Simple Summary
Sarcomas are a group of cancers with differing clinical features, some of which require long courses of cytotoxic chemotherapy. Therefore, patients with sarcoma may be at high risk of developing severe COVID-19. The aim of our study was to describe risk factors and clinical outcomes for patients with sarcoma and COVID-19. We show that patients with sarcoma have high rates of complications from COVID-19. Risk factors for more severe COVID-19 included older age, poor performance status, and lung metastases. We also compared 30 day mortality rates to a matched cohort of patients with sarcoma without COVID-19 and found that patients with bone sarcoma may be at higher risk of death from COVID-19 than patients with other sarcoma subtypes.
Abstract
Background: Patients with sarcoma often require individualized treatment strategies and are likely to receive aggressive immunosuppressive therapies, which may place them at higher risk for severe COVID-19. We aimed to describe demographics, risk factors, and outcomes for patients with sarcoma and COVID-19. Methods: We performed a retrospective cohort study of patients with sarcoma and COVID-19 reported to the COVID-19 and Cancer Consortium (CCC19) registry (NCT04354701) from 17 March 2020 to 30 September 2021. Demographics, sarcoma histologic type, treatments, and COVID-19 outcomes were analyzed. Results: of 281 patients, 49% (n = 139) were hospitalized, 33% (n = 93) received supplemental oxygen, 11% (n = 31) were admitted to the ICU, and 6% (n = 16) received mechanical ventilation. A total of 23 (8%) died within 30 days of COVID-19 diagnosis and 44 (16%) died overall at the time of analysis. When evaluated by sarcoma subtype, patients with bone sarcoma and COVID-19 had a higher mortality rate than patients from a matched SEER cohort (13.5% vs 4.4%). Older age, poor performance status, recent systemic anti-cancer therapy, and lung metastases all contributed to higher COVID-19 severity. Conclusions: Patients with sarcoma have high rates of severe COVID-19 and those with bone sarcoma may have the greatest risk of death.
1. Introduction
Since March 2020, the COVID-19 pandemic has significantly impacted hospital systems, patients, and public health in the United States, including disruption of cancer screening, diagnosis, and treatment. This is anticipated to increase both morbidity and mortality of individuals with cancer in upcoming years [1]. In addition to the disruption of care, having a cancer diagnosis is a significant negative prognostic indicator for patients with COVID-19. Patients with active malignancies experience more severe COVID-19 and higher death rates from SARS-CoV-2 infection [2,3,4,5]. In March 2020, the COVID-19 and Cancer Consortium (CCC19) was founded by five institutions with the goal of collecting and disseminating uniformly organized information on patients with cancer and COVID-19. Since then, CCC19 has grown to 125 institutions with >600 collaborators [1]. Analyses of the collective CCC19 patient cohort have demonstrated high 30 day all-cause mortality among patients with both cancer and COVID-19, attributed to both general risk factors including advanced age, male sex, former smoker status, higher number of comorbidities and poor Eastern Cooperative Oncology Group (ECOG) performance status, as well as cancer-specific risk factors such as active (measurable) disease, malignancy type, and recent receipt of systemic chemotherapy [6,7].
Sarcomas are a heterogenous group of mesenchymal tumors that range in severity and risk of recurrence or spread [8]. They include over 170 histologic diagnoses that, depending on the specific subtype and extent of disease, may require chemotherapy or targeted therapy, radiotherapy, surgery, or a combination of treatments [9]. Due to the rarity and diversity of sarcoma subtypes, National Comprehensive Cancer Network (NCCN) guidelines state that all patients with sarcoma should have their case reviewed at a multidisciplinary sarcoma center; this often requires patients to travel long distances which has been shown to significantly impact outcomes of patients with sarcoma [10].
In early 2020 the clinical challenges of sarcomas were further complicated with the emergence of the SARS-CoV-2 virus. Questions arose over which patients might be at increased risk of developing more severe cases of COVID-19, and whether or not it might be safe to travel to sub-specialty sarcoma centers. Due to the need to travel to sarcoma referral centers, patients with sarcoma may be more vulnerable and have higher risk of contracting COVID-19 because they could not isolate at home to the same degree as other patients with cancer. Furthermore, some sarcoma subtypes require intense immunosuppressive chemotherapies that can significantly impact the clinical outcomes of a SARS-CoV-2 infection [11,12,13]. Finally, patients with advanced sarcoma often have metastatic involvement in the lungs, the organs most vulnerable to the consequences of COVID-19. Taken together, patients with both sarcoma and COVID-19 may face more challenges than patients with other cancer types. An early case series suggested that patients with sarcoma have a high risk of developing severe COVID-19 [14]. As the response to the COVID-19 pandemic continues to evolve, an understanding of the unique risk factors faced by patients with sarcoma can help inform decision making for patients and guide advice given by treating physicians. The recent omicron wave of COVID-19 demonstrated that, even two years after the first COVID-19 cases were diagnosed, the possibility of additional variants and additional surges remains. No large study has formally assessed the risks faced by patients with sarcoma and COVID-19; herein, we describe the largest such study to date.
2. Materials and Methods
2.1. Study Design and Patient Population
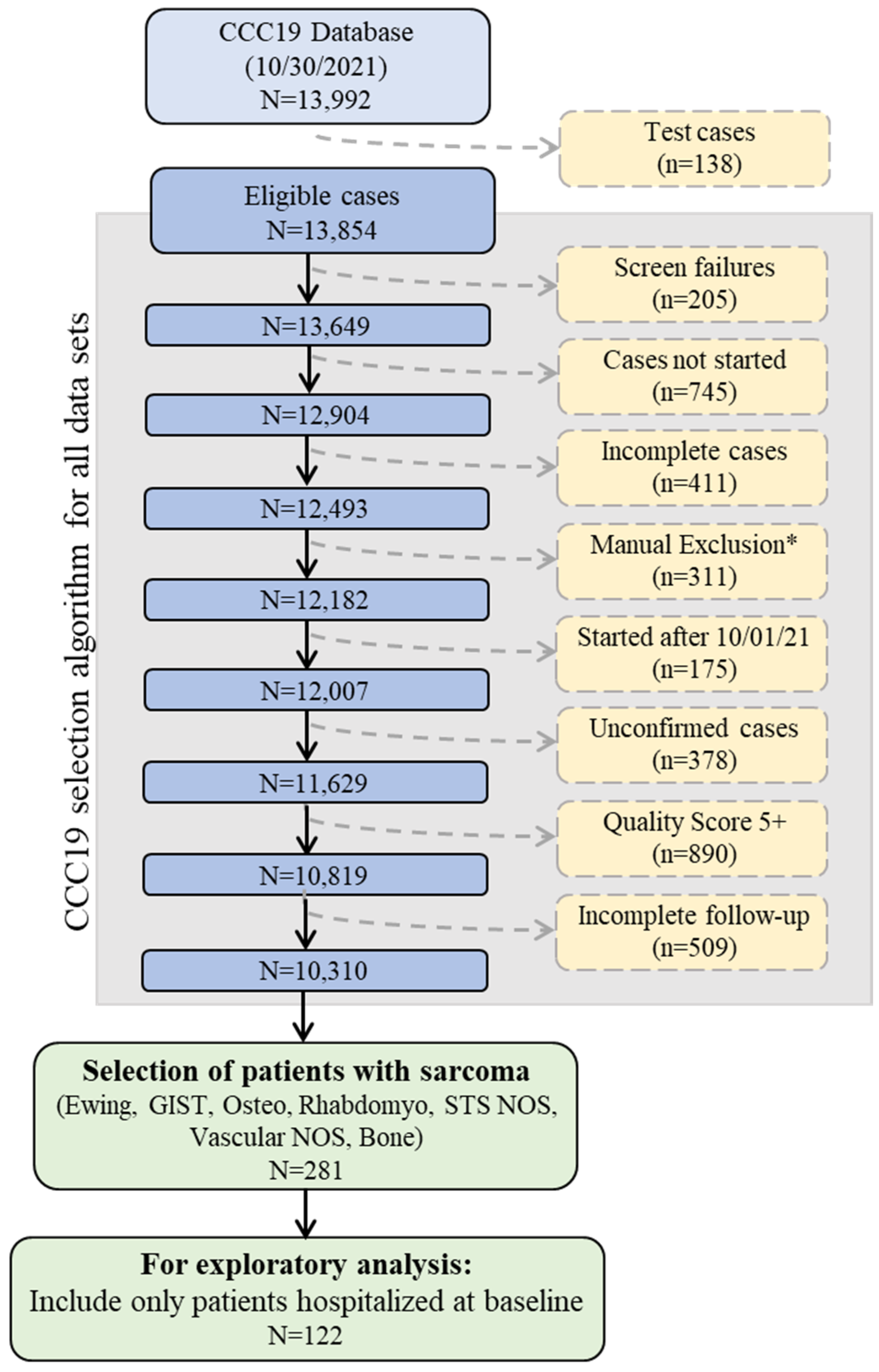
This was a registry-based retrospective cohort study of patients reported to CCC19. Data were collected using a REDCap database housed at the Vanderbilt University Medical Center (VUMC). Details of the CCC19 workflow and data elements have been previously described [1]. Patient reports entered into the CCC19 registry (NCT04354701) between 17 March 2020 and 30 September 2021 were included in the analysis. Supplementary File S1 lists all contributing institutions. Patients with a diagnosis of sarcoma and a laboratory-confirmed SARS-CoV-2 infection were included; those under 18 were truncated to age = 18. Patient reports with inadequate data quality (quality score ≥5 according to our previously published metric) or incomplete primary outcome data were excluded. A diagnosis of sarcoma was further defined as Ewing sarcoma, gastrointestinal stromal tumor (GIST), osteosarcoma, Rhabdomyosarcoma, soft tissue sarcoma not otherwise specified (NOS), vascular sarcoma NOS, or bone sarcoma. Sarcoma subgroups were defined as soft tissue sarcoma (STS), bone sarcoma, GIST, and other/indolent histologies (Figure 1, Supplemental Table S1).
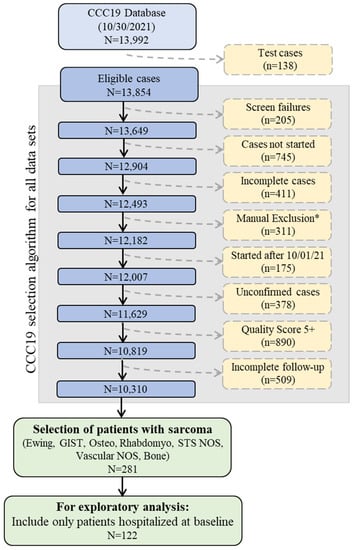
Figure 1.
Schematic of CCC19 Database selection. Abbreviations: Gastrointestinal stromal tumor (GIST), Osteosarcoma (Osteo), Rhabdomyosarcoma (Rhabdomyo), Soft tissue sarcoma not otherwise specified (STS NOS). * Manual Exclusions = Duplicate records; In situ solid malignancy; Precursor hematologic condition; Benign hematologic condition; False positive SARS-CoV-2 test; Non-melanoma skin cancer, non-invasive; and Low quality score, non-CCC19 site.
2.2. Data Elements
The CCC19 data dictionary can be accessed at https://github.com/covidncancer/CCC19_dictionary. Data collected included baseline demographic data, approximate date of COVID-19 diagnosis, comorbidities, cancer-specific data (sarcoma subtype, extent of disease, metastatic sites), and timing and modality of most recent anti-cancer therapy [7]. Laboratory measurements included absolute lymphocyte count, absolute neutrophil count, white blood cell count, creatinine, lactate dehydrogenase (LDH), and D-dimer. The primary outcome was COVID-19 severity, a six-level ordinal variable ranging from: none of the following events; hospitalization, without or with supplemental oxygen; admission to an intensive care unit (ICU); mechanical ventilation; or death due to any cause. This ordinal severity outcome was meant to capture the full range of clinical complications experienced by patients and was assessed over patients’ total follow-up time. The secondary outcome was 30 day all-cause mortality.
2.3. Statistical Analysis
Standard descriptive statistics were used to summarize baseline patient characteristics and outcomes overall and stratified by sarcoma subtype. Laboratory measurements were summarized among patients hospitalized at baseline. Unadjusted descriptive statistics are presented without formal statistical hypothesis testing (i.e., p values). Multiple imputation (twenty iterations; missingness rates were <15% for risk factors other than laboratory measurements) using additive regression, bootstrapping, and predictive mean matching was used to impute missing and unknown data, except unknown ECOG performance status and unknown cancer status, which were included as unknown categories. Imputation was performed on the full dataset before exclusions. Formal statistical hypothesis testing was performed using adjusted odds ratios and 95% confidence intervals for the ordinal COVID-19 severity outcome, which were obtained from ordinal logistic regression models with an offset for (log) follow-up time. Due to the sample size and corresponding degrees of freedom, a reduced set of covariates was included in adjusted models based on a priori clinical relevance [15]. Covariates included age (regression spline with a knot at age 40 years), sex, renal disease, ECOG performance status, site of metastasis (lung vs. none and other vs. none), and time period of COVID-19 diagnosis. Due to substantial collinearity between sarcoma subtype and modality of anti-cancer therapy, two models were fit. The first included sarcoma subtypes along with binary indicators for any recent anti-cancer therapy (0–3 months versus >3 months before COVID-19 diagnosis; never or after COVID-19 diagnosis versus >3 months). The second excluded sarcoma subtypes but included binary indicators for modalities of recent anti-cancer therapy (cytotoxic chemotherapy, targeted therapy, immunotherapy, locoregional therapy—including surgery and radiation, and none). Analyses were performed using R (version 4.0.2), including the Hmisc and rms extension packages. The statistical analysis plan and data elements are provided as supplementary material.
2.4. Comparison to SEER Data
Because sarcoma is an aggressive cancer, it is possible that deaths were due to the underlying cancer rather than due to COVID-19. However, capture of cause of death in CCC19 is incomplete. To isolate the impact of COVID-19 on mortality risk, we compared mortality rates among CCC19 patients with sarcoma and COVID-19 to a matched sample of patients with sarcoma reported to the Surveillance, Epidemiology, and End Results (SEER) Program database between 2000 and 2018 (and therefore before COVID-19). Exact matching was performed based on age (in decades), sex, race (Black, White, other), sarcoma subtype (bone, GIST, STS, other), cancer stage (localized, disseminated, unknown), and recency of cancer diagnosis (<1 year, 1–5 years, >5 years) using the MatchIt package in R; CCC19 patients with no match (n = 5) or only 1 match (n = 4) in SEER were excluded. For SEER patients, we defined a counterfactual time of COVID-19 diagnosis based on the median survival time for groups defined by recency of cancer diagnosis; 30 day mortality rates were calculated from this counterfactual time. Mortality at 30 days was compared between CCC19 patients and their matched SEER counterparts overall and stratified by sarcoma subtype due to expected differences in their clinical behaviors using Fisher’s exact tests.
3. Results
3.1. Clinical Characteristics of Sarcoma Patients Infected with SARS-CoV-2
From 17 March 2020 to 30 September 2021, 281 patients with sarcoma were entered into the CCC-19 registry (Figure 1) with a median follow-up of 90 days (Interquartile rage; IQR, 30–180). Baseline clinical characteristics including demographic characteristics, comorbidities, cancer-specific data, and approximate date of COVID-19 diagnosis are summarized in Table 1. Of the 281 patients, 153 (54%) were classified as having soft tissue sarcoma (STS), 48 (17%) with bone sarcoma, 45 (16%) with GIST, and 35 (13%) with other/indolent subtypes; see Supplemental Table S1 for details. The median patient age was 56 years (IQR, 41–66), 149 (53%) were male, and 133 (47%) patients were non-Hispanic white. A total of 111 (40%) patients were clinically obese and the most common comorbidity was diabetes mellitus (54 patients, 19%). A further 105 (37%) patients were reported to be in remission from their sarcoma and 152 (54%) were reported to have active sarcoma. Of patients with active sarcoma, 79 (52%) had sarcomas that were stable or responsive to current therapy, and 73 (48%) had progressing cancers. Furthermore, 53 (19%) patients had lung metastases and 37 (13%) patients had metastases to other sites. A total of 142 (51%) patients had received anticancer therapy within 3 months of their COVID-19 diagnosis, with the majority (n = 82, 58%) of these patients receiving cytotoxic chemotherapy. Low absolute lymphocyte count and abnormal D-Dimer levels were the most common irregular laboratory result among hospitalized patients (Supplemental Table S2).

Table 1.
Patient demographic and clinical characteristics.
3.2. Clinical Outcomes Stratified by Sarcoma Subgroup
Of the 281 patients analyzed in this study, 49% (n = 139) were hospitalized, 33% (n = 93) were hospitalized and received supplemental oxygen, 11% (n = 31) were admitted to the ICU, and 6% (n = 16) received mechanical ventilation. There were 23 deaths (8%) within 30 days of COVID-19 diagnosis and 44 (16%) deaths overall at the time of analysis. When patients were stratified by sarcoma subtype, there was only slight variation in hospitalization rates, with STS having the highest hospitalization rate at 52% (n = 79/153) and GIST having the lowest hospitalization rate at 44% (n = 20/45) (Table 2). There was no clear difference in ICU admission, supplemented oxygen, or mechanical ventilation. However, there was a small but noticeable difference in the overall death rate. Patients with GIST or other/indolent subtypes had lower rates of death (9% and 3%, respectively) when compared to bone and STS (19% and 20%, respectively).

Table 2.
Outcomes for patients with sarcoma and COVID-19.
3.3. Clinical Risk Factors for COVID-19 Severity
All sarcoma subtypes had lower COVID-19 severity compared to STS after adjustment (Table 3): bone sarcoma OR was 0.68 (95% CI 0.29–1.57), GIST OR was 0.37 (95% CI 0.16–0.82) and other/indolent histologies OR was 0.34 (95% CI 0.14–0.80). Timing of recent anti-cancer therapy was not associated with COVID-19 severity; recent receipt of cytotoxic chemotherapy was associated with a higher COVID-19 severity with on OR 1.97 (95% CI 0.99–3.93). Age was associated with higher COVID-19 severity (OR 1.50 per decade, 95% CI 1.17–1.93 when controlling for sarcoma subtype; OR 1.40 per decade, 95% CI 1.11–1.83 when controlling for anti-cancer modalities). Significantly higher COVID-19 severity was also observed among patients with renal disease, patients with poor ECOG performance status (2 or higher), and patients with metastases to the lung or other sites (Table 3).

Table 3.
Analysis of clinical predictors for patients with sarcoma and COVID19.
3.4. Comparison to Matched SEER Cohort
The overall 30-day mortality rate for the CCC19 sarcoma cohort was 7.8% (20 deaths) in the CCC19 sarcoma cohort and 7.4% (1223 deaths) in the SEER sarcoma cohort. When separated by sarcoma subtype, patients with bone sarcoma and COVID-19 appeared to have a higher mortality rate than counterparts in the SEER cohort (13.5% vs 4.4%, p = 0.030). The 30 day mortality rates for patients with GIST, STS, or other/indolent subtypes were similar (Table 4).

Table 4.
30 day Mortality Rates for CCC19 and matched SEER Sarcoma Patients, Overall and by Subtype.
4. Discussion
We report that patients with sarcoma have an overall high risk of developing severe COVID-19 and a higher risk of death from COVID-19 than is reported in the general population [16]. Patients with STS had higher COVID-19 severity than patients with GIST and more classically indolent histologies. Advanced age, baseline renal disease, poor performance status, and metastatic disease and site were associated with higher rates of COVID-19 complications in our multivariable analysis. Recent (within 3 months) receipt of cancer therapy was associated with high risk of COVID-19 complications, although this was not statistically significant likely due to lack of power with lower bound of the 95% confidence interval 0.99. When separated by sarcoma subtype, patients with STS and bone sarcomas had the highest likelihood of developing severe COVID-19.
One possible explanation for this difference by subtype is that STS and bone sarcomas are more likely to be treated with highly cytotoxic chemotherapy even in the curative setting, leaving some of these patients in a more immunosuppressed state over the longer term. This may be especially true for patients with bone sarcomas, who in our comparison to a matched SEER cohort had the highest excess risk of death from COVID-19. A hypothesis for this excess mortality in patients with bone sarcoma and COVID-19 is that bone sarcomas such as osteosarcoma and Ewing sarcoma are treated with prolonged, highly immunosuppressive chemotherapy that can leave the bone marrow reserve depleted in patients even after completion of therapy [17]. Our results support further investigation to test the hypothesis that depth of prior immunosuppression associates with increased risk from COVID-19. For patients with lung metastasis at presentation, surgery to resect all sites of lung metastasis after completion of chemotherapy is routinely performed, perhaps predisposing these patients to pulmonary complications of COVID-19; prior lung surgery was not routinely captured in the CCC19 registry [18].
As society begins to open without restrictions, patients with sarcoma must take this increased risk into account. COVID-19 vaccines have been shown to be effective at preventing hospitalization and death from COVID-19 [19,20,21,22]. Although some patients with cancer may have less effective immune responses to vaccination, any afforded protection may be important and a majority of those with solid tumors appear to derive benefit [23]. Among patients with sarcoma, those with STS and bone sarcomas appear to be at higher risk than other subtypes that are not typically treated with intensive chemotherapy. A potential confounding factor is that some of the patients in our cohort might have died from progressing cancer or other sequelae of sarcoma. However, a mortality rate of 8% in 30 days exceeds what would be expected from sarcoma alone. We attempted to account for this by comparing our cohort to a matched cohort from the SEER database.
Patient race has also been observed as a risk factor for adverse COVID-19 outcomes [24], including among patients with cancer [25]. Only 47% of our cohort was non-Hispanic white, suggesting that we had high minority representation. This may introduce additional confounding in interpreting our observed high COVID-19 severity rate.
Strategies to mitigate the risks faced by patients with sarcoma will be critical as cancer care delivery continues to evolve [26]. The adoption of telemedicine has the potential to decrease potential exposure to COVID-19 during peaks in cases as a significant number of cancer visits can likely be performed virtually [27]. Other strategies like pre-exposure prophylaxis, and continuing to encourage vaccination and masking in patients, relatives, and caregivers in spite of the removal of mandates can also minimize the risks faced by patients seeking cancer care.
Strengths of this study include the multi-institutional nature of the CCC19 database, inclusion of patients across a range of cancer treatment settings, and incorporation of variables not routinely available in structured electronic medical record data, such as sarcoma subtype, cancer status, and ECOG performance status. Although small compared to other CCC19 analyses, this represents a large dataset for a sarcoma-specific analysis. By comparing the survival of patients in our database to a matched cohort from SEER patients who did not have COVID-19, this is the first analysis of COVID-19 outcomes in patients with cancer to differentiate the mortality attributed to COVID-19 from the expected mortality from the cancer itself. This is important considering the overall poor long-term outcomes for patients with metastatic sarcoma.
Limitations to this analysis include its retrospective nature, selection and unmeasured confounding biases, and the rapidly changing pace of strategies for COVID-19 prevention, treatment, and management. We were not able to collect outcome data from a similar population of patients with COVID-19 who did not have cancer. A small number of those hospitalized with supplemental oxygen were known to be on chronic supplemental oxygen prior to hospitalization, such that these patients would be automatically assigned to the higher levels of ordinal severity. While this could have some type of unintended analytic effect, it is also the case that patients on chronic supplemental oxygen have pulmonary compromise and would therefore be anticipated to have more severe COVID-19 outcomes. Laboratory measurements are only somewhat informative, as most labs are not routinely checked for patients with mild COVID-19, and some labs (e.g., LDH) are even less frequently measured, with missingness >50%. Most of the patients in our cohort are from the pre-vaccination era, and it is possible that that patients at participating sites who were positive for COVID-19 were not captured in the CCC19 database. The data range of the analysis also exclude the most recent waves of cases due to the omicron variant. Future directions include assessing the impact of vaccinations and vaccine boosters, COVID-19 treatments, and practice changes made as a result of the pandemic.
5. Conclusions
Patients with sarcoma have high rates of severe COVID-19. Older age, poor performance status, recent cytotoxic chemotherapy, and lung metastases all contributed to worse outcomes. Patient with STS and bone sarcoma are most at risk, but only patients with bone sarcoma and COVID-19 had a higher mortality rate than patients from a matched SEER cohort. Strategies to mitigate the risk from COVID-19 are encouraged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14174334/s1, Table S1: Sarcoma subtype at diagnosis and classification into sarcoma subgroups; Table S2: Laboratory results among hospitalized patients. File S1: List of participants by institution: Alphabetical list of participants by institution that contributed at least one record to the analysis.
Author Contributions
Conceptualization, M.J.W., M.I., C.A.P., J.C.T., V.S., E.T.L., P.G., J.L.W. and E.J.D.; methodology, M.J.W., C.H. (Cassandra Hennessy), A.B., B.F., D.P.S., J.L.W. and E.J.D.; formal analysis, C.H. (Cassandra Hennessy), A.B. and B.F.; data curation, M.J.W., D.V.-C., E.R.-G., M.I., G.K.S., R.C., L.F., C.P., O.Z., A.R.K., L.T., E.S.N., E.T.L., C.L., R.M.S., R.R.M., A.A., E.A.G., I.P., W.D.T., C.H. (Clara Hwang), S.T., S.R.J., B.H.-L., E.W.-B., A.K., D.Y.R., G.N., M.J., H.P., A.A.K., K.E., D.H.K., L.P., M.A.B., E.B.D., P.G., J.L.W. and E.J.D.; writing—original draft preparation, M.J.W., S.C. and E.J.D.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/National Institute of Health (NIH)). This study was partly supported by grant number P30 CA068485 from the National Cancer Institute to Vanderbilt University Medical Center. Igor Puzanov is supported by NIH (P30CA016056). Petros Grivas is supported by NIH (P30CA015704). The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to de-identification of the dataset.
Informed Consent Statement
Patient consent was waived due to use of a de-identified database.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from CCC19 and are available from the authors with the permission of the CCC19 Steering Committee.
Acknowledgments
We thank all members of the CCC19 steering committee: Toni K. Choueiri, Narjust Duma, Solange Peters, Dimitrios Farmakiotis, Brian I. Rini, Michael A. Thompson, and Gilberto de Lima Lopes Jr. for their invaluable guidance of the CCC19 consortium. We also thank Nicole Kuderer and Gary Lyman for thoughtful discussions and guidance during the planning stages of this project.
Conflicts of Interest
M.J.W.—Advisory board Deciphera, Adaptimmune, Epizyme; L.F.—research funding to institution: BMS, Kartos, EMD Serono/Pfizer, Array/Pfizer. Consultant: Else-vier. All outside submitted work; J.C.T.—Blueprint, Deciphera, Bayer, c4 therapeutics, Aadi, Foghorn, Daiichi-Sankyo, Adcendo; A.A.K.—stock ownership Merck, Fate Therapeutics, Exact Sciences, Gilead Sciences, Novavaxi, advisory board Genentech, Research funding to institution Astra Zeneca; A.R.K.—Within the last three years (all unrelated to this manuscript): stock ownership Merck and Sanofi; honoraria from MJH Life Sciences/OncLive and HMP Global; research collaborations with Tempus Labs and Natera; Uncompensated advisory board with Seagen/Astellas; E.A.G.—Honoraria to EAG—Abbvie, Alexion Pharmaceuticals, Genentech, Novartis, CTI biopharma, Apellis, Celgene/BMS, Takeda Oncology, Taiho Oncology, Physician Educational Resource, Med-iCom Worldwide, American Society of Hematology, Picnic Health, AAMDSIF; Research Support to Roswell Park: Astex Pharmaceuticals, Genentech, Blueprint Medicine, Alexion Pharmaceuticals, Apellis, BMS/Celgene, Celldex Therapeutics; I.P.—Within the last three years (all unrelated to this manuscript): consultant fees from Regeneron, Oncorus, Iovance, and Nouscom; stock options from Seneca Therapeutics; W.T.—Personal fees from Eli Lilly, EMD Serono, Mundipharma, C4 Therapeutics, Daiichi Sankyo, Blueprint, Agios Pharmaceuticals, Deciphera, Adcendo, Ayala Pharmaceuticals, Kowa, Servier, Bayer Pharmaceuticals, Epizyme, Cogent, Medpacto, Foghorn Therapeutics, Amgen, AmMax Bio, outside the submitted work; patent Companion Diagnostic for CDK4 inhibitors - 14/854,329 pending to MSKCC/SKI, and a patent Enigma and CDH18 as companion Diagnostics for CDK4 inhibition–SKI2016-021-03 pending to MSKCC/SKI and Scientific Advisory Board - Certis Oncology Solutions, Stock Ownership, Co-Founder - Atropos Therapeutics, Stock Ownership, Scientific Advisory Board Innova Therapeutics; C.H.—Stock holdings in Johnson and Johnson; research funding to institution from Merck, Bausch Health, Genentech, Bayer, and AstraZeneca, consultant fees from Tempus, Genzyme, and EMD Sorono, speaking fees from OncLive/MJH Life Sciences, travel fees from Merck, all outside the submitted work; SRJ—institutional research funding from Varian Medical Systems; M.J.—Research grant—Astrazeneca, Pfizer, Eisai, Advisory board for Seagen, Sanofi; A.K.—Stock holdings Fate Therapeutics, Exact Sciences, Gilead Sciences, Mitati Therapeutics, Novavax; Research funding to institution from Astra Zeneca, Advisory board: Genentech; K.E.—Honoraria from BMS, Pfizer, Merck, Roche, EMD Serono, Astrazeneca, Ipsen, Sanofi, all outside submitted work. Research grants from Pfizer, outside submitted work; L.P.—workshop-Curio Science; advisory board-Epizyme; P.G.—consulting: 4D Pharma PLC, Astellas Pharma, AstraZeneca, Boston Gene, Bristol Myers Squibb, Dyania Health, EMD Serono, Exelixis, Fresenius Kabi, Genentech, Gilead Sciences, Guardant Health, Infinity Pharmaceuticals, Janssen, Lucence Health, Merck, Mirati Therapeutics, Pfizer, Puretech, QED Therapeutics, Regeneron Pharmaceuticals, Roche, Seattle Genetics, Silverback Therapeutics, Uro-Gen; institutional research funding: Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm Group, EMD Serono, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Merck, Mirati Therapeutics, Pfizer, QED Therapeutics; J.L.W.—Consulting-Westat, Roche, Flatiron Health, Melax Tech, outside the submitted work; Ownership-HemOnc.org LLC, outside the submitted work.; E.J.D.- Consulting with Deciphera and Aadi, Speaking fees MJH Life Sciences, Research funding to institution Karyopharm, Top Alliance Biosciences, Cogent, Inhibrx, Actuate, BioAtla, Cornerstone, Incyte; C.H., S.C., B.F., A.B., D.V., E.R., M.I., G.K.S., C.A.P., R.C., C.P., O.Z., V.S., L.T., E.N., E.T.L., C.L., R.M.S., R.R.M., A.A., S.T., B.H., A.A.K., D.Y.R., G.N., H.P., D.K., M.A.B., D.P.S. declared no conflicts relevant to this manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- The COVID-19 and Cancer Consortium. A Systematic Framework to Rapidly Obtain Data on Patients with Cancer and COVID-19: CCC19 Governance, Protocol, and Quality Assurance. Cancer Cell 2020, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef]
- Saini, K.S.; Tagliamento, M.; Lambertini, M.; McNally, R.; Romano, M.; Leone, M.; Curigliano, G.; de Azambuja, E. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur. J. Cancer 2020, 139, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021, 32, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A. Sarcoma classification: An update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer 2014, 120, 1763–1774. [Google Scholar] [CrossRef]
- Crago, A.M.; Brennan, M.F. Principles in Management of Soft Tissue Sarcoma. Adv. Surg. 2015, 49, 107–122. [Google Scholar] [CrossRef]
- Onega, T.; Duell, E.J.; Shi, X.; Wang, D.; Demidenko, E.; Goodman, D. Geographic access to cancer care in the U.S. Cancer 2008, 112, 909–918. [Google Scholar] [CrossRef]
- Seynaeve, C.; Verweij, J. High-dose chemotherapy in adult sarcomas: No standard yet. Semin. Oncol. 1999, 26, 119–133. [Google Scholar] [PubMed]
- Demetri, G.D. High-dose ifosfamide in the treatment of sarcomas of soft tissues and bone. Semin. Oncol. 1996, 23, 22–26. [Google Scholar] [PubMed]
- Verma, S.; Bramwell, V. Dose-intensive chemotherapy in advanced adult soft tissue sarcoma. Expert Rev. Anticancer Ther. 2002, 2, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Pollack, S.M.; Cranmer, L.D.; Thompson, M.J.; Maxwell, S.; Wright, S.; Khaki, A.R.; Madeleine, M.M.; Grivas, P.; Kuderer, N.M.; et al. Outcomes of Patients with Sarcoma and COVID-19 Infection: A Single Institution Cohort Analysis. Cancer Investig. 2021, 39, 315–320. [Google Scholar] [CrossRef]
- French, B.; Shotwell, M.S. Regression Models for Ordinal Outcomes. JAMA 2022, 328, 772–773. [Google Scholar] [CrossRef]
- Elo, I.T.; Luck, A.; Stokes, A.C.; Hempstead, K.; Xie, W.; Preston, S.H. Evaluation of Age Patterns of COVID-19 Mortality by Race and Ethnicity from March 2020 to October 2021 in the US. JAMA Netw. Open 2022, 5, e2212686. [Google Scholar] [CrossRef]
- Wagner, M.J.; Livingston, J.A.; Patel, S.R.; Benjamin, R.S. Chemotherapy for Bone Sarcoma in Adults. J. Oncol. Pract. 2016, 12, 208–216. [Google Scholar] [CrossRef]
- Picci, P. Osteosarcoma (osteogenic sarcoma). Orphanet J. Rare Dis. 2007, 2, 6. [Google Scholar] [CrossRef]
- Harder, T.; Kulper-Schiek, W.; Reda, S.; Treskova-Schwarzbach, M.; Koch, J.; Vygen-Bonnet, S.; Wichmann, O. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: Second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Eurosurveillance 2021, 26, 2100920. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.L.; Yasawardene, P.; Wijerathne, W.; Somawardana, B. COVID-19 vaccination in cancer patients: A narrative review. J. Int. Med. Res. 2022, 50, 3000605221086155. [Google Scholar] [CrossRef] [PubMed]
- Magesh, S.; John, D.; Li, W.T.; Li, Y.; Mattingly-app, A.; Jain, S.; Chang, E.Y.; Ongkeko, W.M. Disparities in COVID-19 Outcomes by Race, Ethnicity, and Socioeconomic Status: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2134147. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Reid, S.A.; French, B.; Hennessy, C.; Hwang, C.; Gatson, N.T.; Duma, N.; Mishra, S.; Nguyen, R.; Hawley, J.E.; et al. Racial Disparities in COVID-19 Outcomes Among Black and White Patients with Cancer. JAMA Netw. Open 2022, 5, e224304. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Chalian, M.; Ricciotti, R.; Roberts, J.L.; Thompson, M.J.; Schaub, S. Sarcoma Care Collaborations: Models for patient centered sub-specialty oncology care in the COVID-19 era and beyond. IJCCD 2022, 2. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Chandereng, T.; Osterman, T.; Arafat, W.; Smerage, J.; Polubriaginof, F.C.G.; Heinrichs, T.; Sugalski, J.; Martin, D.B. Oncologist Perspectives on Telemedicine for Patients with Cancer: A National Comprehensive Cancer Network Survey. JCO Oncol. Pract. 2021, 17, e1318–e1326. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).