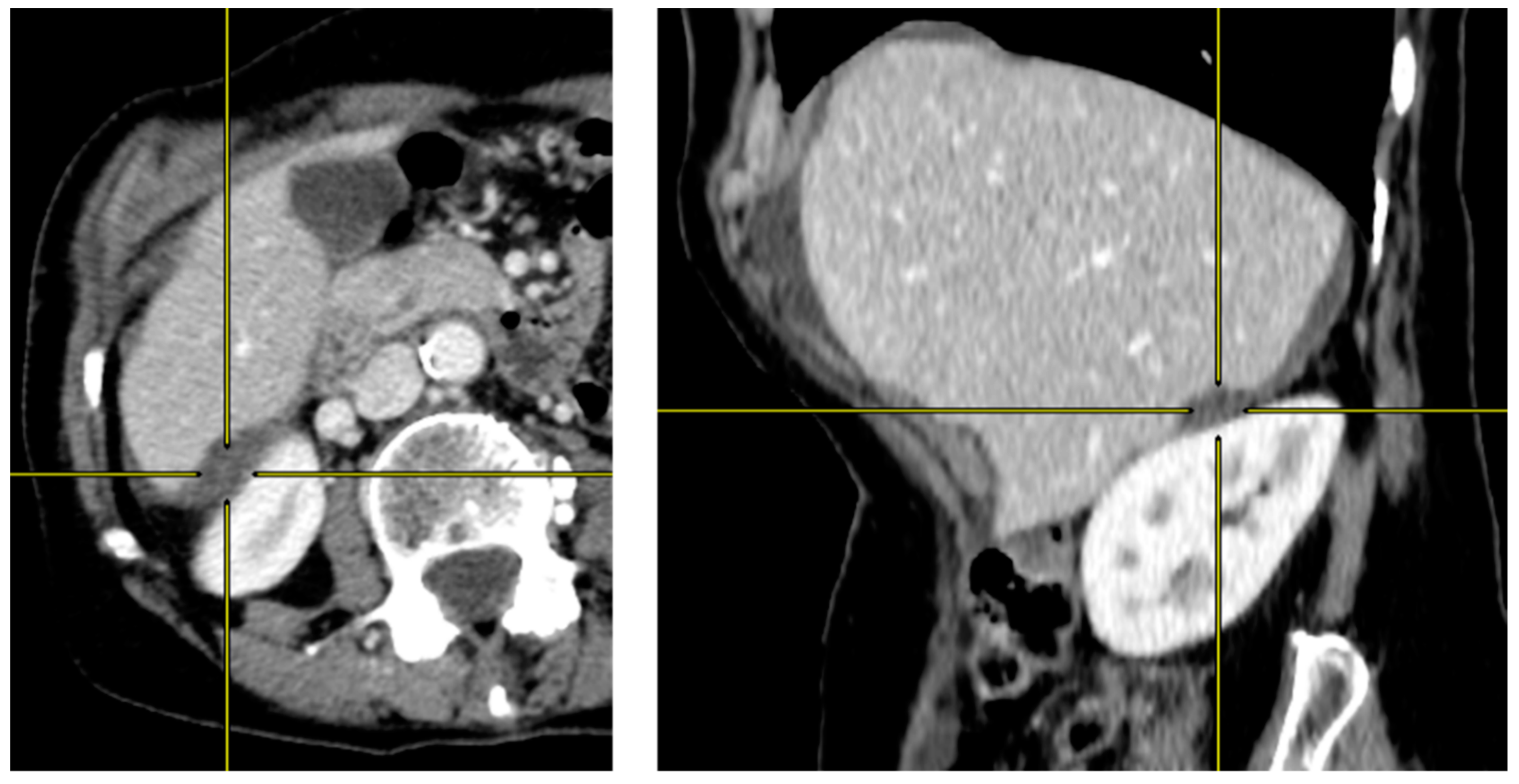
2.2. Preoperative CT-Scan Protocol and Image Analysis
2.3. Cytoreductive Surgery and HIPEC
2.4. Statistical Analysis
3. Results
3.1. Pseudomyxoma Peritonei: Complete vs. Incomplete Cytoreduction
3.1.1. Population Characteristics
3.1.2. Predictive Value of Scalloping on Resectability and Histologic Grade
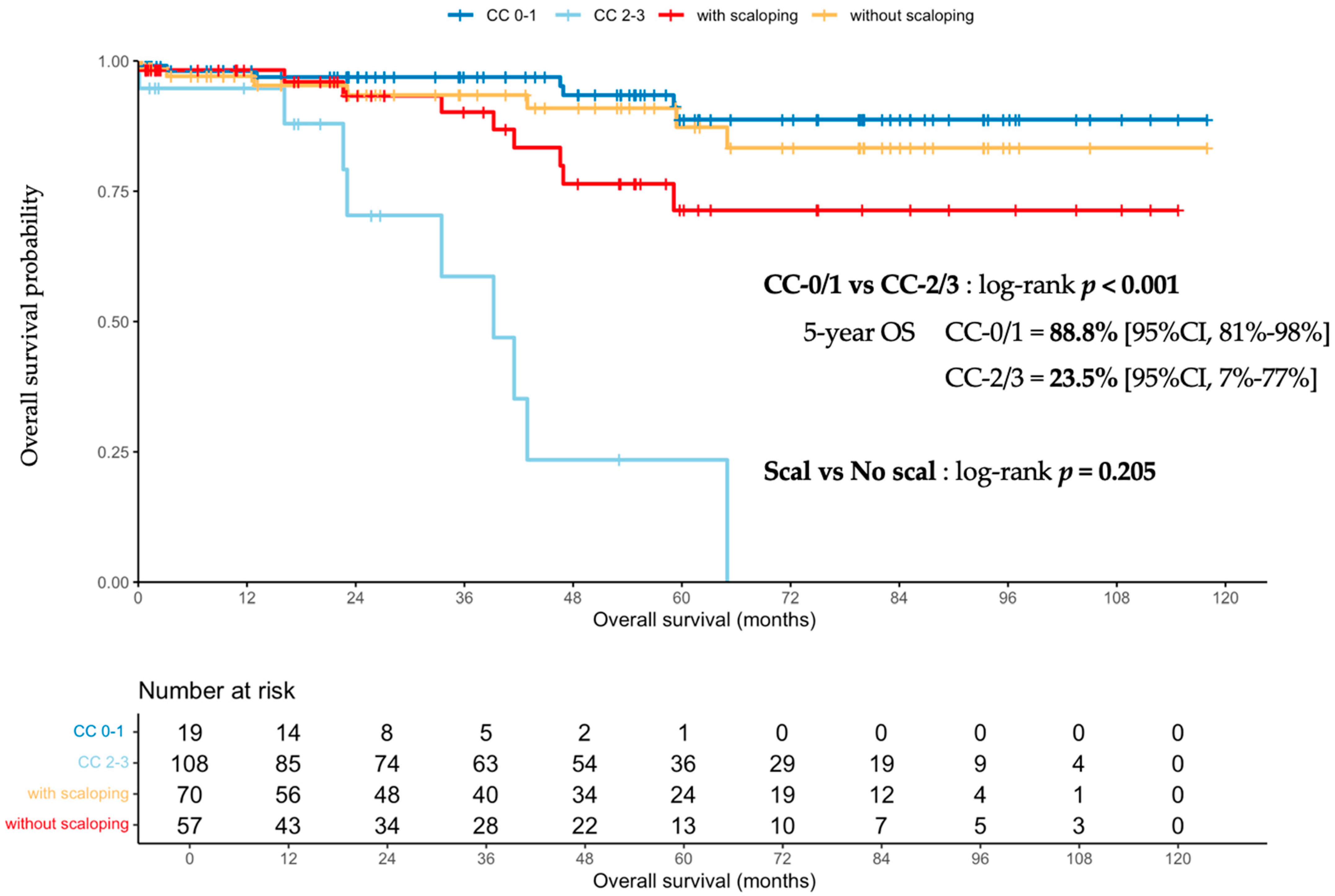
3.1.3. Scalloping Prognosis Impact on Overall Survival
3.2. Scalloping Predictive Value in the Completely Resected Population (CC-0/1)
3.2.1. Population Characteristics
3.2.2. Prognostic Impact of Scalloping on Overall and Recurrence-Free Survivals and Severe Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugarbaker, P.H. Pseudomyxoma Peritonei A Cancer Whose Biology Is Characterized by a Redistribution Phenomenon. Ann. Surg. 1994, 219, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Sugarbaker, P.H. Clinical Presentation of the Pseudomyxoma Peritonei Syndrome. Br. J. Surg. 2000, 87, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Peritonectomy Procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and Long-Term Outcome Data of Patients with Pseudomyxoma Peritonei from Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Al-Zahrani, A.; Saxena, A.; Liauw, W.; Zhao, J.; Morris, D.L. Secondary Cytoreduction and Perioperative Intraperitoneal Chemotherapy after Initial Debulking of Pseudomyxoma Peritonei: A Study of Timing and the Impact of Malignant Dedifferentiation. J. Am. Coll. Surg. 2010, 211, 526–535. [Google Scholar] [CrossRef]
- Lord, A.C.; Shihab, O.; Chandrakumaran, K.; Mohamed, F.; Cecil, T.D.; Moran, B.J. Recurrence and Outcome after Complete Tumour Removal and Hyperthermic Intraperitoneal Chemotherapy in 512 Patients with Pseudomyxoma Peritonei from Perforated Appendiceal Mucinous Tumours. Eur. J. Surg. Oncol. 2015, 41, 396–399. [Google Scholar] [CrossRef]
- Delhorme, J.B.; Severac, F.; Averous, G.; Glehen, O.; Passot, G.; Bakrin, N.; Marchal, F.; Pocard, M.; Dico, R.L.; Eveno, C.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei of Appendicular and Extra-Appendicular Origin. Br. J. Surg. 2018, 105, 668–676. [Google Scholar] [CrossRef]
- Baratti, D.; Milito, P.; Kusamura, S.; Roman, L.M.; Guaglio, M.; Deraco, M. Systemic Metastases from Low-Grade and High-Grade Pseudomyxoma Peritonei: Treatments and Outcomes. Eur. J. Surg. Oncol. 2022, 48, 1590–1597. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Peritoneal Carcinomatosis: Principles of Management. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef]
- Carr, N.J.; Cecil, T.D.; Mohamed, F.; Sobin, L.H.; Sugarbaker, P.H.; González-Moreno, S.; Taflampas, P.; Chapman, S.; Moran, B.J. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am. J. Surg. Pathol. 2016, 40, 14–26. [Google Scholar] [CrossRef]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.H.J.T.; Speeten, K.V.D.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J.; et al. Appendiceal Tumours and Pseudomyxoma Peritonei: Literature Review with PSOGI/EURACAN Clinical Practice Guidelines for Diagnosis and Treatment. Eur. J. Surg. Oncol. 2020, 47, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Fabio, F.D.; Mehta, A.; Chandrakumaran, K.; Mohamed, F.; Cecil, T.; Moran, B. Advanced Pseudomyxoma Peritonei Requiring Gastrectomy to Achieve Complete Cytoreduction Results in Good Long-Term Oncologic Outcomes. Ann. Surg. Oncol. 2016, 23, 4316–4321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mizumoto, A.; Ishibashi, H.; Takeshita, K.; Hirano, M.; Ichinose, M.; Takegawa, S.; Yonemura, Y. Should Total Gastrectomy and Total Colectomy Be Considered for Selected Patients with Severe Tumor Burden of Pseudomyxoma Peritonei in Cytoreductive Surgery? Eur. J. Surg. Oncol. 2016, 42, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Benhaim, L.; Honoré, C.; Goéré, D.; Delhorme, J.B.; Elias, D. Huge Pseudomyxoma Peritonei: Surgical Strategies and Procedures to Employ to Optimize the Rate of Complete Cytoreductive Surgery. Eur. J. Surg. Oncol. 2016, 42, 552–557. [Google Scholar] [CrossRef]
- Kitai, T.; Yamanaka, K.; Sugimoto, N.; Inamoto, O. Surgical Management for Peritoneal Carcinomatosis of Appendiceal Origin with a High-Tumor Burden. Surg. Today 2020, 50, 171–177. [Google Scholar] [CrossRef]
- Mercier, F.; Dagbert, F.; Goéré, D.; Quenet, F.; Wernert, R.; Dumont, F.; Brigand, C.; Passot, G.; Glehen, O.; Network, R.; et al. Recurrence of Pseudomyxoma Peritonei after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. BJS Open 2019, 3, 195–202. [Google Scholar] [CrossRef]
- Kusamura, S.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Elias, D.; Baratti, D.; Morris, D.L.; Sardi, A.; Glehen, O.; Deraco, M.; et al. Multicentre Study of the Learning Curve and Surgical Performance of Cytoreductive Surgery with Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei. Br. J. Surg. 2014, 101, 1758–1765. [Google Scholar] [CrossRef]
- Govaerts, K.; Chandrakumaran, K.; Carr, N.J.; Cecil, T.D.; Dayal, S.; Mohamed, F.; Thrower, A.; Moran, B.J. Single Centre Guidelines for Radiological Follow-up Based on 775 Patients Treated by Cytoreductive Surgery and HIPEC for Appendiceal Pseudomyxoma Peritonei. Eur. J. Surg. Oncol. 2018, 44, 1371–1377. [Google Scholar] [CrossRef]
- Kirby, R.; Liauw, W.; Zhao, J.; Morris, D. Quality of Life Study Following Cytoreductive Surgery and Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei Including Redo Procedures. Int. J. Surg. Oncol. 2013, 2013, 461041. [Google Scholar] [CrossRef]
- Stearns, A.T.; Malcomson, L.; Punnett, G.; Abudeeb, H.; Aziz, O.; Selvasekar, C.R.; Fulford, P.E.; Wilson, M.S.; Renehan, A.G.; O’Dwyer, S.T. Long-Term Quality of Life After Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei: A Prospective Longitudinal Study. Ann. Surg. Oncol. 2018, 25, 965–973. [Google Scholar] [CrossRef]
- Ahmadi, N.; Kostadinov, D.; Sakata, S.; Ball, W.R.; Gandhi, J.; Carr, N.J.; Tzivanakis, A.; Dayal, S.P.; Mohamed, F.; Cecil, T.D.; et al. Managing Recurrent Pseudomyxoma Peritonei in 430 Patients After Complete Cytoreduction and HIPEC: A Dilemma for Patients and Surgeons. Ann. Surg. Oncol. 2021, 28, 7809–7820. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Barone, R.M.; Rousset, P. Peritoneal MRI in Patients Undergoing Cytoreductive Surgery and HIPEC: History, Clinical Applications, and Implementation. Eur. J. Surg. Oncol. 2019, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Menassel, B.; Duclos, A.; Passot, G.; Dohan, A.; Payet, C.; Isaac, S.; Valette, P.J.; Glehen, O.; Rousset, P. Preoperative CT and MRI Prediction of Non-Resectability in Patients Treated for Pseudomyxoma Peritonei from Mucinous Appendiceal Neoplasms. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2016, 42, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Minamimoto, R.; Gohda, Y.; Tajima, T.; Kiyomatsu, T.; Yano, H. Pseudomyxoma Peritonei: Visceral Scalloping on CT Is a Predictor of Recurrence after Complete Cytoreductive Surgery. Eur. Radiol. 2020, 33, 4193–4200. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, X.; Wang, L.; Wang, R.; Du, X. Enhanced Computed Tomography Imaging Features Predict Tumor Grade in Pseudomyxoma Peritonei. Quant. Imaging Med. Surg. 2022, 12, 2321–2331. [Google Scholar] [CrossRef]
- Bouquot, M.; Dohan, A.; Gayat, E.; Barat, M.; Glehen, O.; Pocard, M.; Rousset, P.; Eveno, C. Prediction of Resectability in Pseudomyxoma Peritonei with a New CT Score. Ann. Surg. Oncol. 2017, 25, 694–701. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Gurney, J.M.; Muller, W.D. Mucinous Appendiceal Neoplasms: Preoperative MR Staging and Classification Compared with Surgical and Histopathologic Findings. Am. J. Roentgenol. 2008, 190, 656–665. [Google Scholar] [CrossRef]
- Chua, T.C.; Al-Zahrani, A.; Saxena, A.; Glenn, D.; Liauw, W.; Zhao, J.; Morris, D.L. Determining the Association Between Preoperative Computed Tomography Findings and Postoperative Outcomes After Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2011, 18, 1582–1589. [Google Scholar] [CrossRef]
- Dineen, S.P.; Royal, R.E.; Hughes, M.S.; Sagebiel, T.; Bhosale, P.; Overman, M.; Matamoros, A.; Mansfield, P.F.; Fournier, K.F. A Simplified Preoperative Assessment Predicts Complete Cytoreduction and Outcomes in Patients with Low-Grade Mucinous Adenocarcinoma of the Appendix. Ann. Surg. Oncol. 2015, 22, 3640–3646. [Google Scholar] [CrossRef]
- Milovanov, V.; Sardi, A.; Aydin, N.; Nieroda, C.; Sittig, M.; Gushchin, V. External Validation of the Simplified Preoperative Assessment for Low-Grade Mucinous Adenocarcinoma of the Appendix. Ann. Surg. Oncol. 2017, 24, 1783–1786. [Google Scholar] [CrossRef]
- Sabesan, A.; Felder, S.; Feuerlein, S.; Lam, C.; McGettigan, M.; Powers, B.D.; Dessureault, S.; Dineen, S.P. Preoperative Radiographic Assessment Predicts Incomplete Cytoreduction in Patients with Low Grade Mucinous Adenocarcinoma of the Appendix. Ann. Surg. Oncol. 2020, 27, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Seshul, M.; Coulam, C. Pseudomyxoma Peritonei: Computed Tomography and Sonography. Am. J. Roentgenol. 1981, 136, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, L.; Passot, G.; Glehen, O.; Isaac, S.; Bibeau, F.; Rousset, P.; Gilly, F.N.; Network, R. The RENAPE Observational Registry: Rationale and Framework of the Rare Peritoneal Tumors French Patient Registry. Orphanet J. Rare Dis. 2017, 12, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 15 July 2022).
- Mercier, F.; Mohamed, F.; Cazauran, J.-B.; Képénékian, V.; Vaudoyer, D.; Cotte, E.; Glehen, O.; Passot, G. An Update of Peritonectomy Procedures Used in Cytoreductive Surgery for Peritoneal Malignancy. Int. J. Hyperth. 2019, 36, 744–752. [Google Scholar] [CrossRef]
- Passot, G.; Kim, B.J.; Vaudoyer, D.; Képénékian, V.; Bonnefoy, I.; Bakrin, N.; Cotte, E.; Glehen, O. Digital Glissonectomy: A Safe Perihepatic Peritonectomy. Ann. Surg. Oncol. 2016, 23, 3978–3985. [Google Scholar] [CrossRef]
- Passot, G.; Vaudoyer, D.; Villeneuve, L.; Wallet, F.; Beaujard, A.-C.; Boschetti, G.; Rousset, P.; Bakrin, N.; Cotte, E.; Glehen, O. A Perioperative Clinical Pathway Can Dramatically Reduce Failure-to-Rescue Rates After Cytoreductive Surgery for Peritoneal Carcinomatosis: A Retrospective Study of 666 Consecutive Cytoreductions. Ann. Surg. 2017, 265, 806–813. [Google Scholar] [CrossRef]
- Bai, M.; Wang, S.; Liang, G.; Cai, Y.; Lu, Y.; Hou, N.; Ma, R.; Xu, H.; Zhang, M. Nomogram to Predict Incomplete Cytoreduction for Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2022, 29, 885–892. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Bijelic, L.; Chang, D.; Yoo, D. Neoadjuvant FOLFOX Chemotherapy in 34 Consecutive Patients with Mucinous Peritoneal Carcinomatosis of Appendiceal Origin. J. Surg. Oncol. 2010, 102, 576–581. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Nonaka, D.; Langer, M.; Andreola, S.; Favaro, M.; Gavazzi, C.; Laterza, B.; Deraco, M. Pseudomyxoma Peritonei: Clinical Pathological and Biological Prognostic Factors in Patients Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann. Surg. Oncol. 2007, 15, 526–534. [Google Scholar] [CrossRef]
- Bijelic, L.; Kumar, A.S.; Stuart, O.A.; Sugarbaker, P.H. Systemic Chemotherapy Prior to Cytoreductive Surgery and HIPEC for Carcinomatosis from Appendix Cancer: Impact on Perioperative Outcomes and Short-Term Survival. Gastroenterol. Res. Pract. 2012, 2012, 163284. [Google Scholar] [CrossRef]
- Sgarbura, O.; Hosni, M.A.; Petruzziello, A.; Figueroa, R.; Khellaf, L.; Pissas, M.-H.; Carrère, S.; Nougaret, S.; Bibeau, F.; Quénet, F. Complete Pathologic Response after Two-Stage Cytoreductive Surgery with HIPEC for Bulky Pseudomyxoma Peritonei: Proof of Concept. Int. J. Hyperther. 2020, 37, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Taflampas, P.; Riss, S.; Chandrakumaran, K.; Cecil, T.D.; Mohamed, F.; Moran, B.J. Complete Cytoreduction for Pseudomyxoma Peritonei Is Optimal but Maximal Tumor Debulking May Be Beneficial in Patients in Whom Complete Tumor Removal Cannot Be Achieved. Dis. Colon Rectum 2013, 56, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, R.E.; Chen, M.Y.M.; Loggie, B.W.; Jackson, S.L.; Geisinger, K. CT Appearance of Disseminated Peritoneal Adenomucinosis. Abdom. Imaging 2001, 26, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Mekkawy, A.; Chua, T.C.; Morris, D.L. Physical and Chemical Characteristics of Mucin Secreted by Pseudomyxoma Peritonei (PMP). Int. J. Med. Sci. 2017, 14, 18–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.; Alzahrani, N.A.; Fisher, O.M.; Chua, T.C.; Kozman, M.A.; Liauw, W.; Arrowaili, A.; Valle, S.J.; Morris, D.L. Intraoperative Macroscopic Tumour Consistency Is Associated with Overall Survival after Cytoreductive Surgery and Intraperitoneal Chemotherapy for Appendiceal Adenocarcinoma with Peritoneal Metastases: A Retrospective Observational Study. Am. J. Surg. 2019, 217, 704–712. [Google Scholar] [CrossRef]
- Zhou, N.; Dou, R.; Zhai, X.; Fang, J.; Wang, J.; Ma, R.; Xu, J.; Cui, B.; Liang, L. Radiomics Analysis Based on CT’s Greater Omental Caking for Predicting Pathological Grading of Pseudomyxoma Peritonei. Sci. Rep. 2022, 12, 4401. [Google Scholar] [CrossRef]


| Overall Population | Completely Resected Population | |||||||
|---|---|---|---|---|---|---|---|---|
| CC-0/1 N = 109 | CC-2/3 N = 20 | Missing N (%) | p2 | With Scalloping N = 43 | Without Scalloping N = 66 | Missing N (%) | p2 | |
| Age 1 | 57.1 [46.2, 65.4] | 67.1 [58.0, 77.5] | 0 | 0.003 | 55.6 [47.4, 65.5] | 57.3 [40.7, 64.8] | 0 | 0.7 |
| Gender, male | 38 (35%) | 12 (60%) | 0 | 0.034 | 12 (28%) | 26 (39%) | 0 | 0.2 |
| ASA | 19 (15%) | 0.2 | 13 (12%) | 0.4 | ||||
| 1 | 44 (46%) | 5 (36%) | 21 (50%) | 23 (43%) | ||||
| 2 | 46 (48%) | 6 (43%) | 20 (48%) | 26 (48%) | ||||
| 3 | 6 (6.2%) | 3 (21%) | 1 (2.4%) | 5 (9.3%) | ||||
| PCI 1 | 14.0 [6.0, 24.0] | 31.5 [29.8, 33.8] | 0 | <0.001 | 24.0 [17.0, 27.0] | 8.0 [3.2, 14.8] | 0 | <0.001 |
| Histologic grade, high | 18 (21%) | 13 (93%) | 30 (23%) | <0.001 | 9 (24%) | 9 (19%) | 24 (22%) | 0.5 |
| BMI (kg/m2) 1 | 23.8 [21.5, 27.9] | 24.2 [22.5, 26.5] | 8 (6.2%) | >0.9 | 22.7 [21.5, 26.2] | 24.0 [21.8, 28.4] | 6 (5.5%) | 0.3 |
| Splenectomy rate | 50 (46%) | 5 (26%) | 1 (0.8%) | 0.11 | 32 (74%) | 18 (27%) | 0 | <0.001 |
| CA-19.9 1 | 15.0 [7.0, 36.0] | 176.0 [77.0, 1,597.0] | 32 (25%) | <0.001 | 28.0 [10.5, 52.8] | 10.0 [6.0, 19.0] | 28 (26%) | 0.001 |
| CEA 1 | 2.9 [1.3, 10.7] | 53.4 [32.0, 147.0] | 29 (22%) | <0.001 | 5.9 [2.2, 18.8] | 1.8 [0.9, 3.6] | 25 (23%) | <0.001 |
| CA-125 1 | 28.0 [17.2, 65.0] | 85.0 [70.0, 168.0] | 51 (40%) | <0.001 | 48.4 [27.2, 83.0] | 20.0 [10.0, 28.7] | 42 (39%) | <0.001 |
| Operative time (min) 1 | 300.0 [240.0, 390.0] | 210.0 [195.0, 225.0] | 30 (23%) | 0.026 | 330.0 [300.0, 420.0] | 270.0 [210.0, 360.0] | 17 (16%) | 0.001 |
| HIPEC | 103 (94%) | 5 (25%) | 0 | <0.001 | 41 (95%) | 62 (94%) | 0 | >0.9 |
| Severe complications (CD ≥ 3) | 52 (48%) | 10 (50%) | 1 (0.8%) | 0.9 | 21 (49%) | 31 (48%) | 1 (0.9%) | >0.9 |
| Postoperative mortality | 1 (0.9%) | 1 (5.0%) | 1 (0.8%) | 0.3 | 0 (0%) | 1 (1.5%) | 1 (0.9%) | >0.9 |
| Hospital length of stay 1 | 19.0 [14.0, 29.0] | 17.5 [13.2, 21.8] | 8 (6.2%) | 0.5 | 23.0 [17.0, 33.0] | 16.0 [12.0, 22.8] | 6 (5.5%) | <0.001 |
| Scalloping rate | 43 (39%) | 15 (75%) | 0 | 0.003 | NA | |||
| Scal (Any Kind) Y vs. N | Scal H + S vs. No Scal | Scal H > 20 mm | Scal S > 10 mm | |
|---|---|---|---|---|
| Resectability (CC-0/1 vs. CC-2/3) | ||||
| Se | 75% | 55% | 53% | 91% |
| Sp | 61% | 73% | 88% | 28% |
| PPV | 26% | 28% | 62% | 32% |
| NPV | 93% | 90% | 84% | 89% |
| Histologic grade (low vs. high) | ||||
| Se | 58% | 42% | 39% | 8% |
| Sp | 57% | 72% | 93% | 89% |
| PPV | 38% | 41% | 78% | 33% |
| NPV | 75% | 73% | 71% | 59% |
| Characteristics | Cytoreduction Completeness | Histologic Grade | ||||||
|---|---|---|---|---|---|---|---|---|
| CC-0/1 N = 109 | CC-2/3 N = 20 | Missing N (%) | p2 | Low N = 68 | High N = 31 | Missing N (%) | p3 | |
| PCI 1 | 14.0 [6.0, 24.0] | 31.5 [29.8, 33.8] | 0 | <0.001 | 14.0 [6.0, 24.0] | 24.0 [18.0, 31.0] | 0 | <0.001 |
| Scalloping | 0 | 0.008 | 0 | 0.3 | ||||
| None | 66 (61%) | 5 (25%) | 39 (57%) | 13 (42%) | ||||
| Hepatic scal | 14 (13%) | 4 (20%) | 10 (15%) | 5 (16%) | ||||
| Hepatic + Splenic scal | 29 (27%) | 11 (55%) | 19 (28%) | 13 (42%) | ||||
| Liver scalloping 1 | ||||||||
| max depth (mm) | 11.0 [7.0, 17.0] | 21.0 [11.5, 24.0] | 71 (55%) | 0.007 | 11.0 [7.0, 17.0] | 13.5 [10.0, 24.5] | 52 (53%) | 0.2 |
| max depth Hotta (mm) | 14.0 [8.5, 27.0] | 23.0 [18.0, 32.5] | 71 (55%) | 0.038 | 14.0 [7.0, 26.0] | 15.5 [10.8, 25.0] | 52 (53%) | 0.4 |
| max length (mm) | 50.0 [32.5, 66.0] | 60.0 [40.0, 80.0] | 71 (55%) | 0.3 | 48.0 [35.0, 70.0] | 60.0 [43.2, 66.5] | 52 (53%) | 0.3 |
| ratio max depth/length | 0.2 [0.2, 0.4] | 0.4 [0.2, 0.5] | 71 (55%) | 0.10 | 0.2 [0.2, 0.4] | 0.3 [0.2, 0.4] | 52 (53%) | >0.9 |
| Splenic scalloping 1 | ||||||||
| max depth (mm) | 10.0 [8.0, 12.0] | 12.0 [10.0, 15.0] | 89 (69%) | 0.3 | 12.0 [10.0, 13.5] | 9.0 [7.0, 10.0] | 67 (68%) | 0.014 |
| max depth Hotta (mm) | 11.0 [9.0, 22.0] | 17.0 [11.0, 25.0] | 89 (69%) | 0.3 | 14.0 [10.0, 23.5] | 12.0 [7.0, 18.0] | 67 (68%) | 0.3 |
| max length (mm) | 40.0 [30.0, 47.0] | 30.0 [20.5, 41.5] | 89 (69%) | 0.2 | 40.0 [30.0, 60.0] | 28.0 [17.0, 35.0] | 67 (68%) | 0.019 |
| ratio max depth/length | 0.3 [0.2, 0.3] | 0.4 [0.3, 0.7] | 89 (69%) | 0.013 | 0.3 [0.2, 0.4] | 0.3 [0.3, 0.6] | 67 (68%) | 0.4 |
| Liver + Splenic scalloping 1 | ||||||||
| max depth (mm) | 19.0 [16.0, 34.0] | 32.0 [24.5, 38.0] | 89 (69%) | 0.040 | 27.0 [17.5, 35.0] | 22.0 [16.0, 32.0] | 67 (68%) | 0.6 |
| max length (mm) | 91.0 [70.0, 110.0] | 113.0 [70.0, 128.0] | 89 (69%) | 0.5 | 91.0 [70.0, 107.5] | 88.0 [75.0, 113.0] | 67 (68%) | 0.9 |
| ratio max depth/length | 0.2 [0.2, 0.3] | 0.3 [0.2, 0.4] | 89 (69%) | 0.065 | 0.3 [0.2, 0.3] | 0.2 [0.2, 0.4] | 67 (68%) | 0.4 |
| ROI density (HU) | ||||||||
| ratio ROI scal/aorta ≥ 0.3 | 8 (19%) | 4 (27%) | 71 (55%) | 0.5 | 5 (17%) | 4 (22%) | 52 (53%) | 0.7 |
| ratio ROI scal/liver ≥ 0.6 | 13 (30%) | 8 (53%) | 71 (55%) | 0.13 | 9 (31%) | 8 (44%) | 52 (53%) | 0.4 |
| Overall Survival | Recurrence-Free Survival | Severe Complications | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | p | HR | 95% CI | p | OR | 95% CI | p | |
| Age, ≥60 yo | 108 | 0.55 | 0.11, 2.83 | 0.5 | 0.99 | 0.46, 2.16 | >0.9 | 1.43 | 0.67, 3.09 | 0.4 |
| Sex, Male | 108 | 2.84 | 0.63, 12.8 | 0.2 | 0.91 | 0.40, 2.10 | 0.8 | 0.95 | 0.43, 2.10 | >0.9 |
| CEA, >15 IU | 83 | 4.28 | 0.94, 19.4 | 0.059 | 2.50 | 0.94, 6.61 | 0.065 | 1.14 | 0.41, 3.30 | 0.8 |
| CA-19.9, >20 IU | 80 | 2.04 | 0.41, 10.1 | 0.4 | 1.67 | 0.62, 4.49 | 0.3 | 0.72 | 0.29, 1.80 | 0.5 |
| CA-125, >41 IU | 66 | 6.47 | 1.23, 34.1 | 0.028 | 3.17 | 1.08, 9.32 | 0.036 | 1.32 | 0.49, 3.59 | 0.6 |
| Histologic grade, high | 84 | 2.31 | 0.32, 16.5 | 0.4 | 4.20 | 1.74, 10.2 | 0.001 | 2.06 | 0.72, 6.22 | 0.2 |
| PCI, >25 | 108 | 7.00 | 1.51, 32.5 | 0.013 | 1.37 | 0.47, 4.01 | 0.6 | 1.43 | 0.52, 4.06 | 0.5 |
| Scalloping | 108 | |||||||||
| none | - | - | - | - | - | - | ||||
| hepatic | 0.00 | 0.00, Inf | >0.9 | 0.78 | 0.23, 2.68 | 0.7 | 0.30 | 0.06, 1.06 | 0.083 | |
| hepatic + splenic | 1.78 | 0.40, 7.94 | 0.5 | 0.88 | 0.35, 2.23 | 0.8 | 1.79 | 0.74, 4.49 | 0.2 | |
| Liver scalloping | ||||||||||
| max depth, >15 mm | 43 | 1.19 | 0.11, 13.1 | 0.9 | 1.12 | 0.28, 4.49 | 0.9 | 1.64 | 0.46, 6.17 | 0.5 |
| max depth Hotta, >20 mm | 43 | 1.33 | 0.12, 14.9 | 0.8 | 1.24 | 0.31, 4.99 | 0.8 | 1.64 | 0.46, 6.17 | 0.5 |
| max length, >60 mm | 43 | 4.28 | 0.39, 47.3 | 0.2 | 1.32 | 0.33, 5.28 | 0.7 | 1.33 | 0.36, 5.07 | 0.7 |
| ratio max depth/length, >0.4 | 43 | 0.00 | 0.00, Inf | >0.9 | 0.48 | 0.06, 3.88 | 0.5 | 2.53 | 0.57, 13.6 | 0.2 |
| Splenic scalloping | ||||||||||
| max depth, >8 mm | 29 | 0.21 | 0.02, 2.37 | 0.2 | 0.43 | 0.09, 2.13 | 0.3 | 0.16 | 0.01, 1.11 | 0.11 |
| max depth Hotta, >20 mm | 29 | 0.00 | 0.00, Inf | >0.9 | 0.56 | 0.06, 4.79 | 0.6 | 0.34 | 0.06, 1.72 | 0.2 |
| max length, >40 mm | 29 | 3.62 | 0.33, 40.3 | 0.3 | 0.96 | 0.17, 5.25 | >0.9 | 0.32 | 0.06, 1.51 | 0.2 |
| ratio max depth/length, >0.4 | 29 | 0.00 | 0.00, Inf | >0.9 | 0.00 | 0.00, Inf | >0.9 | 1.29 | 0.20, 10.7 | 0.8 |
| ROI density (HU) | ||||||||||
| ratio ROI scal/aorta ≥ 0.3 | 43 | 1.37 | 0.12, 15.2 | 0.8 | 1.52 | 0.38, 6.11 | 0.6 | 4.00 | 0.79, 30.0 | 0.12 |
| ratio ROI scal/healthy liver ≥ 0.6 | 43 | 2.95 | 0.26, 32.9 | 0.4 | 1.37 | 0.36, 5.12 | 0.6 | 3.37 | 0.88, 14.9 | 0.085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kepenekian, V.; Kefleyesus, A.; Keskin, D.; Benzerdjeb, N.; Bonnefoy, I.; Villeneuve, L.; Alhadeedi, O.; Al-Otaibi, A.; Galan, A.; Glehen, O.; et al. Scalloping of the Liver and Spleen on Preoperative CT-Scan of Pseudomyxoma Peritonei Patients: Impact on Prediction of Resectability, Grade, Morbidity and Survival. Cancers 2022, 14, 4434. https://doi.org/10.3390/cancers14184434
Kepenekian V, Kefleyesus A, Keskin D, Benzerdjeb N, Bonnefoy I, Villeneuve L, Alhadeedi O, Al-Otaibi A, Galan A, Glehen O, et al. Scalloping of the Liver and Spleen on Preoperative CT-Scan of Pseudomyxoma Peritonei Patients: Impact on Prediction of Resectability, Grade, Morbidity and Survival. Cancers. 2022; 14(18):4434. https://doi.org/10.3390/cancers14184434
Chicago/Turabian StyleKepenekian, Vahan, Amaniel Kefleyesus, David Keskin, Nazim Benzerdjeb, Isabelle Bonnefoy, Laurent Villeneuve, Omar Alhadeedi, Abeer Al-Otaibi, Alexandre Galan, Olivier Glehen, and et al. 2022. "Scalloping of the Liver and Spleen on Preoperative CT-Scan of Pseudomyxoma Peritonei Patients: Impact on Prediction of Resectability, Grade, Morbidity and Survival" Cancers 14, no. 18: 4434. https://doi.org/10.3390/cancers14184434
APA StyleKepenekian, V., Kefleyesus, A., Keskin, D., Benzerdjeb, N., Bonnefoy, I., Villeneuve, L., Alhadeedi, O., Al-Otaibi, A., Galan, A., Glehen, O., Péron, J., & Rousset, P. (2022). Scalloping of the Liver and Spleen on Preoperative CT-Scan of Pseudomyxoma Peritonei Patients: Impact on Prediction of Resectability, Grade, Morbidity and Survival. Cancers, 14(18), 4434. https://doi.org/10.3390/cancers14184434







