Surgical Management of Recurrent Brain Metastasis: A Systematic Review of Laser Interstitial Thermal Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
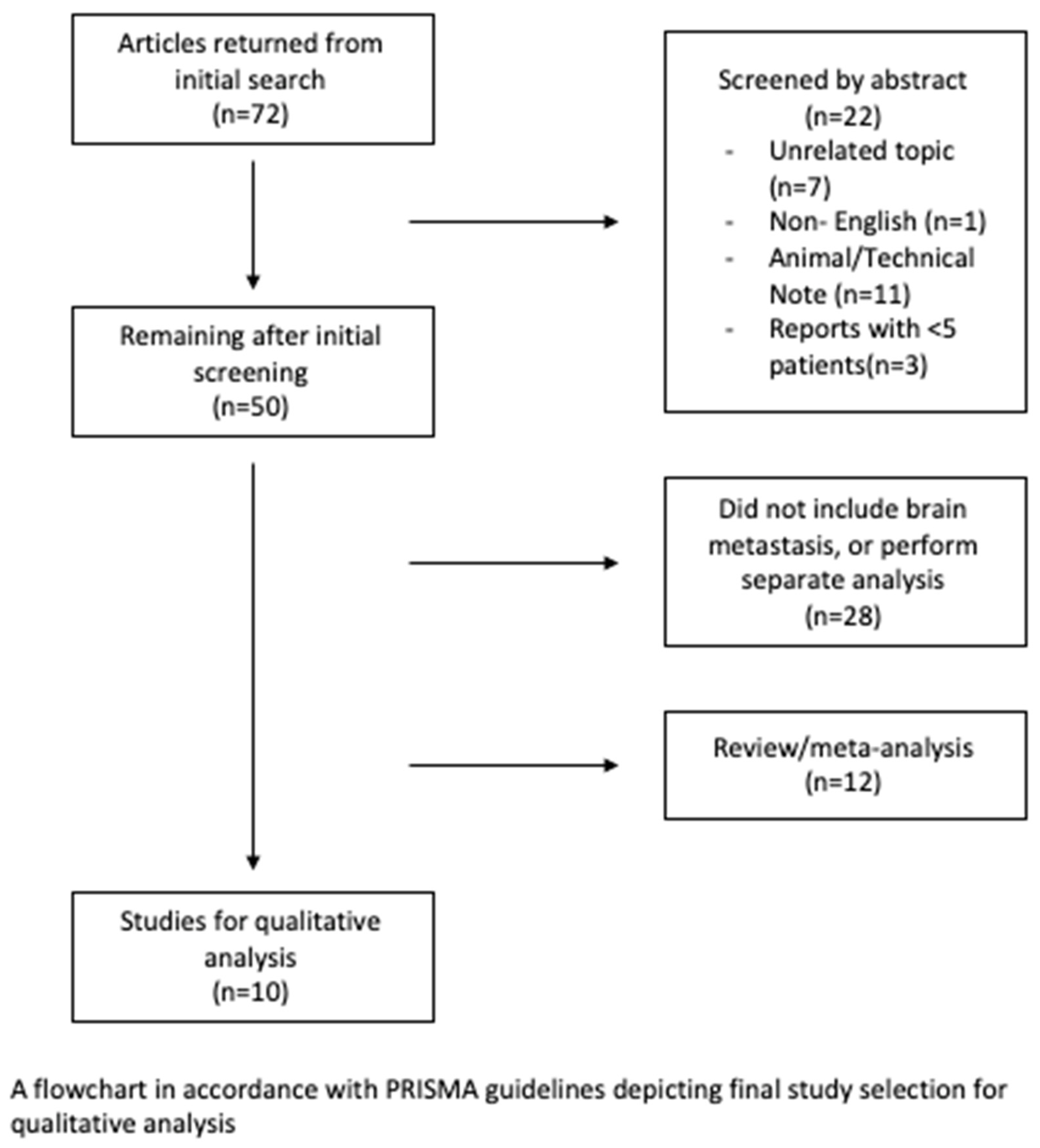
2. Materials and Methods
Eligibility Criteria and Study Selection
3. Results
4. Discussion
4.1. Re-Resection for Brain Metastasis
4.2. Laser Interstitial Thermal Therapy for Recurrent Disease
4.3. Limitations of LITT
4.4. Current Research and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Huntoon, K.; Damante, M.; Wang, J.; Olencki, T.; Elder, J.B. Survival benefit with resection of brain metastases from renal cell carcinoma in the setting of molecular targeted therapy and/or immune therapy. Curr. Probl. Cancer 2022, 46, 100805. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann. Neurol. 1993, 33, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Bindal, R.K.; Sawaya, R.; Leavens, M.E.; Hess, K.R.; Taylor, S.H. Reoperation for recurrent metastatic brain tumors. J. Neurosurg. 1995, 83, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Bindal, R.K.; Sawaya, R.; Leavens, M.E.; Lee, J.J. Surgical treatment of multiple brain metastases. J. Neurosurg. 1993, 79, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.; Sachdev, V.P.; DiGiacinto, G.V.; Hughes, J.E. Reoperation for brain metastases. J. Clin. Oncol. 1988, 6, 1625–1629. [Google Scholar] [CrossRef]
- McTyre, E.; Ayala-Peacock, D.; Contessa, J.; Corso, C.; Chiang, V.; Chung, C.; Fiveash, J.; Ahluwalia, M.; Kotecha, R.; Chao, S.; et al. Multi-institutional competing risks analysis of distant brain failure and salvage patterns after upfront radiosurgery without whole brain radiotherapy for brain metastasis. Ann. Oncol. 2018, 29, 497–503. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Salehi, A.; Kamath, A.A.; Leuthardt, E.C.; Kim, A.H. Management of Intracranial Metastatic Disease with Laser Interstitial Thermal Therapy. Front. Oncol. 2018, 8, 499. [Google Scholar] [CrossRef]
- Heisterkamp, J.; van Hillegersberg, R.; Zondervan, P.E.; JN, I.J. Metabolic activity and DNA integrity in human hepatic metastases after interstitial laser coagulation (ILC). Lasers Surg. Med. 2001, 28, 80–86. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Gazelle, G.S.; Mueller, P.R. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am. J. Roentgenol. 2000, 174, 323–331. [Google Scholar] [CrossRef]
- Yaroslavsky, A.N.; Schulze, P.C.; Yaroslavsky, I.V.; Schober, R.; Ulrich, F.; Schwarzmaier, H.J. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 2002, 47, 2059–2073. [Google Scholar] [CrossRef]
- Ali, M.A.; Carroll, K.T.; Rennert, R.C.; Hamelin, T.; Chang, L.; Lemkuil, B.P.; Sharma, M.; Barnholtz-Sloan, J.S.; Myers, C.; Barnett, G.H.; et al. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: A multiinstitutional experience. Neurosurg. Focus 2016, 41, E11. [Google Scholar] [CrossRef]
- Ahluwalia, M.; Barnett, G.H.; Deng, D.; Tatter, S.B.; Laxton, A.W.; Mohammadi, A.M.; Leuthardt, E.; Chamoun, R.; Judy, K.; Asher, A.; et al. Laser ablation after stereotactic radiosurgery: A multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J. Neurosurg. 2018, 130, 804–811. [Google Scholar] [CrossRef]
- Luther, E.; McCarthy, D.; Shah, A.; Semonche, A.; Borowy, V.; Burks, J.; Eichberg, D.G.; Komotar, R.; Ivan, M. Radical Laser Interstitial Thermal Therapy Ablation Volumes Increase Progression-Free Survival in Biopsy-Proven Radiation Necrosis. World Neurosurg. 2020, 136, e646–e659. [Google Scholar] [CrossRef]
- Luther, E.; Lu, V.M.; Morell, A.A.; Elarjani, T.; Mansour, S.; Echeverry, N.; Gaztanaga, W.; King, H.; McCarthy, D.; Eichberg, D.G.; et al. Supralesional Ablation Volumes Are Feasible in the Posterior Fossa and May Provide Enhanced Symptomatic Relief. Oper. Neurosurg. 2021, 21, 418–425. [Google Scholar] [CrossRef]
- Chaunzwa, T.L.; Deng, D.; Leuthardt, E.C.; Tatter, S.B.; Mohammadi, A.M.; Barnett, G.H.; Chiang, V.L. Laser Thermal Ablation for Metastases Failing Radiosurgery: A Multicentered Retrospective Study. Neurosurgery 2018, 82, 56–63. [Google Scholar] [CrossRef]
- Hong, C.S.; Deng, D.; Vera, A.; Chiang, V.L. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J. Neurooncol. 2019, 142, 309–317. [Google Scholar] [CrossRef]
- Kaye, J.; Patel, N.V.; Danish, S.F. Laser interstitial thermal therapy for in-field recurrence of brain metastasis after stereotactic radiosurgery: Does treatment with LITT prevent a neurologic death? Clin. Exp. Metastasis 2020, 37, 435–444. [Google Scholar] [CrossRef]
- Lucas, J.T., Jr.; Colmer, H.G.t.; White, L.; Fitzgerald, N.; Isom, S.; Bourland, J.D.; Laxton, A.W.; Tatter, S.B.; Chan, M.D. Competing Risk Analysis of Neurologic versus Nonneurologic Death in Patients Undergoing Radiosurgical Salvage after Whole-Brain Radiation Therapy Failure: Who Actually Dies of Their Brain Metastases? Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.H.; McTyre, E.R.; Okoukoni, C.; Alphonse-Sullivan, N.K.; Ruiz, J.; Munley, M.T.; Qasem, S.; Lo, H.W.; Xing, F.; Laxton, A.W.; et al. Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J. Neurosurg. 2017, 127, 148–156. [Google Scholar] [CrossRef]
- Traylor, J.I.; Patel, R.; Habib, A.; Muir, M.; de Almeida Bastos, D.C.; Rao, G.; Prabhu, S.S. Laser Interstitial Thermal Therapy to the Posterior Fossa: Challenges and Nuances. World Neurosurg. 2019, 132, e124–e132. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, O.; Arzumanov, G.; Luther, E.; McMahon, J.T.; Malcolm, J.G.; Mansour, S.; Lee, I.Y.; Willie, J.T.; Komotar, R.J.; Danish, S.F. Magnetic resonance-guided laser interstitial thermal therapy for posterior fossa neoplasms. J. Neurooncol. 2020, 149, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Semonche, A.; Eichberg, D.G.; Borowy, V.; Luther, E.; Sarkiss, C.A.; Morell, A.; Mahavadi, A.K.; Ivan, M.E.; Komotar, R.J. The Role of Laser Interstitial Thermal Therapy in Surgical Neuro-Oncology: Series of 100 Consecutive Patients. Neurosurgery 2020, 87, 266–275. [Google Scholar] [CrossRef]
- Hafez, D.M.; Liekweg, C.; Leuthardt, E.C. Staged Laser Interstitial Thermal Therapy (LITT) Treatments to Left Insular Low-Grade Glioma. Neurosurgery 2020, 86, E337–E342. [Google Scholar] [CrossRef] [PubMed]
- Hajtovic, S.; Mogilner, A.; Ard, J.; Gautreaux, J.E.; Britton, H.; Fatterpekar, G.; Young, M.G.; Placantonakis, D.G. Awake Laser Ablation for Patients with Tumors in Eloquent Brain Areas: Operative Technique and Case Series. Cureus 2020, 12, e12186. [Google Scholar] [CrossRef]
- Siomin, V.E.; Vogelbaum, M.A.; Kanner, A.A.; Lee, S.Y.; Suh, J.H.; Barnett, G.H. Posterior fossa metastases: Risk of leptomeningeal disease when treated with stereotactic radiosurgery compared to surgery. J. Neurooncol. 2004, 67, 115–121. [Google Scholar] [CrossRef]
- Sabahi, M.; Bordes, S.J.; Najera, E.; Mohammadi, A.M.; Barnett, G.H.; Adada, B.; Borghei-Razavi, H. Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: A Systematic Review and Analysis of Multi-Institutional Outcomes. Cancers 2022, 14, 456. [Google Scholar] [CrossRef]
| Author | Year | #Total Lesions | #Total Recurrent Lesions | Location(s) Supratentorial Infratentorial Deep Seated (Respectively) | PFS | OS | Follow Up (Median, Months) |
|---|---|---|---|---|---|---|---|
| Ali et al. | 2016 | 23 | 26 | 17 1 8 | 100% when ≥80% ablation | NA | 4.7 |
| Ahluwalia et al. | 2018 | 42 | 20 | Includes all, but unable to distinguish | 74% | 72% | 6.5 |
| Traylor et al. | 2019 | 13 | 8 | 8 infratentorial only | 7 m | 40 m | NA |
| Kamath et al. | 2017 | 25 | 25 | Includes all, but unable to distinguish | NR | 17.2 m | 9.8 m |
| Ashraf et al. | 2020 | 60 | 44 | 44 infratentorial only | 11.5 m (89.7% at 12 m) | NA | NA |
| Salehi et al. | 2018 | 25 | 25 | 22 1 2 | 6.3 m | 13.3 m | 16.1 m |
| Hong et al. | 2019 | 75 | 16 | Includes all, but unable to distinguish | 54.7% at 12 m | NA | NA |
| Kaye et al. | 2020 | 97 | 97 | 73 8 4 | 5.6 m | 11.9 m | 12.0 m |
| Dabeco et al. | 2021 | 19 | 12 | Includes all, but unable to distinguish | 7 m | 25 m | 10 m |
| Chaunzwa et al. | 2018 | 30 | 30 | Includes all, but unable to distinguish | 83% overall | 26.1% at 12 m | 5.6 m |
| Overall | 412 | 303 | 112 62 14 | 7.5 m | 21.5 m | 6.5 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damante, M.A., Jr.; Wang, J.L.; Elder, J.B. Surgical Management of Recurrent Brain Metastasis: A Systematic Review of Laser Interstitial Thermal Therapy. Cancers 2022, 14, 4367. https://doi.org/10.3390/cancers14184367
Damante MA Jr., Wang JL, Elder JB. Surgical Management of Recurrent Brain Metastasis: A Systematic Review of Laser Interstitial Thermal Therapy. Cancers. 2022; 14(18):4367. https://doi.org/10.3390/cancers14184367
Chicago/Turabian StyleDamante, Mark A., Jr., Joshua L. Wang, and J. Bradley Elder. 2022. "Surgical Management of Recurrent Brain Metastasis: A Systematic Review of Laser Interstitial Thermal Therapy" Cancers 14, no. 18: 4367. https://doi.org/10.3390/cancers14184367
APA StyleDamante, M. A., Jr., Wang, J. L., & Elder, J. B. (2022). Surgical Management of Recurrent Brain Metastasis: A Systematic Review of Laser Interstitial Thermal Therapy. Cancers, 14(18), 4367. https://doi.org/10.3390/cancers14184367






