Farnesoid X Receptor Overexpression Decreases the Migration, Invasion and Angiogenesis of Human Bladder Cancers via AMPK Activation and Cholesterol Biosynthesis Inhibition
Abstract
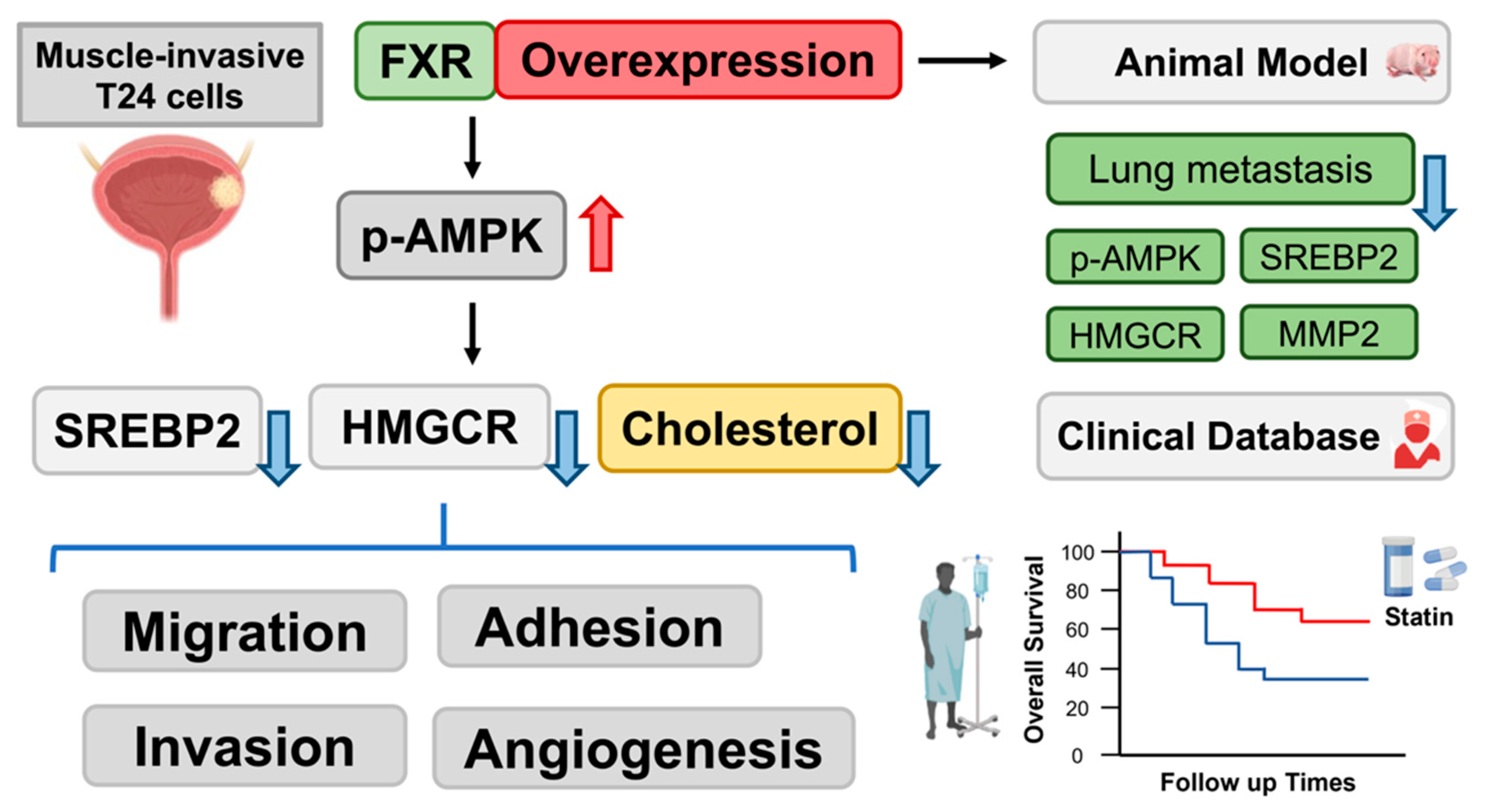
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Doxycycline-Induced Overexpression System
2.3. Drugs and Reagents
2.4. MTT Assays
2.5. Cholesterol Cell-Based Assay
2.6. Total Cholesterol Level Measurement
2.7. Wound-Healing Migration Assay
2.8. Adhesion Assays
2.9. Transwell Assays
2.10. Tube Formation Assays
2.11. Western Blotting
2.12. Xenograft Mouse Model
2.13. HE and IHC Staining
2.14. Kaplan–Meier Analysis of Bladder Cancer Patients Administered Statins
2.15. Statistical Analysis
3. Results
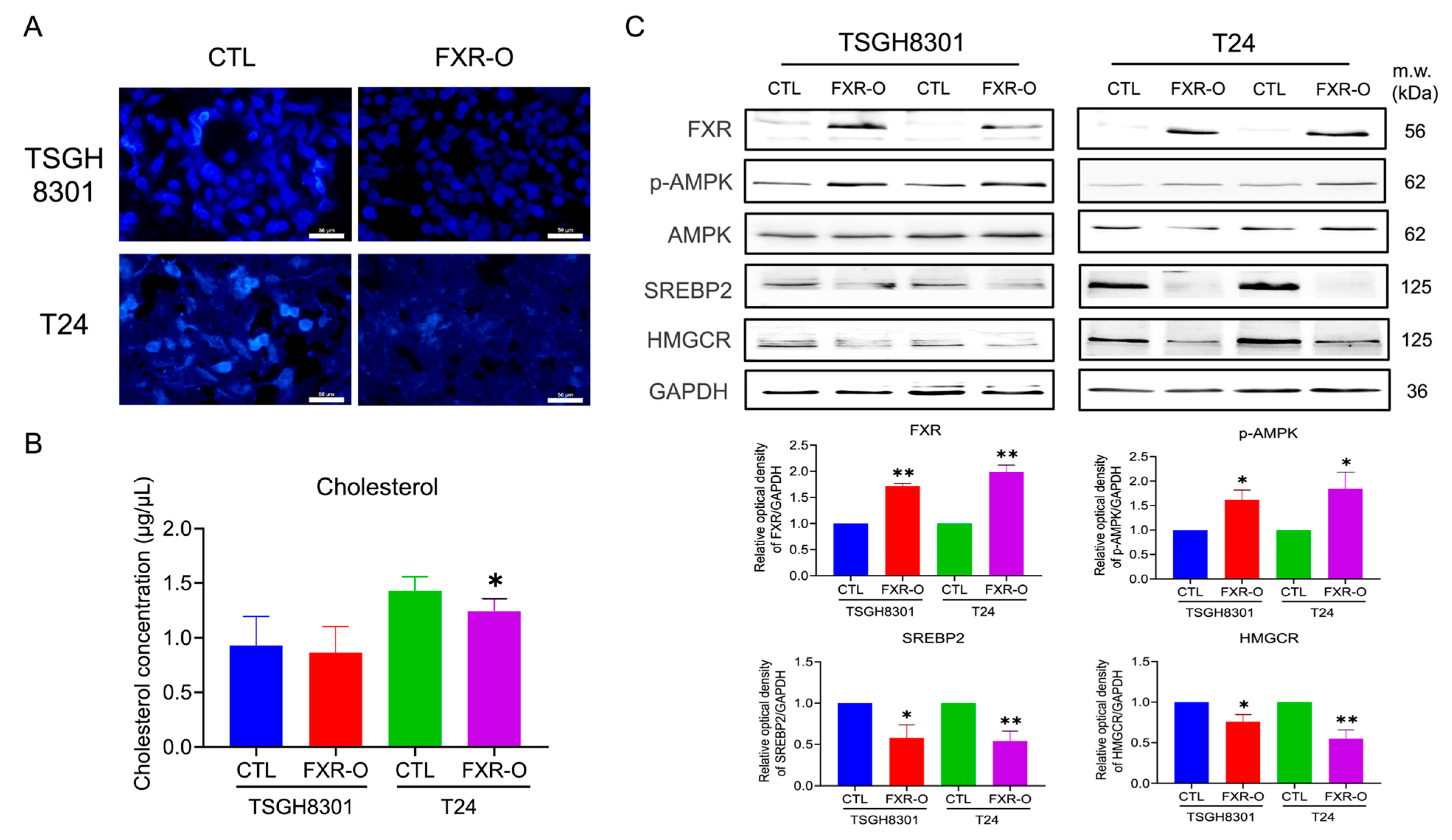
3.1. Overexpression of FXR Reduced the Cholesterol Content in TSGH8301 and T24 Cells
3.2. Survival Rate and Expressions of PRKAA1 (AMPK), SREBP2 and HMGCR in Bladder Cancer Patients and Bladder Cancer Cell Lines
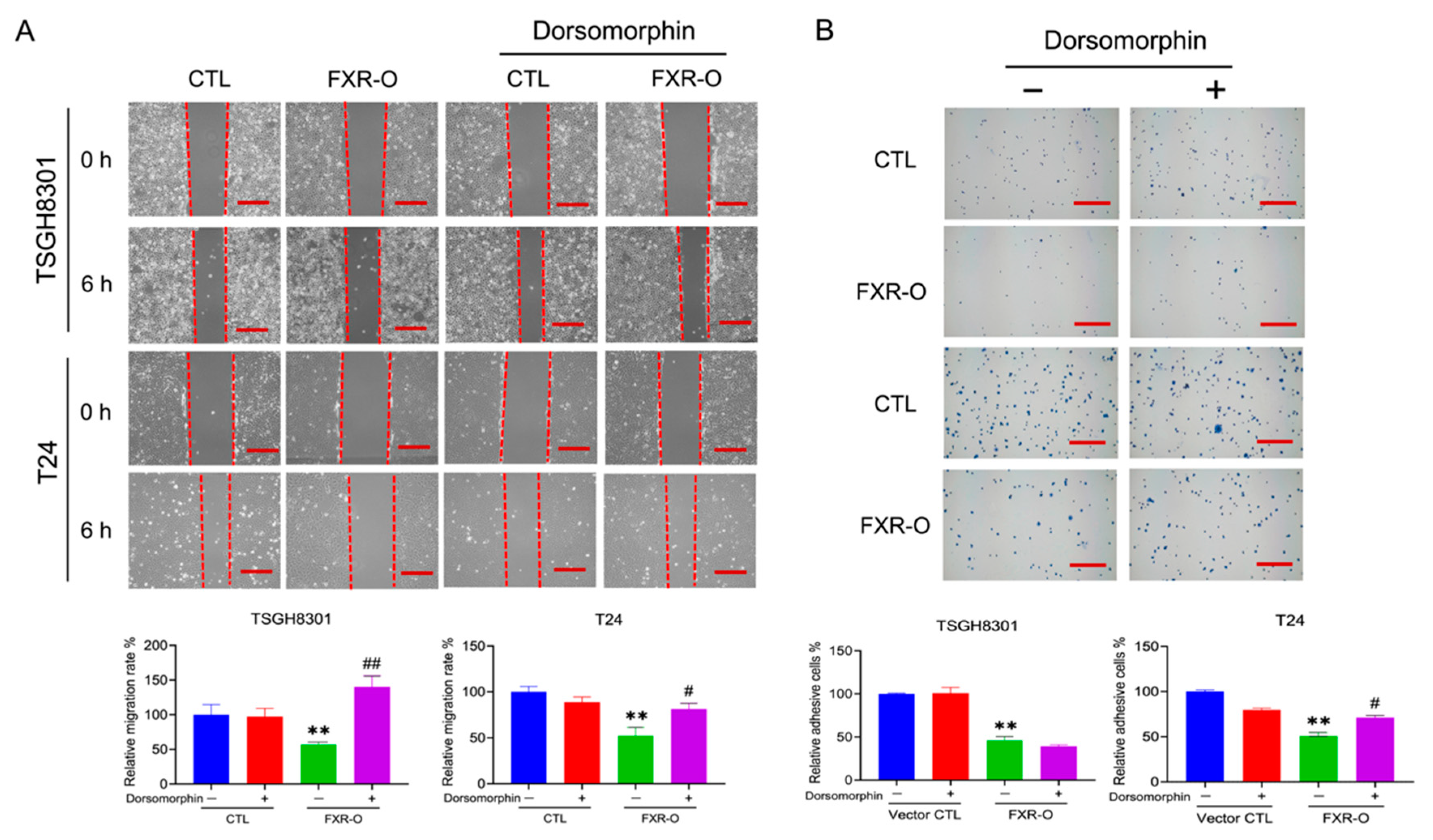
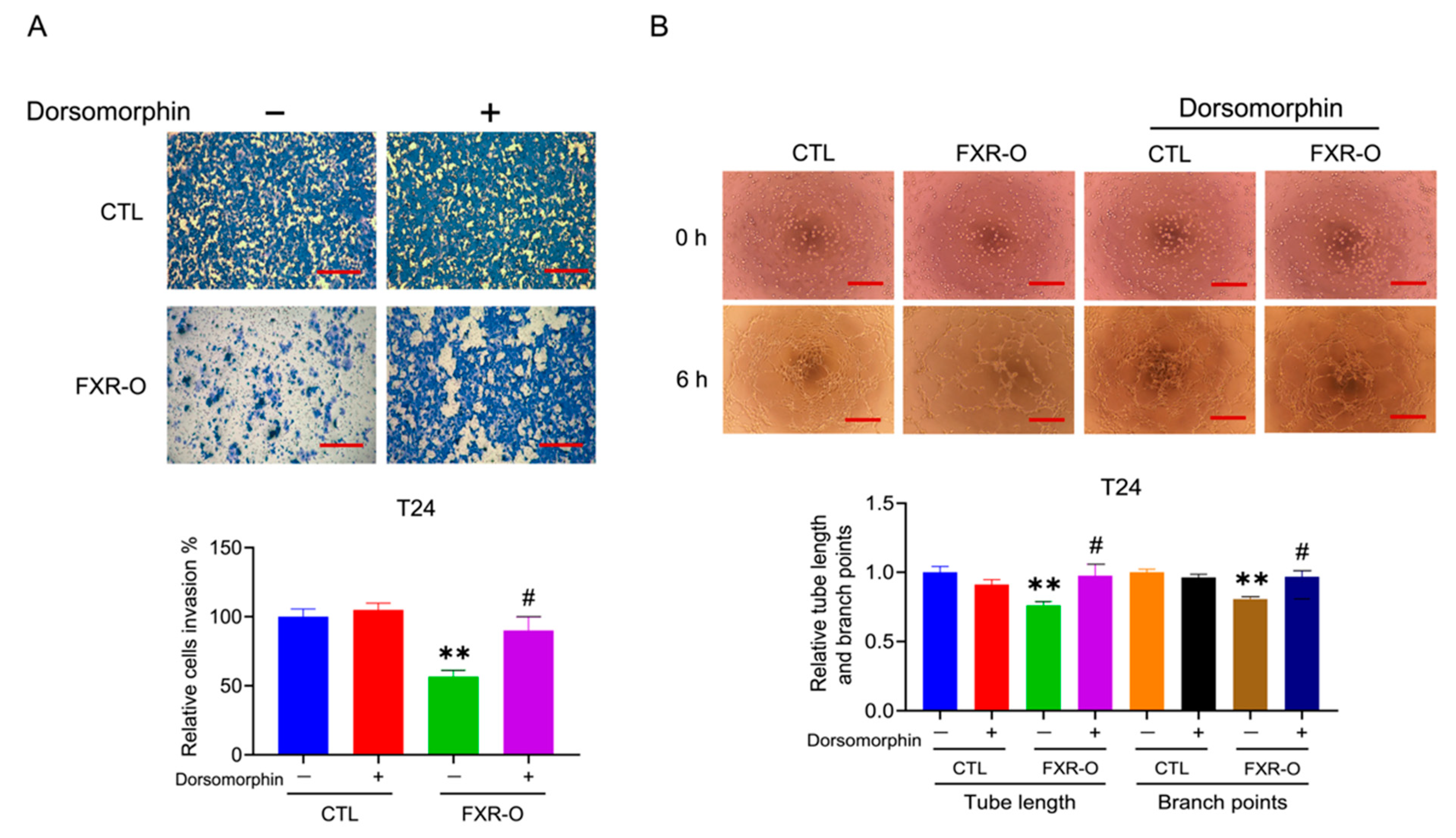
3.3. The AMPK Inhibitor Dorsomorphin Reversed the FXR Overexpression-Mediated Inhibition of Migration, Adhesion, Invasion and Angiogenesis
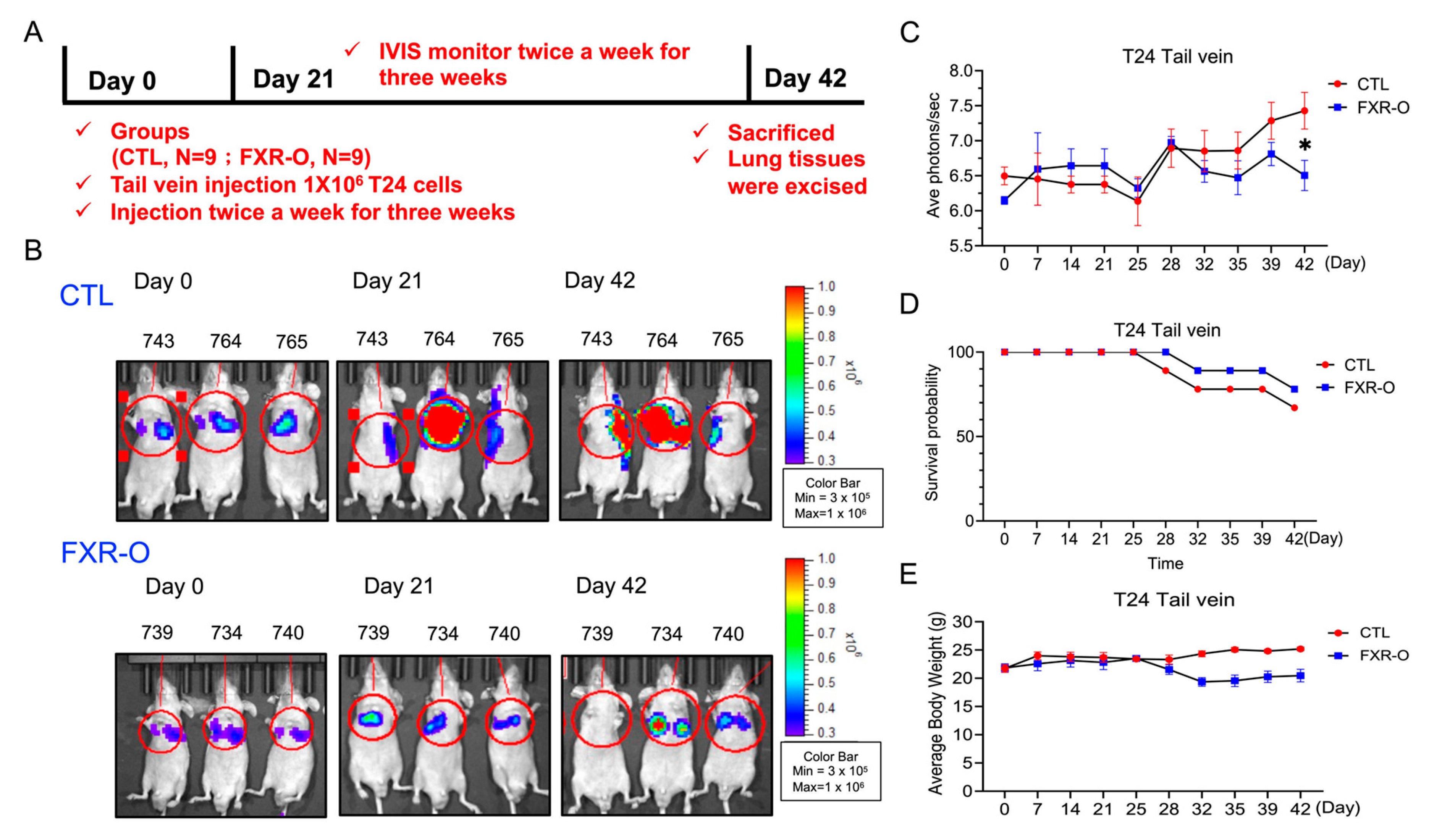
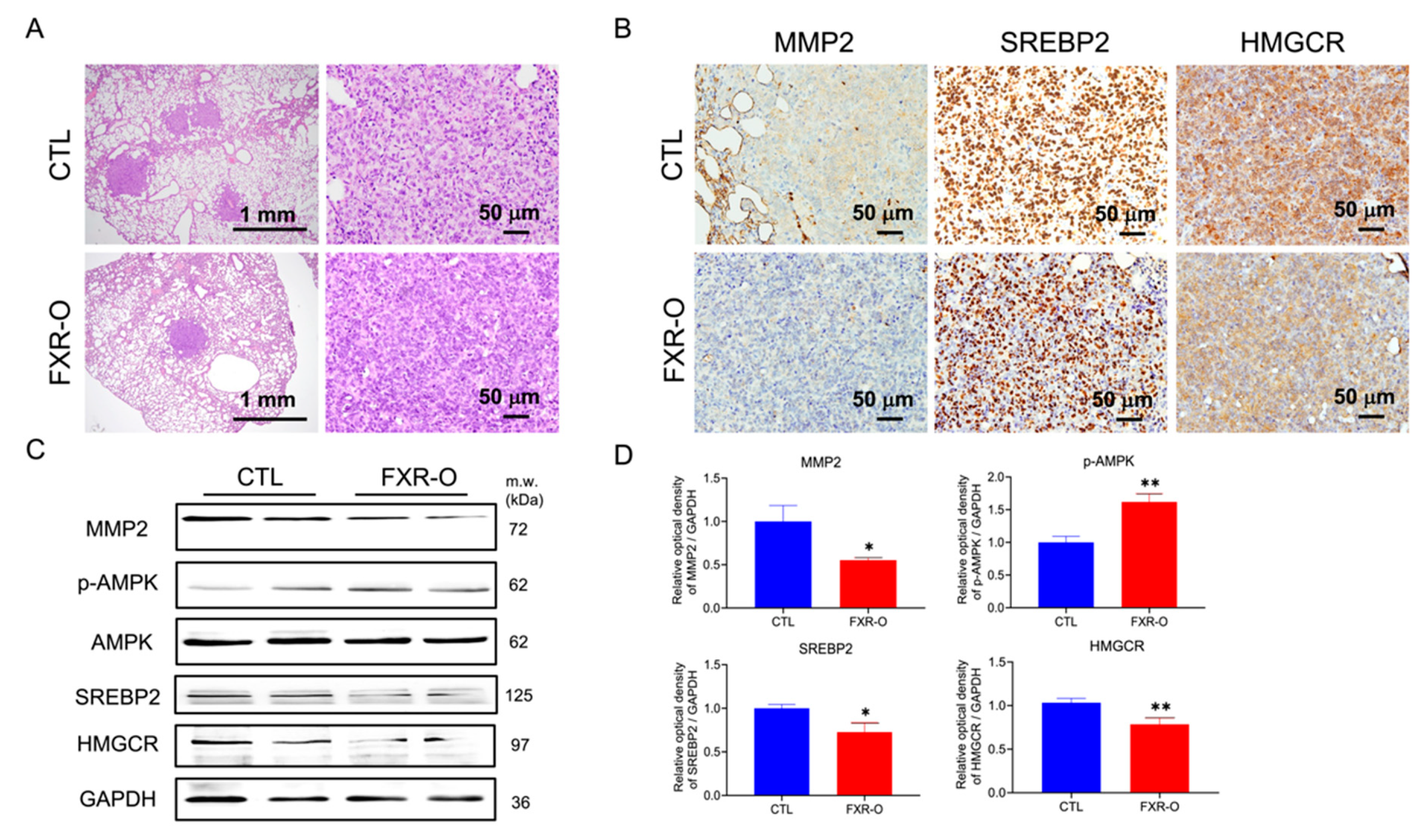
3.4. FXR Overexpression Attenuated Tumor Metastasis by Reducing MMP2, SREBP2, and HMGCR Expression and Enhancing p-AMPK Expression in a Tail Vein Metastatic Xenograft Model
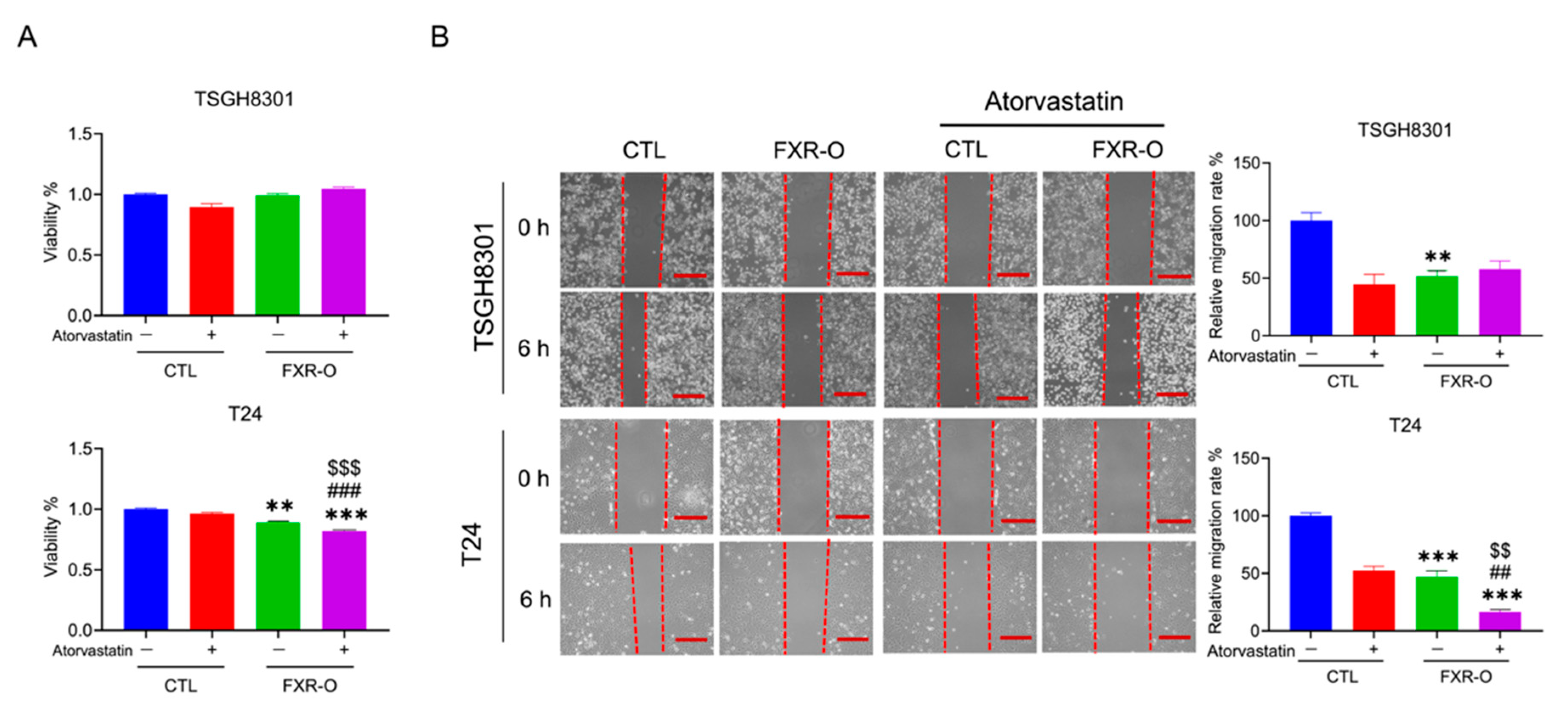
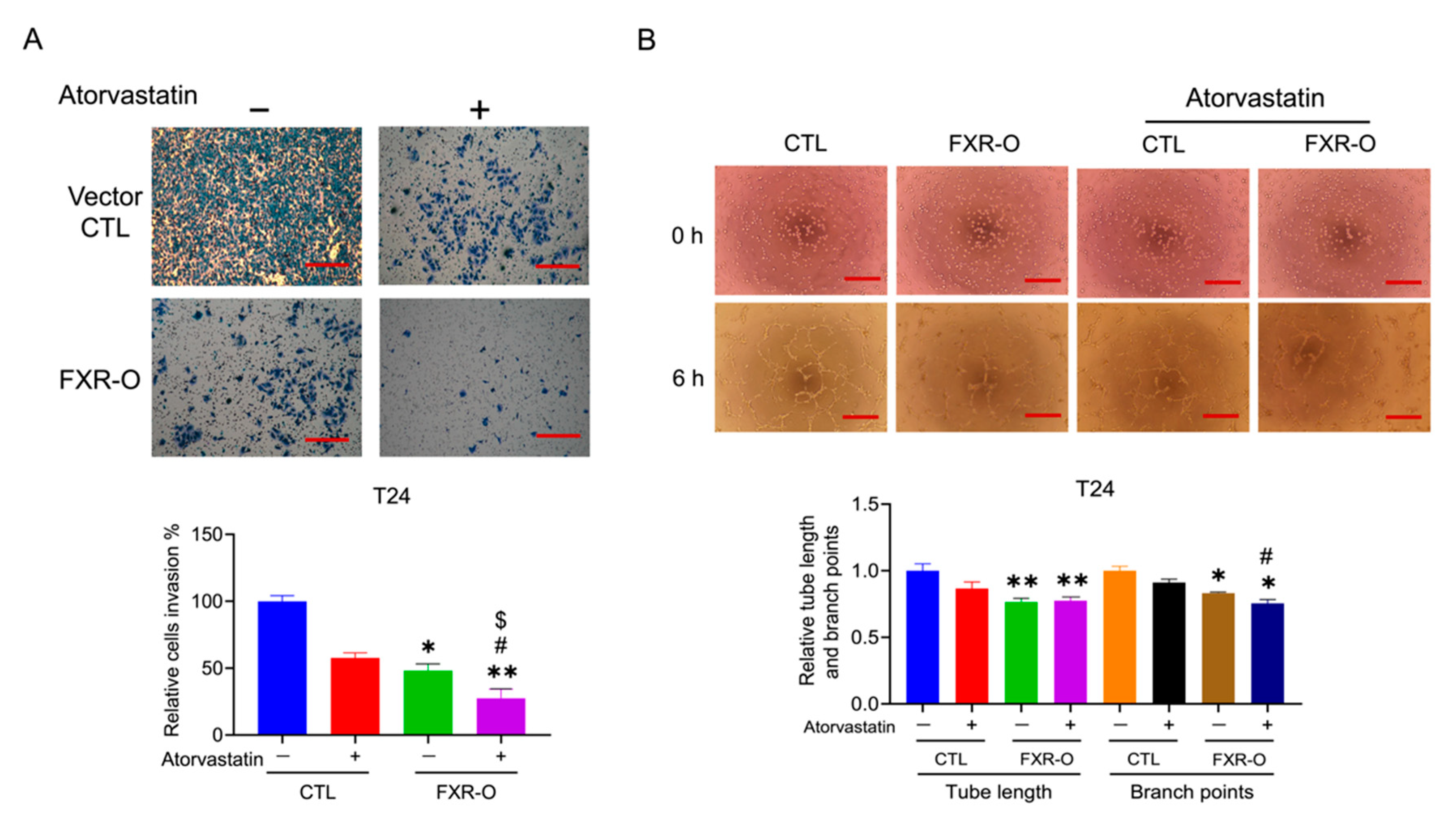
3.5. The HMGCR Inhibitor Atorvastatin Enhanced the FXR Overexpression-Mediated Decreases in the Migration, Adhesion, Invasion and Angiogenesis of T24 Cells
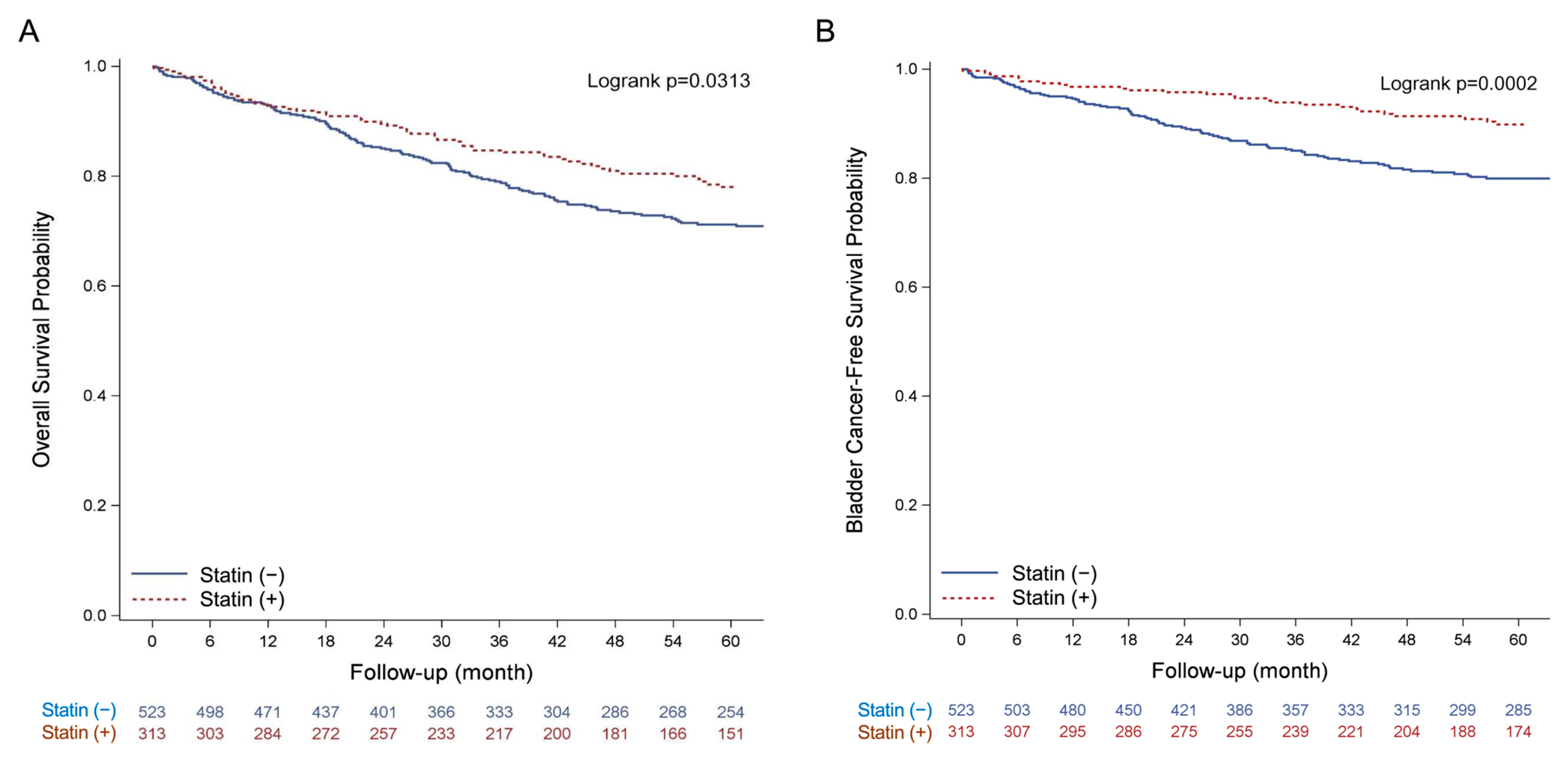
3.6. Clinical Effect of Statin Usage on the Overall Survival of BLCA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letašiová, S.; Medveďová, A.; Šovčíková, A.; Dušinská, M.; Volkovová, K.; Mosoiu, C.; Bartonová, A. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kuper, H.; Boffetta, P.; Adami, H.O. Tobacco use and cancer causation: Association by tumour type. J. Intern. Med. 2002, 252, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Millan-Rodriguez, F.; Chechile-Toniolo, G.; Salvador-Bayarri, J.; Palou, J.; Algaba, F.; Vicente-Rodriguez, J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J. Urol. 2000, 164, 680–684. [Google Scholar] [CrossRef]
- Holmang, S.; Hedelin, H.; Anderstrom, C.; Johansson, S.L. The relationship among multiple recurrences, progression and prognosis of patients with stages Ta and T1 transitional cell cancer of the bladder followed for at least 20 years. J. Urol. 1995, 153, 1823–1827. [Google Scholar] [CrossRef]
- Forman, B.M.; Goode, E.; Chen, J.; Oro, A.E.; Bradley, D.J.; Perlmann, T.; Noonan, D.J.; Burka, L.T.; McMorris, T.; Lamph, W.W. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995, 81, 687–693. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, F.Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1006–1011. [Google Scholar] [CrossRef]
- Lambert, G.; Amar, M.J.; Guo, G.; Brewer, H.B.; Gonzalez, F.J.; Sinal, C.J. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 2003, 278, 2563–2570. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef]
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000, 6, 507–515. [Google Scholar] [CrossRef]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Fang, S. Bile acid receptor farnesoid X Receptor: A novel therapeutic target for metabolic diseases. J. Lipid Atheroscler. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Clendening, J.W.; Pandyra, A.; Boutros, P.C.; El Ghamrasni, S.; Khosravi, F.; Trentin, G.A.; Martirosyan, A.; Hakem, A.; Hakem, R.; Jurisica, I. Dysregulation of the mevalonate pathway promotes transformation. Proc. Natl. Acad. Sci. USA 2010, 107, 15051–15056. [Google Scholar] [CrossRef] [PubMed]
- Thurnher, M.; Nussbaumer, O.; Gruenbacher, G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin. Cancer Res. 2012, 18, 3524–3531. [Google Scholar] [CrossRef]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef]
- Luo, Z.; Saha, A.K.; Xiang, X.; Ruderman, N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005, 26, 69–76. [Google Scholar] [CrossRef]
- Hardie, D.G. New roles for the LKB1—AMPK pathway. Curr. Opin. Cell Biol. 2005, 17, 167–173. [Google Scholar] [CrossRef]
- Lai, C.-R.; Wang, H.-H.; Chang, H.-H.; Tsai, Y.-L.; Tsai, W.-C.; Lee, C.-R.; Changchien, C.-Y.; Cheng, Y.-C.; Wu, S.-T.; Chen, Y. Enhancement of Farnesoid X Receptor Inhibits Migration, Adhesion and Angiogenesis through Proteasome Degradation and VEGF Reduction in Bladder Cancers. Int. J. Mol. Sci. 2022, 23, 5259. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.-L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.P.; McTavish, D. Atorvastatin. Drugs 1997, 53, 828–847. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Mo, Z.; Eid, W.; Courtney, K.C.; Zha, X. Akt inhibition promotes ABCA1-mediated cholesterol efflux to ApoA-I through suppressing mTORC1. PLoS ONE 2014, 9, e113789. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Thysell, E.; Surowiec, I.; Hörnberg, E.; Crnalic, S.; Widmark, A.; Johansson, A.I.; Stattin, P.; Bergh, A.; Moritz, T.; Antti, H. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS ONE 2010, 5, e14175. [Google Scholar] [CrossRef]
- Roethle, P.A.; Chen, I.T.; Trauner, D. Total synthesis of (−)-archazolid B. J. Am. Chem. Soc. 2007, 129, 8960–8961. [Google Scholar] [CrossRef]
- Hamm, R.; Chen, Y.-R.; Seo, E.-J.; Zeino, M.; Wu, C.-F.; Müller, R.; Yang, N.-S.; Efferth, T. Induction of cholesterol biosynthesis by archazolid B in T24 bladder cancer cells. Biochem. Pharmacol. 2014, 91, 18–30. [Google Scholar] [CrossRef]
- Miyata, M.; Hata, T.; Yamazoe, Y.; Yoshinari, K. SREBP-2 negatively regulates FXR-dependent transcription of FGF19 in human intestinal cells. Biochem. Biophys. Res. Commun. 2014, 443, 477–482. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, M.J.; Kim, E.J.; Yang, Y.; Lee, M.S.; Lim, J.S. Berberine-induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX-2 protein expression. Biochem. Pharm. 2012, 83, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, A.; Arbiser, J.L.; Bonner, M.Y.; Saxena, N.K.; Sharma, D. Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast Cancer Res. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Tichet, M.; Abbe, P.; Ohanna, M.; Lehraiki, A.; Rouaud, F.; Allegra, M.; Giacchero, D.; Bahadoran, P.; Bertolotto, C. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol. Cancer Ther. 2013, 12, 1605–1615. [Google Scholar] [CrossRef]
- Herold, G.; Jungwirth, R.; Rogler, G.; Geerling, I.; Stange, E.F. Influence of cholesterol supply on cell growth and differentiation in cultured enterocytes (CaCo-2). Digestion 1995, 56, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.G.; Kung, H.; Longo, D.L.; Smith, M.R. Regulation of intracellular actin polymerization by prenylated cellular proteins. J. Cell Biol. 1992, 117, 347–356. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.; Martel, M. Effect of statin therapy on colorectal cancer. Gut 2010, 59, 1572–1585. [Google Scholar] [CrossRef]
- Park, H.-J.; Kong, D.; Iruela-Arispe, L.; Begley, U.; Tang, D.; Galper, J.B. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ. Res. 2002, 91, 143–150. [Google Scholar] [CrossRef]
- Denoyelle, C.; Vasse, M.; Körner, M.; Mishal, Z.; Ganné, F.; Vannier, J.-P.; Soria, J.; Soria, C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: An in vitro study. Carcinogenesis 2001, 22, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Kocyk, M.; Kijewska, M. TGF beta signaling and its role in glioma pathogenesis. Glioma Signal. 2013, 986, 171–187. [Google Scholar]
- Richard, P.O.; Ahmad, A.E.; Bashir, S.; Hamilton, R.J.; Nam, R.K.; Leao, R.; Jeldres, C.; Kulkarni, G.S. Effect of Statins as a Secondary Chemopreventive Agent among Individuals with Non–Muscle-Invasive Bladder Cancer: A Population-Based Analysis. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tsai, H.K.; Katz, M.S.; Coen, J.J.; Zietman, A.L.; Kaufman, D.S.; Shipley, W.U. Association of statin use with improved local control in patients treated with selective bladder preservation for muscle-invasive bladder cancer. Urology 2006, 68, 1188–1192. [Google Scholar] [CrossRef]
| Variable | Statins Use of Patients (%) | p-Value | |
|---|---|---|---|
| Statin (+) (n = 313) | Statin (−) (n = 523) | ||
| Age, Mean (SD) | 69.6 (12.6) | 68.4 (14.0) | 0.22 |
| Gender | 0.42 | ||
| Male | 236 (75.4) | 407 (77.8) | |
| Female | 77 (24.6) | 116 (22.2) | |
| T category | 0.53 | ||
| T1 | 313 (100.0) | 521 (99.6) | |
| T2 | 0 (0.0) | 2 (0.4) | |
| N category | - | ||
| N0 | 313 (100.0) | 523 (100.0) | |
| Cell type | 0.0017 | ||
| Urothelial carcinoma | 296 (94.6) | 455 (87.0) | |
| Adenocarcinoma, Squamous carcinoma, Neuroendocrine carcinoma | 3 (1.0) | 7 (1.3) | |
| Others | 14 (4.5) | 61 (11.7) | |
| BMI, kg/m | <0.0001 | ||
| <25 | 167 (53.4) | 381 (72.8) | |
| ≥25 | 146 (46.6) | 142 (27.2) | |
| CCI | <0.0001 | ||
| 0 | 142 (45.4) | 410 (78.4) | |
| 1–2 | 92 (29.4) | 74 (14.1) | |
| 3+ | 79 (25.2) | 39 (7.5) | |
| Lipid profiles, mg/dL | |||
| Total Cholesterol, mg/dL | 206.9 (44.8) | 182.3 (33.5) | <0.0001 |
| Normal or low (<200) | 82 (26.2) | 59 (11.3) | |
| High (≥200) | 105 (33.5) | 26 (5.0) | |
| No. | Events (%) | Crude HR (95% CI) | p-Value | Adjusted † HR (95% CI) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | |||||||||
| Statin (−) | 523 | 134 | (25.6) | 1.00 | (Ref.) | 1.00 | (Ref.) | ||
| Statin (+) | 313 | 60 | (19.2) | 0.72 | (0.53–0.97) | 0.0321 | 0.69 | (0.48–0.99) | 0.0433 |
| Bladder cancer-related mortality | |||||||||
| Statin (−) | 523 | 93 | (17.8) | 1.00 | (Ref.) | 1.00 | (Ref.) | ||
| Statin (+) | 313 | 27 | (8.6) | 0.46 | (0.30–0.70) | 0.0003 | 0.60 | (0.37–0.96) | 0.0335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-R.; Tsai, Y.-L.; Tsai, W.-C.; Chen, T.-M.; Chang, H.-H.; Changchien, C.-Y.; Wu, S.-T.; Wang, H.-H.; Chen, Y.; Lin, Y.-H. Farnesoid X Receptor Overexpression Decreases the Migration, Invasion and Angiogenesis of Human Bladder Cancers via AMPK Activation and Cholesterol Biosynthesis Inhibition. Cancers 2022, 14, 4398. https://doi.org/10.3390/cancers14184398
Lai C-R, Tsai Y-L, Tsai W-C, Chen T-M, Chang H-H, Changchien C-Y, Wu S-T, Wang H-H, Chen Y, Lin Y-H. Farnesoid X Receptor Overexpression Decreases the Migration, Invasion and Angiogenesis of Human Bladder Cancers via AMPK Activation and Cholesterol Biosynthesis Inhibition. Cancers. 2022; 14(18):4398. https://doi.org/10.3390/cancers14184398
Chicago/Turabian StyleLai, Chien-Rui, Yu-Ling Tsai, Wen-Chiuan Tsai, Tzu-Min Chen, Hsin-Han Chang, Chih-Ying Changchien, Sheng-Tang Wu, Hisao-Hsien Wang, Ying Chen, and Yu-Huei Lin. 2022. "Farnesoid X Receptor Overexpression Decreases the Migration, Invasion and Angiogenesis of Human Bladder Cancers via AMPK Activation and Cholesterol Biosynthesis Inhibition" Cancers 14, no. 18: 4398. https://doi.org/10.3390/cancers14184398
APA StyleLai, C.-R., Tsai, Y.-L., Tsai, W.-C., Chen, T.-M., Chang, H.-H., Changchien, C.-Y., Wu, S.-T., Wang, H.-H., Chen, Y., & Lin, Y.-H. (2022). Farnesoid X Receptor Overexpression Decreases the Migration, Invasion and Angiogenesis of Human Bladder Cancers via AMPK Activation and Cholesterol Biosynthesis Inhibition. Cancers, 14(18), 4398. https://doi.org/10.3390/cancers14184398










