Pathological Evaluation of Resected Colorectal Liver Metastases: mFOLFOX6 Plus Bevacizumab versus mFOLFOX6 Plus Cetuximab in the Phase II ATOM Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Endpoints
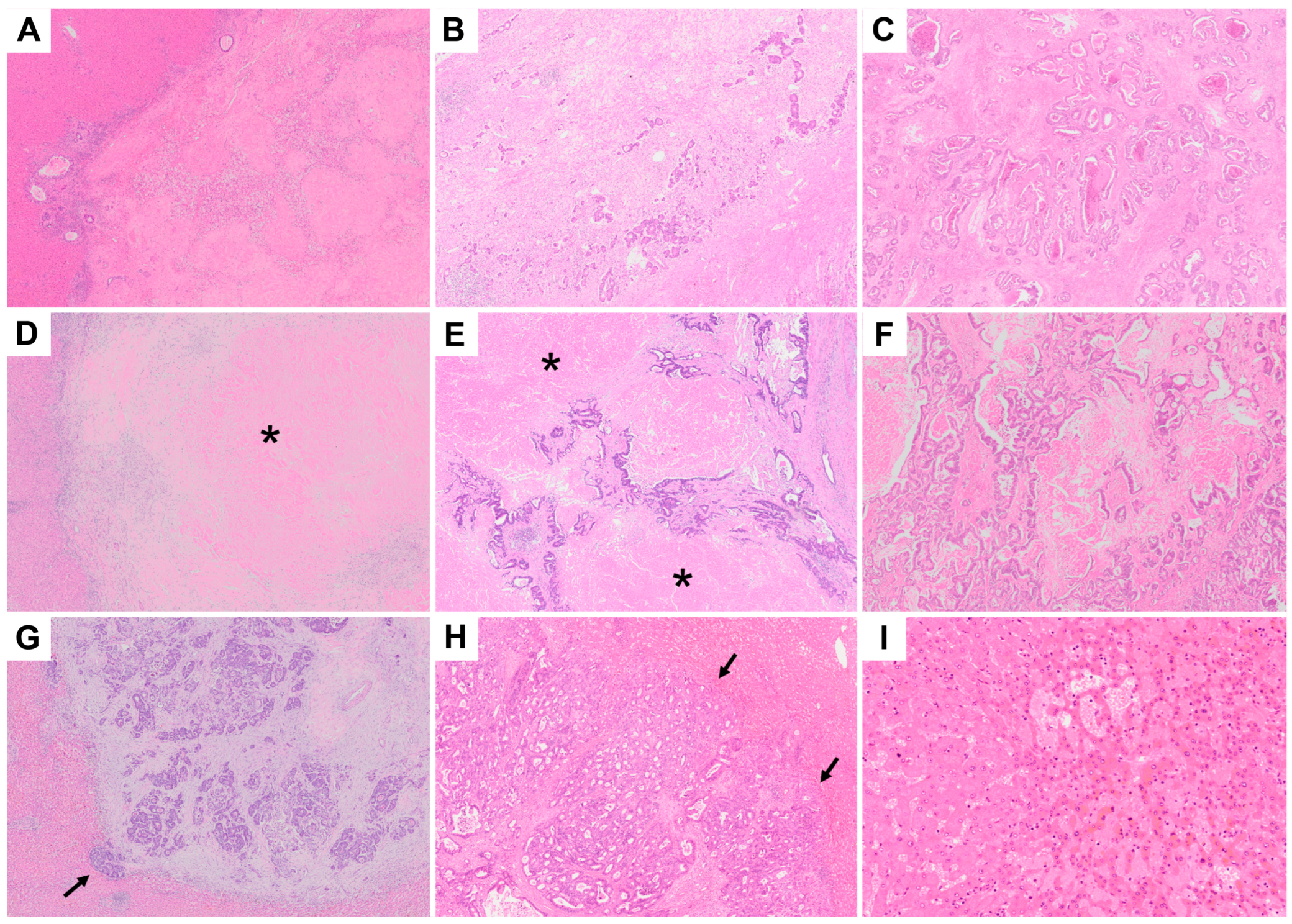
2.3. Pathological Assessment and Radiological Assessment
2.4. Classification of Histopathological Response
2.5. Statistical Analyses
3. Results
3.1. Patients
3.2. Differences in TRG, mTRG, DH, and SOS
3.3. Relationship between Histological Response Based on TRG/mTRG and Radiological Response According to RECIST (Version 1.1)
3.4. TRG and mTRG Classifications as Predictors of RFS and OS
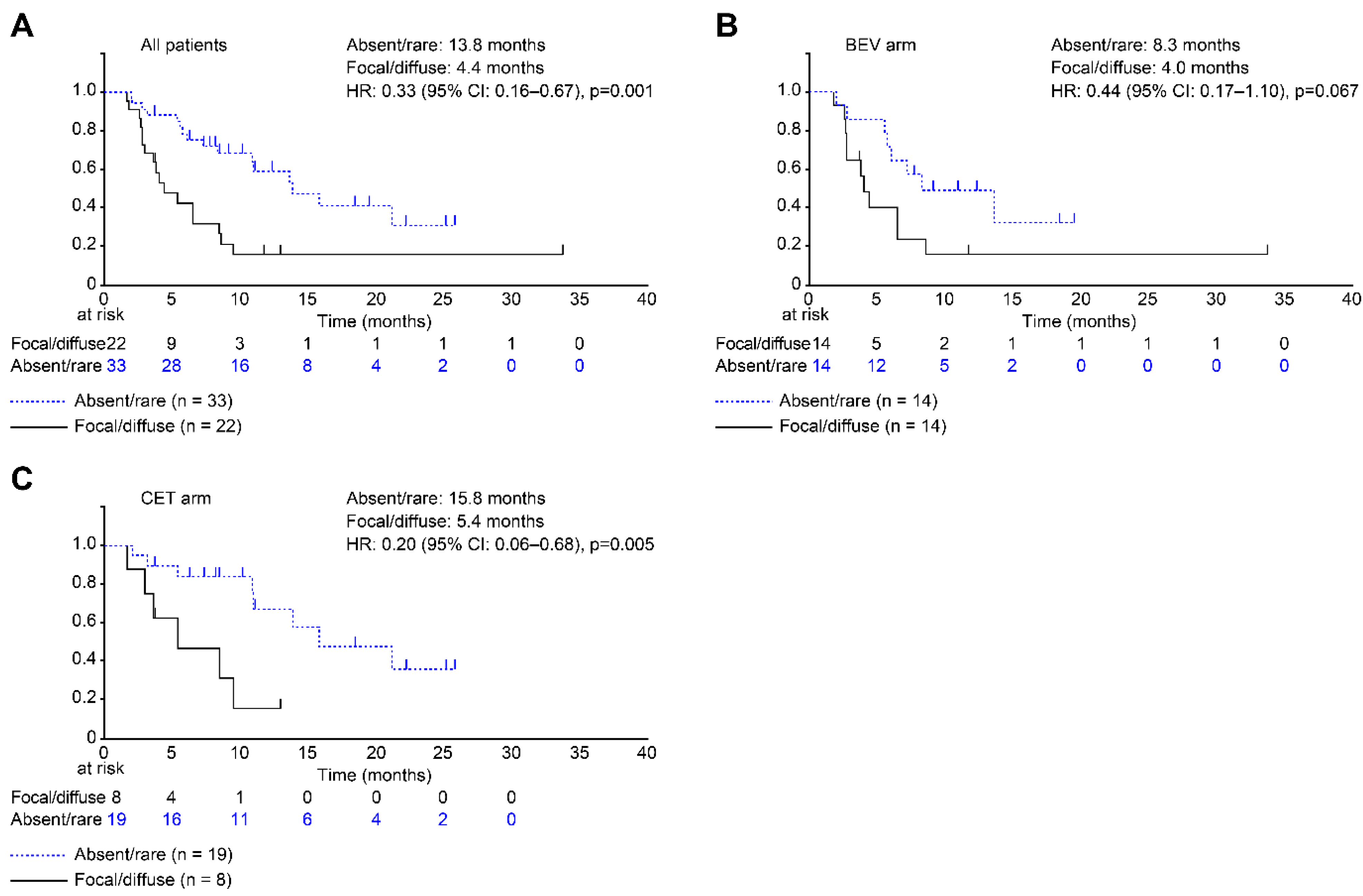
3.5. DH Classifications as Predictors of RFS and OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2019 for the Treatment of Colorectal Cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Delvart, V.; Pascal, G.; Valeanu, A.; Castaing, D.; Azoulay, D.; Giacchetti, S.; Paule, B.; Kunstlinger, F.; Ghémard, O.; et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann. Surg. 2004, 240, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Alberts, S.R.; Horvath, W.L.; Sternfeld, W.C.; Goldberg, R.M.; Mahoney, M.R.; Dakhil, S.R.; Levitt, R.; Rowland, K.; Nair, S.; Sargent, D.J.; et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group Phase II Study. J. Clin. Oncol. 2005, 23, 9243–9249. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.O.; Raab, H.R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- Garufi, C.; Torsello, A.; Tumolo, S.; Ettorre, G.M.; Zeuli, M.; Campanella, C.; Vennarecci, G.; Mottolese, M.; Sperduti, I.; Cognetti, F. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER Trial. Br. J. Cancer 2010, 103, 1542–1547. [Google Scholar]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; García Alfonso, P.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef]
- Wong, R.; Cunningham, D.; Barbachano, Y.; Saffery, C.; Valle, J.; Hickish, T.; Mudan, S.; Brown, G.; Khan, A.; Wotherspoon, A.; et al. A Multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann. Oncol. 2011, 22, 2042–2048. [Google Scholar] [CrossRef]
- Oki, E.; Emi, Y.; Yamanaka, T.; Uetake, H.; Muro, K.; Takahashi, T.; Nagasaka, T.; Hatano, E.; Ojima, H.; Manaka, D.; et al. Randomised phase II trial of mFOLFOX6 plus bevacizumab versus mFOLFOX6 plus cetuximab as first-line treatment for colorectal liver metastasis (ATOM Trial). Br. J. Cancer 2019, 121, 222–229. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Giostra, E.; Brezault, C.; Roth, A.D.; Andres, A.; Audard, V.; Sartoretti, P.; Dousset, B.; Majno, P.E.; Soubrane, O.; et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 2007, 18, 299–304. [Google Scholar] [CrossRef]
- Chang, H.H.; Leeper, W.R.; Chan, G.; Quan, D.; Driman, D.K. Infarct-like necrosis: A distinct form of necrosis seen in colorectal carcinoma liver metastases treated with perioperative chemotherapy. Am. J. Surg. Pathol. 2012, 36, 570–576. [Google Scholar] [CrossRef]
- Mentha, G.; Terraz, S.; Morel, P.; Andres, A.; Giostra, E.; Roth, A.; Rubbia-Brandt, L.; Majno, P. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br. J. Surg. 2009, 96, 95–103. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Audard, V.; Sartoretti, P.; Roth, A.D.; Brezault, C.; Le Charpentier, M.; Dousset, B.; Morel, P.; Soubrane, O.; Chaussade, S.; et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann. Oncol. 2004, 15, 460–466. [Google Scholar] [CrossRef]
- Ribero, D.; Wang, H.; Donadon, M.; Zorzi, D.; Thomas, M.B.; Eng, C.; Chang, D.Z.; Curley, S.A.; Abdalla, E.K.; Ellis, L.M.; et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007, 110, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Loyer, E.M.; Kopetz, S.; Boonsirikamchai, P.; Maru, D.M.; Chun, Y.S.; Zimmitti, G.; Curley, S.A.; Charnsangavej, C.; Aloia, T.A.; et al. Optimal morphologic response to preoperative chemotherapy: An alternate outcome end point before resection of hepatic colorectal metastases. J. Clin. Oncol. 2012, 30, 4566–4572. [Google Scholar] [CrossRef]
- Yoshita, H.; Hosokawa, A.; Ueda, A.; Ando, T.; Kajiura, S.; Kato, H.; Kawabe, H.; Tomizawa, G.; Horikawa, N.; Yabuhita, K.; et al. Predictive value of optimal morphologic response to first-line chemotherapy in patients with colorectal liver metastases. Digestion 2014, 89, 43–48. [Google Scholar] [CrossRef]
- Klinger, M.; Tamandl, D.; Eipeldauer, S.; Hacker, S.; Herberger, B.; Kaczirek, K.; Dorfmeister, M.; Gruenberger, B.; Gruenberger, T. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann. Surg. Oncol. 2010, 17, 2059–2065. [Google Scholar] [CrossRef]
- Klinger, M.; Eipeldauer, S.; Hacker, S.; Herberger, B.; Tamandl, D.; Dorfmeister, M.; Koelblinger, C.; Gruenberger, B.; Gruenberger, T. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur. J. Surg. Oncol. 2009, 35, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Blazer, D.G., III; Kishi, Y.; Maru, D.M.; Kopetz, S.; Chun, Y.S.; Overman, M.J.; Fogelman, D.; Eng, C.; Chang, D.Z.; Wang, H.; et al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J. Clin. Oncol. 2008, 26, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Schirripa, M.; Caparello, C.; Funel, N.; Pollina, L.; Vasile, E.; Cremolini, C.; Salvatore, L.; Morvillo, M.; Antoniotti, C.; et al. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br. J. Cancer 2013, 108, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Vauthey, J.N.; Boonsirikamchai, P.; Maru, D.M.; Kopetz, S.; Palavecino, M.; Curley, S.A.; Abdalla, E.K.; Kaur, H.; Charnsangavej, C.; et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009, 302, 2338–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremolini, C.; Milione, M.; Marmorino, F.; Morano, F.; Zucchelli, G.; Mennitto, A.; Prisciandaro, M.; Lonardi, S.; Pellegrinelli, A.; Rossini, D.; et al. Differential histopathologic parameters in colorectal cancer liver metastases resected after triplets plus bevacizumab or cetuximab: A pooled analysis of five prospective trials. Br. J. Cancer 2018, 118, 955–965. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Mazzaferro, V.; Miceli, R.; Cotsoglou, C.; Melotti, F.; Fanetti, G.; Perrone, F.; Biondani, P.; Muscarà, C.; Di Bartolomeo, M.; et al. Pathological response after neoadjuvant bevacizumab- or cetuximab-based chemotherapy in resected colorectal cancer liver metastases. Med. Oncol. 2015, 32, 182. [Google Scholar] [CrossRef]
- Stremitzer, S.; Stift, J.; Singh, J.; Starlinger, P.; Gruenberger, B.; Tamandl, D.; Gruenberger, T. Histological response, pattern of tumor destruction and clinical outcome after neoadjuvant chemotherapy including bevacizumab or cetuximab in patients undergoing liver resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2015, 41, 868–874. [Google Scholar] [CrossRef]
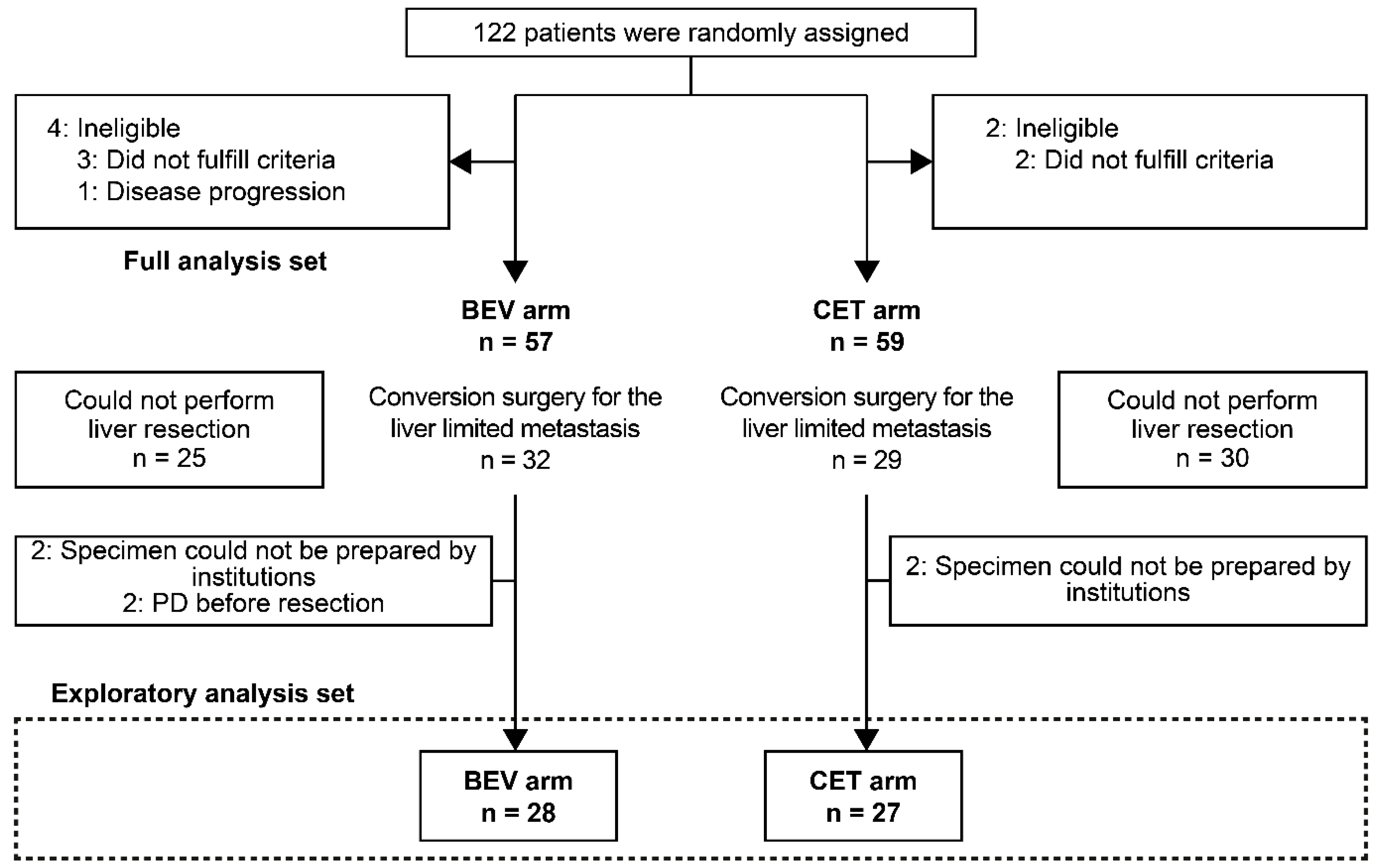

| Characteristic | BEV Arm (n = 28) | CET Arm (n = 27) | p-Value | |
|---|---|---|---|---|
| Age (years) | Median (range) | 61.0 (32.0–79.0) | 63.0 (50.0–77.0) | 0.316 |
| Sex | Male | 16 (57.1%) | 17 (63.0%) | 0.660 |
| Female | 12 (42.9%) | 10 (37.0%) | ||
| ECOG PS | 0 | 25 (89.3%) | 25 (92.6%) | 0.670 |
| 1 | 3 (10.7%) | 2 (7.4%) | ||
| Adjuvant chemotherapy | Yes | 3 (10.7%) | 2 (7.4%) | 0.670 |
| Prior oxaliplatin | Yes | 1 (3.6%) | 2 (7.4%) | 0.531 |
| Tumor location | Right | 3 (10.7%) | 8 (29.6%) | 0.080 |
| Left | 25 (89.3%) | 19 (70.4%) | ||
| Tumor status | Synchronous, with primary tumor | 4 (14.3%) | 5 (18.5%) | 0.476 |
| Synchronous, without primary tumor | 19 (67.9%) | 19 (70.4%) | ||
| Metachronous | 5 (17.9%) | 3 (11.1%) | ||
| Number of liver metastases (at the time of registration) | 1–4 | 10 (35.7%) | 13 (48.1%) | 0.350 |
| ≥5 | 18 (64.3%) | 14 (51.9%) | ||
| Diameter of liver metastases (at the time of registration) | ≤5 cm | 10 (35.7%) | 10 (37.0%) | 0.919 |
| >5 cm | 18 (64.3%) | 17 (63.0%) | ||
| Chemotherapy course up to hepatectomy | Median (range) | 8 (6–22) | 8 (4–31) | 0.1088 |
| Period from registration to hepatectomy (days) | Median (range) | 160 (116–439) | 158 (92–465) | 0.7173 |
| Number of liver metastases before hepatectomy | Median (range) | 6 (1–15) | 4 (1–18) | 0.8458 |
| Pathological Response | All Patients (n = 55) | BEV Arm (n = 28) | CET Arm (n = 27) | p-Value | |
|---|---|---|---|---|---|
| TRG | Low TRG (TRG 1–3) | 36 (65.5%) | 13 (46.4%) | 23 (85.2%) | 0.003 |
| High TRG (TRG 4–5) | 19 (34.5%) | 15 (53.6%) | 4 (14.8%) | ||
| mTRG | Low mTRG (mTRG 1–3) | 47 (85.5%) | 23 (82.1%) | 24 (88.9%) | 0.478 |
| High mTRG (mTRG 4–5) | 8 (14.5%) | 5 (17.9%) | 3 (11.1%) | ||
| DH | Absent/rare | 33 (60.0%) | 14 (50.0%) | 19 (70.4%) | 0.123 |
| Focal/diffuse | 22 (40.0%) | 14 (50.0%) | 8 (29.6%) | ||
| SOS | Grade 1 | 50 (90.9%) | 28 (100%) | 22 (81.5%) | 0.017 |
| Grade 2/3 | 5 (9.1%) | 0 | 5 (18.5%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, T.; Ishida, K.; Emi, Y.; Sakamoto, M.; Imura, J.; Aishima, S.; Muro, K.; Uetake, H.; Oki, E.; Katayose, Y.; et al. Pathological Evaluation of Resected Colorectal Liver Metastases: mFOLFOX6 Plus Bevacizumab versus mFOLFOX6 Plus Cetuximab in the Phase II ATOM Trial. Cancers 2022, 14, 4392. https://doi.org/10.3390/cancers14184392
Takahashi T, Ishida K, Emi Y, Sakamoto M, Imura J, Aishima S, Muro K, Uetake H, Oki E, Katayose Y, et al. Pathological Evaluation of Resected Colorectal Liver Metastases: mFOLFOX6 Plus Bevacizumab versus mFOLFOX6 Plus Cetuximab in the Phase II ATOM Trial. Cancers. 2022; 14(18):4392. https://doi.org/10.3390/cancers14184392
Chicago/Turabian StyleTakahashi, Takao, Kazuyuki Ishida, Yasunori Emi, Michiie Sakamoto, Johji Imura, Shinichi Aishima, Kei Muro, Hiroyuki Uetake, Eiji Oki, Yu Katayose, and et al. 2022. "Pathological Evaluation of Resected Colorectal Liver Metastases: mFOLFOX6 Plus Bevacizumab versus mFOLFOX6 Plus Cetuximab in the Phase II ATOM Trial" Cancers 14, no. 18: 4392. https://doi.org/10.3390/cancers14184392
APA StyleTakahashi, T., Ishida, K., Emi, Y., Sakamoto, M., Imura, J., Aishima, S., Muro, K., Uetake, H., Oki, E., Katayose, Y., Yoshida, K., Unno, M., Hyodo, I., Tomita, N., Sugihara, K., & Maehara, Y. (2022). Pathological Evaluation of Resected Colorectal Liver Metastases: mFOLFOX6 Plus Bevacizumab versus mFOLFOX6 Plus Cetuximab in the Phase II ATOM Trial. Cancers, 14(18), 4392. https://doi.org/10.3390/cancers14184392






