Investigating the Impact of Ultra-Radical Surgery on Survival in Advanced Ovarian Cancer Using Population-Based Data in a Multicentre UK Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. SOCQER 2 Study
2.2. Defining Centre Surgical Complexity Score Pattern
2.3. Statistics
3. Results
3.1. Characteristics of Cohort
3.2. Treatment Patterns
3.3. Survival
4. Discussion
4.1. Limitations
4.2. Implications for Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fotopoulou, C.; Concin, N.; Planchamp, F.; Morice, P.; Vergote, I.; du Bois, A.; Querleu, D. Quality indicators for advanced ovarian cancer surgery from the European Society of Gynaecological Oncology (ESGO): 2020 update. Int. J. Gynecol. Cancer 2020, 30, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Hall, M.; Cruickshank, D.; Gabra, H.; Ganesan, R.; Hughes, C.; Kehoe, S.; Ledermann, J.; Morrison, J.; Naik, R.; et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Chi, D.S.; Franklin, C.C.; Levine, D.A.; Akselrod, F.; Sabbatini, P.; Jarnagin, W.R.; DeMatteo, R.; Poynor, E.A.; Abu-Rustum, N.R.; Barakat, R.R. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: A change in surgical approach. Gynecol. Oncol. 2004, 94, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.P.; Adib, T.; Butler, J. Surgical practice of UK gynaecological oncologists in the treatment of primary advanced epithelial ovarian cancer (PAEOC): A questionnaire survey. Gynecol. Oncol. 2013, 131, 347–351. [Google Scholar] [CrossRef]
- Turnbull, H.L.; Akrivos, N.; Wemyss-Holden, S.; Maiya, B.; Duncan, T.J.; Nieto, J.J.; Burbos, N. The impact of ultra-radical surgery in the management of patients with stage IIIC and IV epithelial ovarian, fallopian tube, and peritoneal cancer. Arch. Gynecol. Obstet. 2017, 295, 681–687. [Google Scholar] [CrossRef]
- Dahm-Kähler, P.; Holmberg, E.; Holtenman, M.; Rådestad, A.F.; Borgfeldt, C.; Hjerpe, E.; Marcickiewicz, J.; Bjurberg, M.; Tholander, B.; Hellman, K.; et al. Implementation of National Guidelines increased survival in advanced ovarian cancer—A population-based nationwide SweGCG study. Gynecol. Oncol. 2021, 161, 244–250. [Google Scholar] [CrossRef]
- Hall, M.; Savvatis, K.; Nixon, K.; Kyrgiou, M.; Hariharan, K.; Padwick, M.; Owens, O.; Cunnea, P.; Campbell, J.; Farthing, A.; et al. Maximal-Effort Cytoreductive Surgery for Ovarian Cancer Patients with a High Tumor Burden: Variations in Practice and Impact on Outcome. Ann. Surg. Oncol. 2019, 26, 2943–2951. [Google Scholar] [CrossRef]
- Sundar, S.; Cummins, C.; Kumar, S.; Long, J.; Arora, V.; Balega, J.; Broadhead, T.; Duncan, T.; Edmondson, R.; Fotopoulou, C.; et al. Quality of life from cytoreductive surgery in advanced ovarian cancer: Investigating the association between disease burden and surgical complexity in the international, prospective, SOCQER-2 cohort study. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1122–1132. [Google Scholar] [CrossRef]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Phillips, A.; Balega, J.; Nevin, J.; Singh, K.; Elattar, A.; Kehoe, S.; Sundar, S. Reporting ‘Denominator’ data is essential for benchmarking and quality standards in ovarian cancer. Gynecol. Oncol. 2017, 146, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Joneborg, U.; Krawiec, K.; Palsdottir, K.; Bottai, M.; Salehi, S. Ultra-radical upfront surgery does not improve survival in women with advanced epithelial ovarian cancer; a natural experiment in a complete population. Gynecol. Oncol. 2020, 159, 58–65. [Google Scholar] [CrossRef]
- National Cancer Guidance Steering Group. Improving Outcomes in Gynaecological Cancers—The Manual; (Guidance on Commissioning Cancer Services-Improving the Outcomes); NHS Executive, Department of Health: London, UK, 1999. [Google Scholar]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef]
- Henson, K.E.; Elliss-Brookes, L.; Coupland, V.H.; Payne, E.; Vernon, S.; Rous, B.; Rashbass, J. Data Resource Profile: National Cancer Registration Dataset in England. Int. J. Epidemiol. 2020, 49, 16–16h. [Google Scholar] [CrossRef] [PubMed]
- Knott, C. Ovarian Cancer Audit Feasibility Pilot: Geographic Variation in Ovarian, Fallopian Tube and Primary Peritoneal Cancer Treatment in England; PHE Publications Gateway Number: GW-1673; Public Health England: London, UK, 2020. [Google Scholar]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 7, CD005343. [Google Scholar] [CrossRef]
- Reuss, A.; Du Bois, A.; Harter, P.; Fotopoulou, C.; Sehouli, J.; Aletti, G.; Guyon, F.; Greggi, S.; Mosgaard, B.J.; Reinthaller, A.; et al. TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int. J. Gynecol. Cancer 2019, 29, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.H.; Cowan, R.A.; Zhou, Q.; Iasonos, A.; Byrne, M.; Polcino, T.; Polen-De, C.; Gardner, G.J.; Sonoda, Y.; Zivanovic, O.; et al. Continuous improvement in primary Debulking surgery for advanced ovarian cancer: Do increased complete gross resection rates independently lead to increased progression-free and overall survival? Gynecol. Oncol. 2018, 151, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Warren, J.L.; Harlan, L.C.; Trimble, E.L.; Stevens, J.; Grimes, M.; Cronin, K.A. Trends in the receipt of guideline care and survival for women with ovarian cancer: A population-based study. Gynecol. Oncol. 2017, 145, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Cliby, W.A.; Powell, M.A.; Al-Hammadi, N.; Chen, L.; Miller, J.P.; Roland, P.Y.; Mutch, D.G.; Bristow, R.E. Ovarian cancer in the United States: Contemporary patterns of care associated with improved survival. Gynecol. Oncol. 2015, 136, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C. Ovarian Cancer Audit Feasibility Pilot: Disease Profile in England: Incidence, Mortality, Stage and Survival for Ovary, Fallopian Tube and Primary Peritoneal Carcinomas; PHE Publications Gateway Number: GW-919; Public Health England: London, UK, 2020. [Google Scholar]
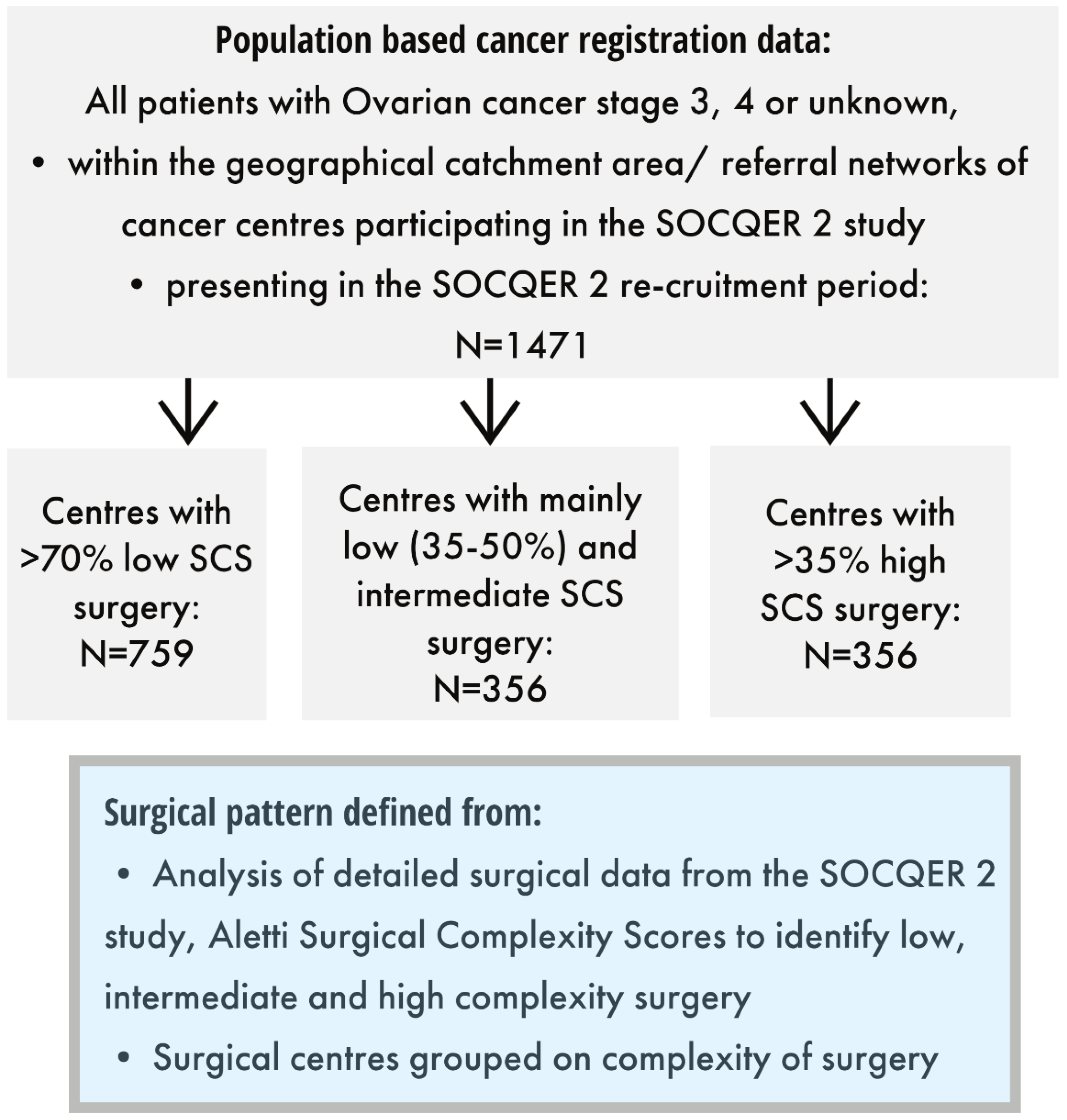
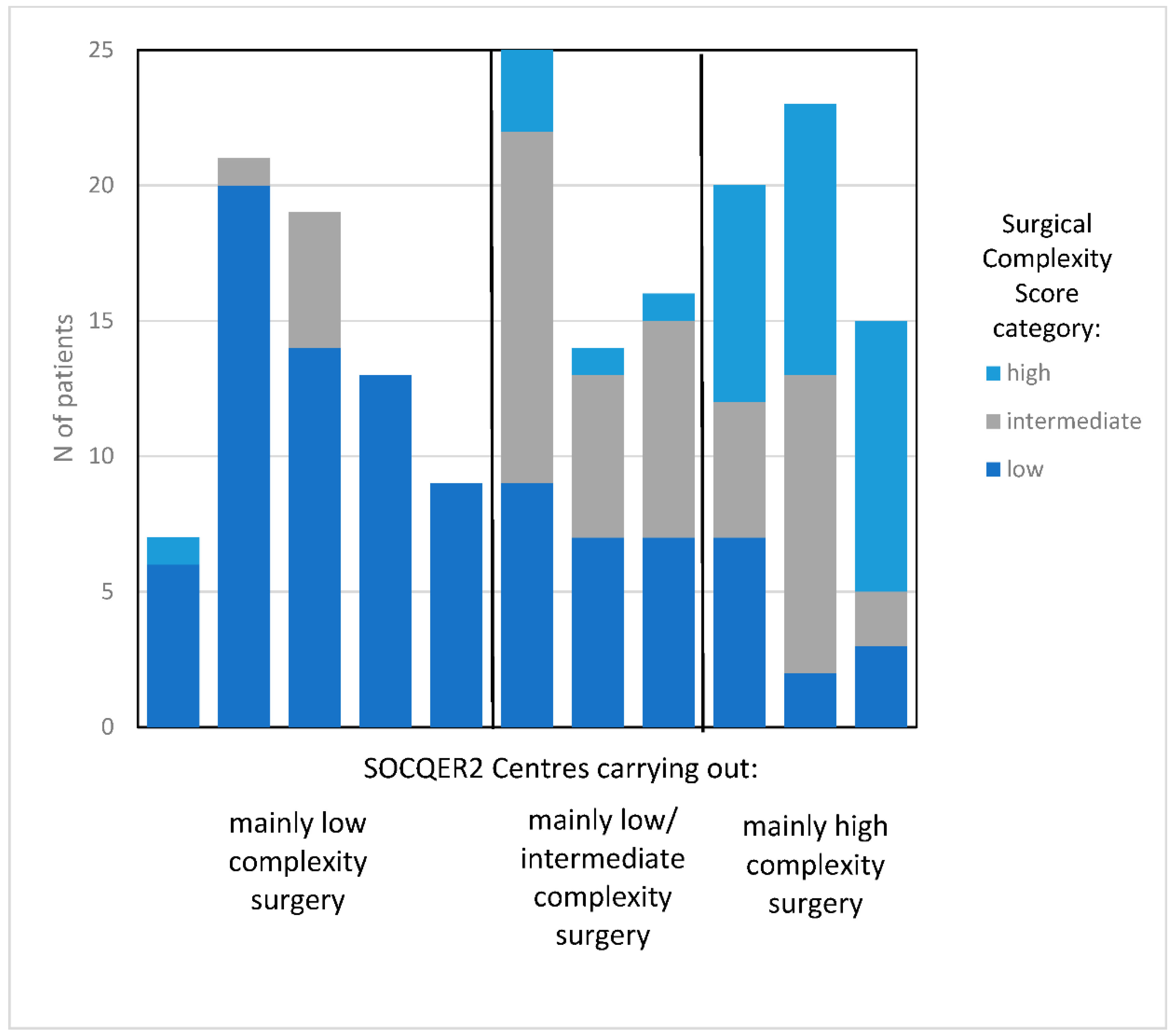
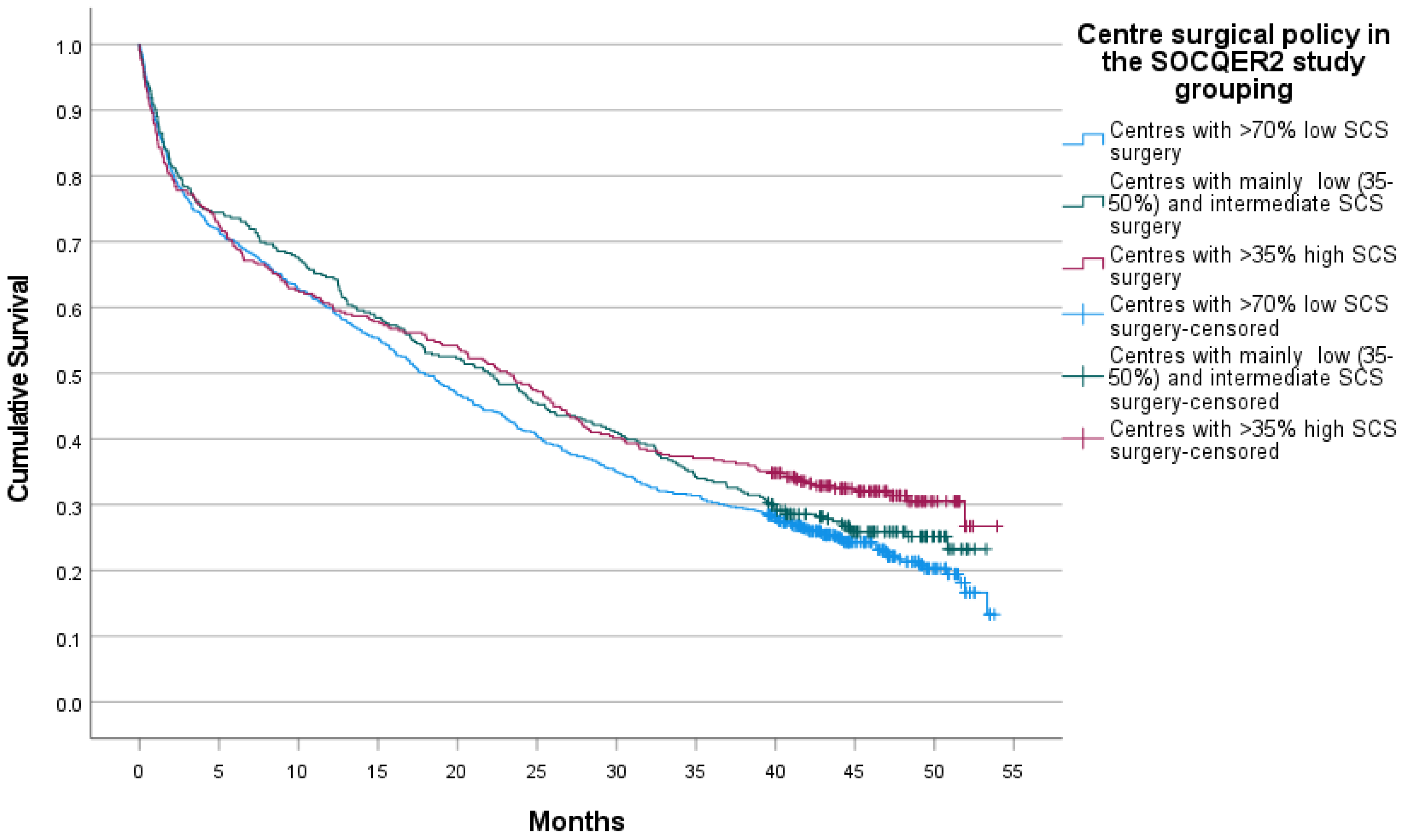
| Patient/Disease Characteristics | Centres with >70% Low SCS Surgery | Centres with Mainly Low (35–50%) and Intermediate SCS Surgery | Centres with >35% High SCS Surgery | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p | ||
| Age group | 0–49 | 51 | 6.7% | 30 | 8.4% | 29 | 8.1% | 0.369 |
| 50–69 | 317 | 41.8% | 163 | 45.8% | 140 | 39.3% | ||
| 70–79 | 245 | 32.3% | 96 | 27.0% | 122 | 34.3% | ||
| 80+ | 146 | 19.2% | 67 | 18.8% | 65 | 18.3% | ||
| Area Income Deprivation Quintile | 1—least deprived | 161 | 21.2% | 62 | 17.4% | 54 | 15.2% | 0.002 |
| 2.00 | 164 | 21.6% | 61 | 17.1% | 56 | 15.7% | ||
| 3.00 | 125 | 16.5% | 75 | 21.1% | 92 | 25.8% | ||
| 4.00 | 157 | 20.7% | 91 | 25.6% | 85 | 23.9% | ||
| 5—most deprived | 152 | 20.0% | 67 | 18.8% | 69 | 19.4% | ||
| Total | 759 | 100.0% | 356 | 100.0% | 356 | 100.0% | ||
| Stage | Unknown | 144 | 19.0% | 52 | 14.6% | 49 | 13.8% | 0.001 |
| 3 | 424 | 55.9% | 182 | 51.1% | 181 | 50.8% | ||
| 4 | 191 | 25.2% | 122 | 34.3% | 126 | 35.4% | ||
| Morphology | Malignant epithelial | 667 | 87.9% | 321 | 90.2% | 325 | 91.3% | 0.302 |
| Miscellaneous and unspecified | 87 | 11.5% | 33 | 9.3% | 31 | 8.7% | ||
| Non-specific site | 5 | 0.7% | 2 | 0.6% | 0 | 0.0% | ||
| Centre Surgical Complexity Pattern | ||||||
|---|---|---|---|---|---|---|
| Treatment | Centres with Mainly Low SCS Surgery | Centres with Mainly Intermediate SCS Surgery | Centres with Mainly High SCS Surgery | |||
| n | % | n | % | n | % | |
| No surgical resection or chemotherapy | 217 | 28.6 | 94 | 26.4 | 93 | 26.1 |
| Chemotherapy only | 214 | 28.2 | 54 | 15.2 | 83 | 23.3 |
| Surgical resection only | 25 | 3.3 | 20 | 5.6 | 41 | 11.5 |
| Surgical resection and chemotherapy | 303 | 39.9 | 188 | 52.8 | 139 | 39.0 |
| Total | 759 | 356 | 356 | |||
| % in each surgical centre grouping | 51.6% | 24.2% | 24.2% | |||
| p | Odds Ratio | 95% CI | ||
|---|---|---|---|---|
| Age group 0–49 (reference) | <0.001 | 1 | ||
| 50–69 | 0.186 | 0.749 | 0.488 | 1.150 |
| 70–79 | 0.000 | 0.309 | 0.199 | 0.480 |
| 80+ | <0.001 | 0.052 | 0.030 | 0.092 |
| Area Income Deprivation Quintile 1 least deprived (reference) | 0.173 | 1 | ||
| 2 | 0.254 | 1.236 | 0.857 | 1.782 |
| 3 | 0.021 | 1.167 | 0.806 | 1.690 |
| 4 | 0.048 | 1.057 | 0.742 | 1.507 |
| 5 most deprived | 0.131 | 0.809 | 0.562 | 1.164 |
| Centres with mainly high SCS surgery (reference) | <0.001 | 1 | ||
| Centres with mainly low SCS surgery | 0.700 | 1.057 | 0.798 | 1.398 |
| Centres with mainly intermediate SCS surgery | <0.001 | 1.833 | 1.322 | 2.540 |
| HR | 95% CI | p | ||
|---|---|---|---|---|
| Aged <50 | 1 | <0.001 | ||
| Aged 50–69 | 1.343 | 1.019 | 1.770 | 0.036 |
| Aged 70–74 | 2.251 | 1.704 | 2.972 | <0.001 |
| Aged 80+ | 4.832 | 3.624 | 6.442 | <0.001 |
| Area Income Deprivation Quintile: 1 (least deprived) | 1 | 0.007 | ||
| 2 | 0.941 | 0.773 | 1.146 | 0.547 |
| 3 | 0.944 | 0.777 | 1.148 | 0.563 |
| 4 | 1.169 | 0.970 | 1.408 | 0.101 |
| 5 (most deprived) | 1.241 | 1.026 | 1.501 | 0.026 |
| SCS score grouping in SOCQER2 study: | ||||
| Centres with mainly high SCS surgery | 1 | 0.054 | ||
| Centres with mainly intermediate SCS surgery | 1.136 | 0.953 | 1.354 | 0.154 |
| Centres with mainly low SCS surgery | 1.205 | 1.036 | 1.401 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cummins, C.; Kumar, S.; Long, J.; Balega, J.; Broadhead, T.; Duncan, T.; Edmondson, R.J.; Fotopoulou, C.; Glasspool, R.M.; Kolomainen, D.; et al. Investigating the Impact of Ultra-Radical Surgery on Survival in Advanced Ovarian Cancer Using Population-Based Data in a Multicentre UK Study. Cancers 2022, 14, 4362. https://doi.org/10.3390/cancers14184362
Cummins C, Kumar S, Long J, Balega J, Broadhead T, Duncan T, Edmondson RJ, Fotopoulou C, Glasspool RM, Kolomainen D, et al. Investigating the Impact of Ultra-Radical Surgery on Survival in Advanced Ovarian Cancer Using Population-Based Data in a Multicentre UK Study. Cancers. 2022; 14(18):4362. https://doi.org/10.3390/cancers14184362
Chicago/Turabian StyleCummins, Carole, Satyam Kumar, Joanna Long, Janos Balega, Tim Broadhead, Timothy Duncan, Richard J. Edmondson, Christina Fotopoulou, Rosalind M. Glasspool, Desiree Kolomainen, and et al. 2022. "Investigating the Impact of Ultra-Radical Surgery on Survival in Advanced Ovarian Cancer Using Population-Based Data in a Multicentre UK Study" Cancers 14, no. 18: 4362. https://doi.org/10.3390/cancers14184362
APA StyleCummins, C., Kumar, S., Long, J., Balega, J., Broadhead, T., Duncan, T., Edmondson, R. J., Fotopoulou, C., Glasspool, R. M., Kolomainen, D., Leeson, S., Manchanda, R., Morrison, J., Naik, R., Tidy, J. A., Wood, N., & Sundar, S. (2022). Investigating the Impact of Ultra-Radical Surgery on Survival in Advanced Ovarian Cancer Using Population-Based Data in a Multicentre UK Study. Cancers, 14(18), 4362. https://doi.org/10.3390/cancers14184362






