PARP Inhibitors for Breast Cancer: Germline BRCA1/2 and Beyond
Abstract
:Simple Summary
Abstract
1. Introduction
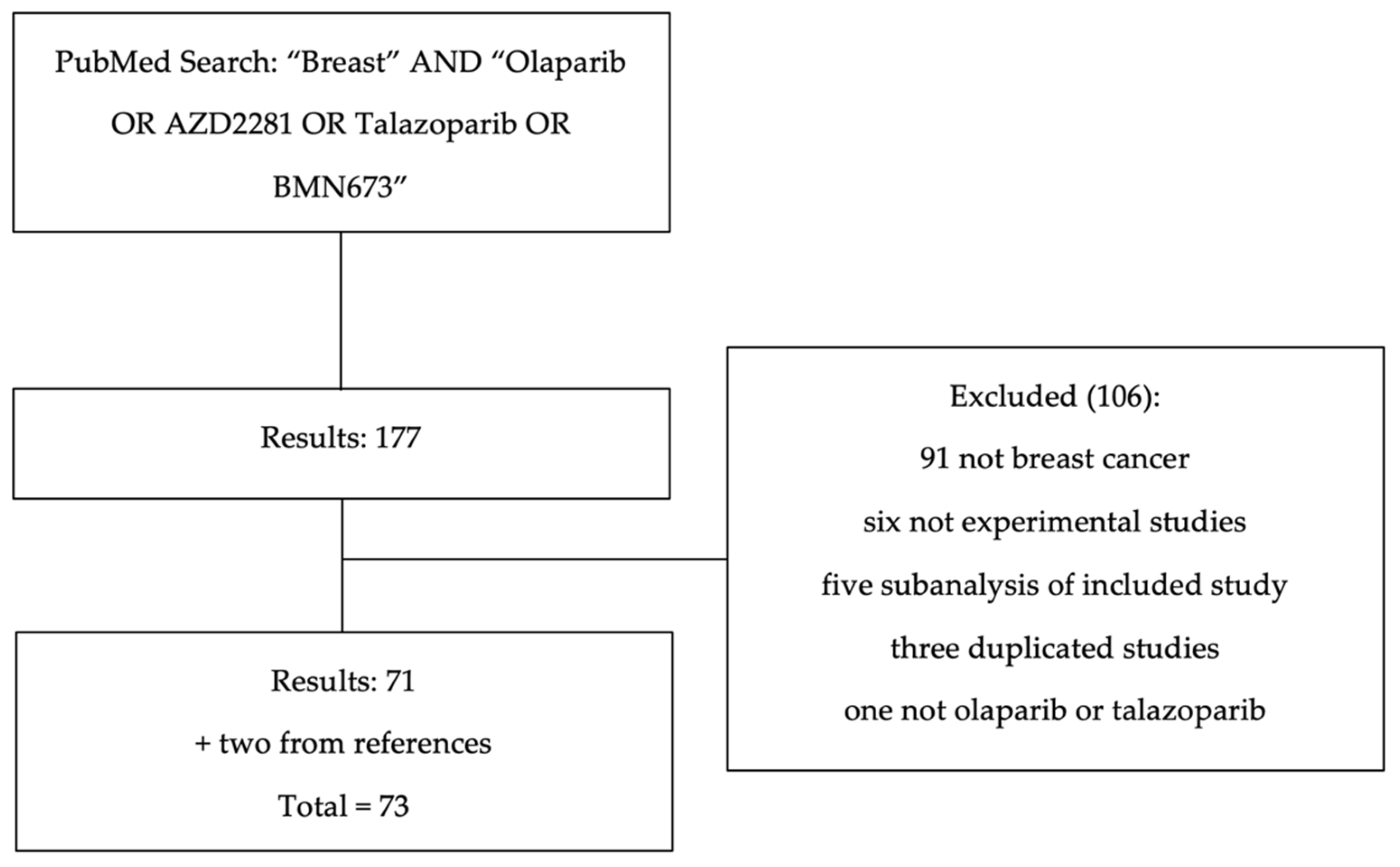
2. Methods
3. Olaparib
3.1. Early-Phase Studies
3.2. Locally Advanced or Metastatic Breast Cancer
3.3. Early-Stage Breast Cancer
3.4. Combination Trials
3.4.1. Olaparib and Eribulin
3.4.2. Olaparib and Paclitaxel
3.4.3. Olaparib and Durvalumab
3.4.4. Olaparib, Durvalumab, and Paclitaxel
4. Talazoparib
4.1. Early-Phase Studies
4.2. Locally Advanced or Metastatic Breast Cancer
4.3. Early-Stage Breast Cancer
5. Biomarkers of Response to PARPi
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEs | adverse events |
| AML | acute myeloid leukemia |
| AUC | area under the receiver operating characteristic curve |
| BC | breast cancer |
| CI | confidence interval |
| Cmax | peak serum concentration |
| CPS + EG | clinical and pathological stage + estrogen receptor status and histological grade |
| CrCl | creatinine clearance |
| gBRCAm | germline BRCA mutation |
| GIS | genomic instability score |
| HER2 | human epidermal growth factor receptor 2 |
| HR | hazard ratio |
| HRD | homologous recombination repair deficiency |
| HRR | homologous recombination repair |
| LOH | loss of heterozygosity |
| MDS | myelodysplastic syndrome |
| ORR | objective response rate |
| OS | overall survival |
| PARP | poly(adenosine diphosphate (ADP)–ribose) polymerase |
| PARPi | poly(adenosine diphosphate (ADP)–ribose) polymerase inhibitors |
| pCR | pathological complete response |
| PFS | progression-free survival |
| PI3K | phosphatidylinositol 3-kinase |
| PI3Ki | phosphatidylinositol 3-kinase inhibitors |
| Sig3 | mutational signature 3 |
| TNBC | triple-negative breast cancer |
References
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A. A synthetic lethal therapeutic approach: Poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.-Y.N.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Buys, S.S.; Sandbach, J.F.; Gammon, A.; Patel, G.; Kidd, J.; Brown, K.L.; Ms, L.S.; Saam, J.; Lancaster, J.; Daly, M.B. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 2017, 123, 1721–1730. [Google Scholar] [CrossRef]
- Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; Wang, Q.; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Hoover, J.; et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016, 44, D862–D868. [Google Scholar] [CrossRef]
- Tutt, A.N.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- FDA Label for Olaparib. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf (accessed on 21 December 2021).
- FDA Label for Rucaparib. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209115s004lbl.pdf (accessed on 21 December 2021).
- FDA Label for Niraparib. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208447s015s017lbledt.pdf (accessed on 21 December 2021).
- FDA Label for Talazoparib. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211651s000lbl.pdf (accessed on 21 December 2021).
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): Post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 710–722. [Google Scholar] [CrossRef]
- Oza, A.M.; Tinker, A.V.; Oaknin, A.; Shapira-Frommer, R.; McNeish, I.A.; Swisher, E.M.; Ray-Coquard, I.; Bell-McGuinn, K.; Coleman, R.L.; O’Malley, D.M.; et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 2017, 147, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Drew, Y.; Ledermann, J.; Hall, G.; Rea, D.; Glasspool, R.; Highley, M.; Jayson, G.; Sludden, J.; Murray, J.; Jamieson, D.; et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br. J. Cancer 2016, 114, 723–730. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.-Y.N.; Renaud, A.; Zhang, Y.; Ji, J.; Takeda, S.; Morris, J.; Teicher, B.; Doroshow, J.H.; Pommier, Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014, 13, 433–443. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 23725625, Olaparib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Olaparib (accessed on 18 August 2022).
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nokihara, H.; Yamada, Y.; Goto, Y.; Tanioka, M.; Shibata, T.; Yamada, K.; Asahina, H.; Kawata, T.; Shi, X.; et al. A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci. 2012, 103, 504–509. [Google Scholar] [CrossRef]
- Bundred, N.; Gardovskis, J.; Jaskiewicz, J.; Eglitis, J.; Paramonov, V.; McCormack, P.; Swaisland, H.; Cavallin, M.; Parry, T.; Carmichael, J.; et al. Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: A phase I multicentre trial in patients scheduled for elective breast cancer surgery. Investig. New Drugs 2013, 31, 949–958. [Google Scholar] [CrossRef]
- Yonemori, K.; Tamura, K.; Kodaira, M.; Fujikawa, K.; Sagawa, T.; Esaki, T.; Shirakawa, T.; Hirai, F.; Yokoi, Y.; Kawata, T.; et al. Safety and tolerability of the olaparib tablet formulation in Japanese patients with advanced solid tumours. Cancer Chemother. Pharmacol. 2016, 78, 525–531. [Google Scholar] [CrossRef]
- Mateo, J.; Moreno, V.; Gupta, A.; Kaye, S.B.; Dean, E.; Middleton, M.R.; Friedlander, M.; Gourley, C.; Plummer, R.; Rustin, G.; et al. An Adaptive Study to Determine the Optimal Dose of the Tablet Formulation of the PARP Inhibitor Olaparib. Target. Oncol. 2016, 11, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Swaisland, H.; Leunen, K.; Van Herpen, C.M.L.; Jerusalem, G.; De Greve, J.; Lolkema, M.P.; Soetekouw, P.; Mau-Sørensen, M.; Nielsen, D.; et al. Olaparib tablet formulation: Effect of food on the pharmacokinetics after oral dosing in patients with advanced solid tumours. Cancer Chemother. Pharmacol. 2015, 76, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Swaisland, H.; Leunen, K.; Rutten, A.; Soetekouw, P.; Slater, S.; Verheul, H.; Fielding, A.; So, K.; Bannister, W.; et al. Effect of Food on the Pharmacokinetics of Olaparib after Oral Dosing of the Capsule Formulation in Patients with Advanced Solid Tumors. Adv. Ther. 2015, 32, 510–522. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca. Available online: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/00997c3f-5912-486f-a7db-930b4639cd51/00997c3f-5912-486f-a7db-930b4639cd51_viewable_rendition__v.pdf (accessed on 19 August 2022).
- Dirix, L.; Swaisland, H.; Verheul, H.M.; Rottey, S.; Leunen, K.; Jerusalem, G.; Rolfo, C.; Nielsen, D.; Molife, L.R.; Kristeleit, R.; et al. Effect of Itraconazole and Rifampin on the Pharmacokinetics of Olaparib in Patients with Advanced Solid Tumors: Results of Two Phase I Open-label Studies. Clin. Ther. 2016, 38, 2286–2299. [Google Scholar] [CrossRef] [PubMed]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Rolfo, C.; Isambert, N.; Italiano, A.; Molife, L.R.; Schellens, J.H.; Blay, J.; Decaens, T.; Kristeleit, R.; Rosmorduc, O.; Demlova, R.; et al. Pharmacokinetics and safety of olaparib in patients with advanced solid tumours and mild or moderate hepatic impairment. Br. J. Clin. Pharmacol. 2020, 86, 1807–1818. [Google Scholar] [CrossRef]
- Rolfo, C.; de Vos-Geelen, J.; Isambert, N.; Molife, L.R.; Schellens, J.H.M.; De Grève, J.; Dirix, L.; Grundtvig-Sørensen, P.; Jerusalem, G.; Leunen, K.; et al. Pharmacokinetics and Safety of Olaparib in Patients with Advanced Solid Tumours and Renal Impairment. Clin. Pharmacokinet. 2019, 58, 1165–1174. [Google Scholar] [CrossRef]
- Zibetti Dal Molin, G.; Westin, S.N.; Msaouel, P.; Gomes, L.M.; Dickens, A.; Coleman, R.L. Discrepancy in calculated and measured glomerular filtration rates in patients treated with PARP inhibitors. Int. J. Gynecol. Cancer 2020, 30, 89–93. [Google Scholar] [CrossRef]
- Bruin, M.A.C.; Korse, C.M.; van Wijnen, B.; de Jong, V.M.T.; Linn, S.C.; van Triest, B.; Rosing, H.; Beijnen, J.H.; Broek, D.V.D.; Huitema, A.D.R. A real or apparent decrease in glomerular filtration rate in patients using olaparib? Eur. J. Clin. Pharmacol. 2021, 77, 179–188. [Google Scholar] [CrossRef]
- Morice, P.-M.; Leary, A.; Dolladille, C.; Chrétien, B.; Poulain, L.; González-Martín, A.; Moore, K.; O’Reilly, E.M.; Ray-Coquard, I.; Alexandre, J. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: A safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021, 8, e122–e134. [Google Scholar] [CrossRef]
- Swaisland, H.; Plummer, R.; So, K.; Garnett, S.; Bannister, W.; Fabre, M.-A.; Dota, C.; Fielding, A. Olaparib does not cause clinically relevant QT/QTc interval prolongation in patients with advanced solid tumours: Results from two phase I studies. Cancer Chemother. Pharmacol. 2016, 78, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dean, E.; Middleton, M.R.; Pwint, T.; Swaisland, H.; Carmichael, J.; Goodege-Kunwar, P.; Ranson, M. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br. J. Cancer 2012, 106, 468–474. [Google Scholar] [CrossRef]
- Liu, J.F.; Tolaney, S.M.; Birrer, M.; Fleming, G.F.; Buss, M.K.; Dahlberg, S.E.; Lee, H.; Whalen, C.; Tyburski, K.; Winer, E.; et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur. J. Cancer 2013, 49, 2972–2978. [Google Scholar] [CrossRef]
- Zimmer, A.S.; Nichols, E.; Cimino-Mathews, A.; Peer, C.; Cao, L.; Lee, M.-J.; Kohn, E.C.; Annunziata, C.M.; Lipkowitz, S.; Trepel, J.B.; et al. A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1-3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. J. Immunother. Cancer 2019, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Cimino-Mathews, A.; Peer, C.J.; Zimmer, A.; Lipkowitz, S.; Annunziata, C.M.; Cao, L.; Harrell, M.I.; Swisher, E.M.; Houston, N.; et al. Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination With Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1-3 Inhibitor Cediranib in Women’s Cancers: A Dose-Escalation, Phase I Study. J. Clin. Oncol. 2017, 35, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.A.; Lindeman, G.J.; Clemons, M.; Wildiers, H.; Chan, A.; McCarthy, N.J.; Singer, C.F.; Lowe, E.S.; Watkins, C.L.; Carmichael, J. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013, 15, R88. [Google Scholar] [CrossRef]
- Yuan, P.; Shentu, J.; Xu, J.; Burke, W.; Hsu, K.; Learoyd, M.; Zhu, M.; Xu, B. Pharmacokinetics and safety of olaparib tablets as monotherapy and in combination with paclitaxel: Results of a Phase I study in Chinese patients with advanced solid tumours. Cancer Chemother. Pharmacol. 2019, 83, 963–974. [Google Scholar] [CrossRef]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021, 39, 989–998.e5. [Google Scholar] [CrossRef] [PubMed]
- Geenen, J.J.J.; Dackus, G.M.H.E.; Schouten, P.C.; Pluim, D.; Marchetti, S.; Sonke, G.S.; Jóźwiak, K.; Huitema, A.D.R.; Beijnen, J.H.; Schellens, J.H.M.; et al. A Phase I dose-escalation study of two cycles carboplatin-olaparib followed by olaparib monotherapy in patients with advanced cancer. Int. J. Cancer 2021, 148, 3041–3050. [Google Scholar] [CrossRef]
- Lee, J.-M.; Peer, C.J.; Yu, M.; Amable, L.; Gordon, N.; Annunziata, C.M.; Houston, N.; Goey, A.K.; Sissung, T.M.; Parker, B.; et al. Sequence-Specific Pharmacokinetic and Pharmacodynamic Phase I/Ib Study of Olaparib Tablets and Carboplatin in Women’s Cancer. Clin. Cancer Res. 2017, 23, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Hays, J.L.; Annunziata, C.M.; Noonan, A.M.; Minasian, L.; Zujewski, J.A.; Yu, M.; Gordon, N.; Ji, J.; Sissung, T.M.; et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J. Natl. Cancer Inst. 2014, 106, dju089. [Google Scholar] [CrossRef] [PubMed]
- Peer, C.J.; Lee, J.-M.; Roth, J.; Rodgers, L.; Nguyen, J.; Annunziata, C.M.; Minasian, L.; Kohn, E.C.; Figg, W.D. Population pharmacokinetic analyses of the effect of carboplatin pretreatment on olaparib in recurrent or refractory women’s cancers. Cancer Chemother. Pharmacol. 2017, 80, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Van der Noll, R.; Jager, A.; Ang, J.E.; Marchetti, S.; Mergui-Roelvink, M.W.J.; Lolkema, M.P.; de Jonge, M.J.A.; van der Biessen, D.A.; Brunetto, A.T.; Arkenau, H.-T.; et al. Phase I study of continuous olaparib capsule dosing in combination with carboplatin and/or paclitaxel (Part 1). Investig. New Drugs 2020, 38, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Van der Noll, R.; Jager, A.; Ang, J.E.; Marchetti, S.; Mergui-Roelvink, M.W.J.; de Bono, J.S.; Lolkema, M.P.; de Jonge, M.J.A.; van der Biessen, D.A.; Brunetto, A.T.; et al. Phase I study of intermittent olaparib capsule or tablet dosing in combination with carboplatin and paclitaxel (part 2). Investig. New Drugs 2020, 38, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Van Der Noll, R.; Marchetti, S.; Steeghs, N.; Beijnen, J.H.; Mergui-Roelvink, M.W.J.; Harms, E.; Rehorst, H.; Sonke, G.; Schellens, J.H.M. Long-term safety and anti-tumour activity of olaparib monotherapy after combination with carboplatin and paclitaxel in patients with advanced breast, ovarian or fallopian tube cancer. Br. J. Cancer 2015, 113, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Scott, C.; Lindeman, G.J.; Hamilton, A.; Lieschke, E.; Gibbs, E.; Asher, R.; Badger, H.; Paterson, R.; Macnab, L.; et al. Phase 1 trial of olaparib and oral cyclophosphamide in BRCA breast cancer, recurrent BRCA ovarian cancer, non-BRCA triple-negative breast cancer, and non-BRCA ovarian cancer. Br. J. Cancer 2019, 120, 279–285. [Google Scholar] [CrossRef]
- Del Conte, G.; Sessa, C.; Von Moos, R.; Viganò, L.; Digena, T.; Locatelli, A.; Gallerani, E.; Fasolo, A.; Tessari, A.; Cathomas, R.; et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br. J. Cancer 2014, 111, 651–659. [Google Scholar] [CrossRef]
- Balmaña, J.; Tung, N.M.; Isakoff, S.J.; Graña, B.; Ryan, P.D.; Saura, C.; Lowe, E.S.; Frewer, P.; Winer, E.; Baselga, J.; et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann. Oncol. 2014, 25, 1656–1663. [Google Scholar] [CrossRef]
- Poveda, A.; Oaknin, A.; Romero, I.; Guerrero-Zotano, A.; Fariñas-Madrid, L.; Rodriguez-Freixinos, V.; Mallol, P.; Lopez-Reig, R.; Lopez-Guerrero, J.A. A phase I dose-finding, pharmacokinetics and genotyping study of olaparib and lurbinectedin in patients with advanced solid tumors. Sci. Rep. 2021, 11, 4433. [Google Scholar] [CrossRef]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.-A.; Park, Y.H.; Delord, J.-P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef]
- Khan, O.A.; Gore, M.; Lorigan, P.; Stone, J.; Greystoke, A.; Burke, W.; Carmichael, J.; Watson, A.J.; McGown, G.; Thorncroft, M.; et al. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br. J. Cancer 2011, 104, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Shimomura, A.; Yasojima, H.; Masuda, N.; Aogi, K.; Takahashi, M.; Naito, Y.; Shimizu, S.; Nakamura, R.; Hashimoto, J.; et al. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur. J. Cancer 2019, 109, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.; Hafez, N.; Doroshow, D.; Sohal, D.; Keedy, V.; Do, K.T.; LoRusso, P.; Jürgensmeier, J.; Avedissian, M.; Sklar, J.; et al. Ceralasertib-Mediated ATR Inhibition Combined With Olaparib in Advanced Cancers Harboring DNA Damage Response and Repair Alterations (Olaparib Combinations). JCO Precis. Oncol. 2021, 5, 1432–1442. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Cheng, S.-C.; Supko, J.G.; Polak, M.; Wahner-Hendrickson, A.E.; Ivy, S.P.; Bowes, B.; Sawyer, H.; Basada, P.; Hayes, M.; et al. Combined PARP and HSP90 inhibition: Preclinical and Phase 1 evaluation in patients with advanced solid tumours. Br. J. Cancer 2022, 126, 1027–1036. [Google Scholar] [CrossRef]
- Do, K.T.; Kochupurakkal, B.S.; Kelland, S.; de Jonge, A.; Hedglin, J.; Powers, A.; Quinn, N.; Gannon, C.; Vuong, L.; Parmar, K.; et al. Phase 1 Combination Study of the CHK1 Inhibitor Prexasertib and the PARP Inhibitor Olaparib in High-grade Serous Ovarian Cancer and Other Solid Tumors. Clin. Cancer Res. 2021, 27, 4710–4716. [Google Scholar] [CrossRef]
- Bendell, J.; O’Reilly, E.M.; Middleton, M.R.; Chau, I.; Hochster, H.; Fielding, A.; Burke, W.; Burris, I.H. Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Ann. Oncol. 2015, 26, 804–811. [Google Scholar] [CrossRef]
- Rajan, A.; Carter, C.A.; Kelly, R.J.; Gutierrez, M.; Kummar, S.; Szabo, E.; Yancey, M.A.; Ji, J.; Mannargudi, B.; Woo, S.; et al. A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin. Cancer Res. 2012, 18, 2344–2351. [Google Scholar] [CrossRef]
- Samol, J.; Ranson, M.; Scott, E.; Macpherson, E.; Carmichael, J.; Thomas, A.; Cassidy, J. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: A phase I study. Investig. New Drugs 2012, 30, 1493–1500. [Google Scholar] [CrossRef]
- Loap, P.; Loirat, D.; Berger, F.; Ricci, F.; Vincent-Salomon, A.; Ezzili, C.; Mosseri, V.; Fourquet, A.; Ezzalfani, M.; Kirova, Y. Combination of Olaparib and Radiation Therapy for Triple Negative Breast Cancer: Preliminary Results of the RADIOPARP Phase 1 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 436–440. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Wulf, G.M.; Barry, W.T.; Birrer, M.; Westin, S.N.; Farooq, S.; Bell-McGuinn, K.M.; Obermayer, E.; Whalen, C.; Spagnoletti, T.; et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann. Oncol. 2017, 28, 512–518. [Google Scholar] [CrossRef]
- Batalini, F.; Xiong, N.; Tayob, N.; Polak, M.; Eismann, J.; Cantley, L.C.; Shapiro, G.I.; Adalsteinsson, V.; Winer, E.P.; Konstantinopoulos, P.A.; et al. Phase 1b Clinical Trial with Alpelisib plus Olaparib for Patients with Advanced Triple-Negative Breast Cancer. Clin. Cancer Res. 2022, 28, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Kristeleit, R.; Michalarea, V.; Pettitt, S.J.; Lim, J.S.; Carreira, S.; Roda, D.; Miller, R.; Riisnaes, R.; Miranda, S.; et al. Phase I Trial of the PARP Inhibitor Olaparib and AKT Inhibitor Capivasertib in Patients with BRCA1/2- and Non-BRCA1/2-Mutant Cancers. Cancer Discov. 2020, 10, 1528–1543. [Google Scholar] [CrossRef]
- Westin, S.N.; Labrie, M.; Litton, J.K.; Blucher, A.; Fang, Y.; Vellano, C.P.; Marszalek, J.R.; Feng, N.; Ma, X.; Creason, A.; et al. Phase Ib Dose Expansion and Translational Analyses of Olaparib in Combination with Capivasertib in Recurrent Endometrial, Triple-Negative Breast, and Ovarian Cancer. Clin. Cancer Res. 2021, 27, 6354–6365. [Google Scholar] [CrossRef]
- Plummer, R.; Verheul, H.M.; De Vos, F.Y.F.L.; Leunen, K.; Molife, L.R.; Rolfo, C.; Grundtvig-Sørensen, P.; De Grève, J.; Rottey, S.; Jerusalem, G.; et al. Pharmacokinetic Effects and Safety of Olaparib Administered with Endocrine Therapy: A Phase I Study in Patients with Advanced Solid Tumours. Adv. Ther. 2018, 35, 1945–1964. [Google Scholar] [CrossRef]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Tischkowitz, M.; Mackay, H.; Swenerton, K.; Robidoux, A.; Tonkin, K.; Hirte, H.; Huntsman, D.; Clemons, M.; Gilks, B.; et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011, 12, 852–861. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Ruddy, K.J.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Li, W.; Tung, N.; Armstrong, A.; et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur. J. Cancer 2019, 120, 20–30. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Fasching, P.A.; Couch, F.J.; Balmaña, J.; Delaloge, S.; Labidi-Galy, I.; Bennett, J.; McCutcheon, S.; Walker, G.; O’Shaughnessy, J.; et al. Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: Phase IIIb LUCY interim analysis. Eur. J. Cancer 2021, 152, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Eikesdal, H.; Yndestad, S.; Elzawahry, A.; Llop-Guevara, A.; Gilje, B.; Blix, E.; Espelid, H.; Lundgren, S.; Geisler, J.; Vagstad, G.; et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann. Oncol. 2021, 32, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Jeruss, J.S.; Tucker, S.L.; Kolli, A.; Newman, L.A.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. 2011, 29, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.; Gelber, R.D.; Phillips, K.-A.; Eisen, A.; Johannsson, O.T.; Rastogi, P.; Cui, K.Y.; Im, S.; Yerushalmi, R.; et al. Prespecified Event-Driven Analysis of Overall Survival in the OlympiA Phase III Trial of Adjuvant Olaparib in Germline BRCA1/2 Mutation Associated Breast Cancer. In ESMO Virtual Plenary. Abstract VP1-2022; ESMO: Lugano, Switzerland, 2022. [Google Scholar]
- Tung, N.M.; Zakalik, D.; Somerfield, M.R.; Hereditary Breast Cancer Guideline Expert Panel. Adjuvant PARP Inhibitors in Patients with High-Risk Early-Stage HER2-Negative Breast Cancer and Germline BRCA Mutations: ASCO Hereditary Breast Cancer Guideline Rapid Recommendation Update. J. Clin. Oncol. 2021, 39, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, A.; Yamashita, S.; Okochi-Takada, E.; Hirakawa, A.; Tsuda, H.; Shimomura, A.; Kojima, Y.; Yonemori, K.; Fujiwara, Y.; Kinoshita, T.; et al. BRCA1 promoter methylation in breast cancer patients is associated with response to olaparib/eribulin combination therapy. Breast Cancer Res. Treat. 2020, 181, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.; Link, T.; Hauke, J.; Seither, F.; Jackisch, C.; Klare, P.; Schmatloch, S.; Hanusch, C.; Huober, J.; Stefek, A.; et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Ann. Oncol. 2021, 32, 49–57. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135565082, Talazoparib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/135565082 (accessed on 18 August 2022).
- De Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef]
- Naito, Y.; Kuboki, Y.; Ikeda, M.; Harano, K.; Matsubara, N.; Toyoizumi, S.; Mori, Y.; Hori, N.; Nagasawa, T.; Kogawa, T. Safety, pharmacokinetics, and preliminary efficacy of the PARP inhibitor talazoparib in Japanese patients with advanced solid tumors: Phase 1 study. Investig. New Drugs 2021, 39, 1568–1576. [Google Scholar] [CrossRef]
- Yu, Y.; Chung, C.; Plotka, A.; Quinn, K.; Shi, H.; Pápai, Z.; Nguyen, L.; Wang, D. A Phase 1 Mass Balance Study of (14) C-Labeled Talazoparib in Patients with Advanced Solid Tumors. J. Clin. Pharmacol. 2019, 59, 1195–1203. [Google Scholar] [CrossRef]
- Durairaj, C.; Chakrabarti, J.; Ferrario, C.; Hirte, H.W.; Babu, S.; Piha-Paul, S.A.; Plotka, A.; Hoffman, J.; Shi, H.; Wang, D.D. The Effect of Renal Impairment on the Pharmacokinetics and Safety of Talazoparib in Patients with Advanced Solid Tumors. Clin. Pharmacokinet. 2021, 60, 921–930. [Google Scholar] [CrossRef]
- Dhawan, M.S.; Bartelink, I.H.; Aggarwal, R.R.; Leng, J.; Zhang, J.Z.; Pawlowska, N.; Terranova-Barberio, M.; Grabowsky, J.A.; Gewitz, A.; Chien, A.J.; et al. Differential Toxicity in Patients with and without DNA Repair Mutations: Phase I Study of Carboplatin and Talazoparib in Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 6400–6410. [Google Scholar] [CrossRef] [Green Version]
- Schafer, E.S.; Rau, R.; Berg, S.L.; Liu, X.; Minard, C.G.; Bishop, A.J.; Romero, J.C.; Hicks, M.J.; Nelson, M.D.; Voss, S.; et al. Phase 1/2 trial of talazoparib in combination with temozolomide in children and adolescents with refractory/recurrent solid tumors including Ewing sarcoma: A Children’s Oncology Group Phase 1 Consortium study (ADVL1411). Pediatr. Blood Cancer 2020, 67, e28073. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.M.; Pappo, A.S.; Sahr, N.; Sykes, A.; Campagne, O.; Stewart, C.F.; Clay, M.R.; Bahrami, A.; McCarville, M.B.; Kaste, S.C.; et al. A phase I trial of talazoparib and irinotecan with and without temozolomide in children and young adults with recurrent or refractory solid malignancies. Eur. J. Cancer 2020, 137, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Elmeliegy, M.; Yu, Y.; Litton, J.K.; Czibere, A.; Wilson, G.G.; Tudor, I.C.; Zheng, J.; Wang, D.D. Exposure-Safety Analyses of Talazoparib in Patients with Advanced Breast Cancer and Germline BRCA1/2 Mutations in the EMBRACA and ABRAZO Trials. J. Clin. Pharmacol. 2020, 60, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Published online 27 November 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 25 May 2022).
- Ettl, J.; Quek, R.; Lee, K.-H.; Rugo, H.; Hurvitz, S.; Gonçalves, A.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.; Martin, M.; et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: Patient-reported outcomes from the EMBRACA phase III trial. Ann. Oncol. 2018, 29, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Antiemetics Version 2.2022; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Turner, N.C.; Telli, M.L.; Rugo, H.S.; Mailliez, A.; Ettl, J.; Grischke, E.-M.; Mina, L.A.; Balmaña, J.; Fasching, P.A.; Hurvitz, S.A.; et al. A Phase II Study of Talazoparib after Platinum or Cytotoxic Nonplatinum Regimens in Patients with Advanced Breast Cancer and Germline BRCA1/2 Mutations (ABRAZO). Clin. Cancer Res. 2019, 25, 2717–2724. [Google Scholar] [CrossRef]
- Hurvitz, S.; Quek, R.; Turner, N.; Telli, M.; Rugo, H.; Mailliez, A.; Ettl, J.; Grischke, E.; Mina, L.; Balmaña, J.; et al. Quality of life with talazoparib after platinum or multiple cytotoxic non-platinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations: Patient-reported outcomes from the ABRAZO phase 2 trial. Eur. J. Cancer 2018, 104, 160–168. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.; Ramirez, D.L.; Murthy, R.K.; Whitman, G.J.; Hess, K.R.; Adrada, B.E.; Moulder, S.L.; Barcenas, C.H.; Valero, V.; et al. A pilot study of neoadjuvant talazoparib for early-stage breast cancer patients with a BRCA mutation. Ann. Oncol. 2016, 27, vi46. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef]
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef]
- Mayer, E.L.; Abramson, V.; Jankowitz, R.; Falkson, C.; Marcom, P.K.; Traina, T.; Carey, L.; Rimawi, M.; Specht, J.; Miller, K.; et al. TBCRC 030: A phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: Evaluating the homologous recombination deficiency (HRD) biomarker. Ann. Oncol. 2020, 31, 1518–1525. [Google Scholar] [CrossRef]
- Blum, J.L.; Laird, A.D.; Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Martin, M.; Roché, H.H.; Lee, K.-H.; Goodwin, A.; et al. Determinants of Response to Talazoparib in Patients with HER2-Negative, Germline BRCA1/2-Mutated Breast Cancer. Clin. Cancer Res. 2022, 28, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Batalini, F.; Gulhan, D.; Mao, V.; Tran, A.; Polak, M.; Xiong, N.; Tayob, N.; Tung, N.M.; Winer, E.P.; Mayer, E.L.; et al. Mutational signature 3 detected from clinical panel sequencing is associated with responses to olaparib in breast and ovarian cancers. Clin. Cancer Res. 2022, CCR-22-0749, (Online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Patsouris, A.; Diop, K.; Tredan, O.; Nenciu, D.; Gonçalves, A.; Arnedos, M.; Sablin, M.-P.; Jézéquel, P.; Jimenez, M.; Droin, N.; et al. Rucaparib in patients presenting a metastatic breast cancer with homologous recombination deficiency, without germline BRCA1/2 mutation. Eur. J. Cancer 2021, 159, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Tovey, H.; Pearson, A.; Cutts, R.; Toms, C.; Proszek, P.; Hubank, M.; Dowsett, M.; Dodson, A.; Daley, F.; et al. Homologous recombination DNA repair deficiency and PARP inhibition activity in primary triple negative breast cancer. Nat. Commun. 2020, 11, 2662. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Balmaña, J.; Poncet, C.; Goulioti, T.; Tryfonidis, K.; Honkoop, A.H.; Zoppoli, G.; Razis, E.; Johannsson, O.T.; Colleoni, M.; et al. Niraparib for Advanced Breast Cancer with Germline BRCA1 and BRCA2 Mutations: The EORTC 1307-BCG/BIG5-13/TESARO PR-30-50-10-C BRAVO Study. Clin. Cancer Res. 2021, 27, 5482–5491. [Google Scholar] [CrossRef]
- Perez-Fidalgo, J.; Cortés, A.; Guerra, E.; García, Y.; Iglesias, M.; Sarmiento, U.B.; García, E.C.; Sánchez, L.M.; Santaballa, A.; Oaknin, A.; et al. Olaparib in combination with pegylated liposomal doxorubicin for platinum-resistant ovarian cancer regardless of BRCA status: A GEICO phase II trial (ROLANDO study). ESMO Open 2021, 6, 100212. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132–1140. [Google Scholar] [CrossRef]
- Han, H.S.; Diéras, V.; Robson, M.; Palácová, M.; Marcom, P.K.; Jager, A.; Bondarenko, I.; Citrin, D.; Campone, M.; Telli, M.L.; et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: Randomized phase II study. Ann. Oncol. 2018, 29, 154–161. [Google Scholar] [CrossRef]
- Xu, J.; Keenan, T.E.; Overmoyer, B.; Tung, N.M.; Gelman, R.S.; Habin, K.; Garber, J.E.; Ellisen, L.W.; Winer, E.P.; Goss, P.E.; et al. Phase II trial of veliparib and temozolomide in metastatic breast cancer patients with and without BRCA1/2 mutations. Breast Cancer Res. Treat. 2021, 189, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J. Clin. Oncol. 2020, 38, 4274–4282. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, A.; Burga, L.N.; Hu, H.; Lunsford, E.P.; Ibrahim, Y.H.; Balmaña, J.; Rajendran, A.; Papa, A.; Spencer, K.; Lyssiotis, C.A.; et al. Combining a PI3K Inhibitor with a PARP Inhibitor Provides an Effective Therapy for BRCA1-Related Breast Cancer. Cancer Discov. 2012, 2, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Gulhan, D.C.; Lee, J.J.-K.; Melloni, G.E.M.; Cortés-Ciriano, I.; Park, P.J. Detecting the mutational signature of homologous recombination deficiency in clinical samples. Nat. Genet. 2019, 51, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Polak, P.; Kim, J.; Braunstein, L.Z.; Karlic, R.; Haradhavala, N.J.; Tiao, G.; Rosebrock, D.; Livitz, D.; Kübler, K.; Mouw, K.W.; et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat. Genet. 2017, 49, 1476–1486. [Google Scholar] [CrossRef]
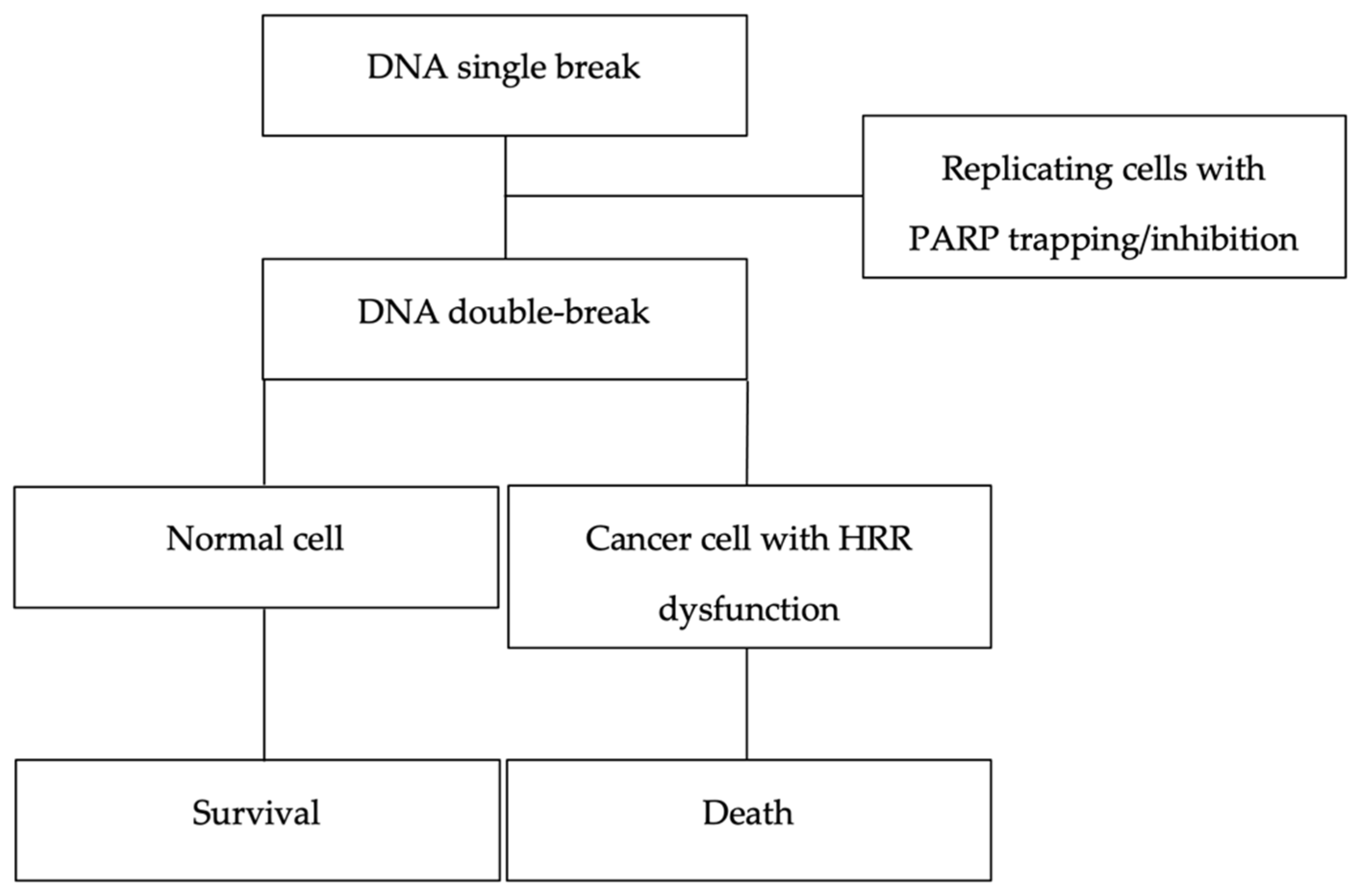
| Pathogenic Variant | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| BRCA1 | 7.62 (5.33–11.27) | <0.001 |
| BRCA2 | 5.23 (4.09–6.77) | <0.001 |
| PALB2 | 3.83 (2.68–5.63) | <0.001 |
| ATM | 1.82 (1.46–2.27) | <0.001 |
| CHEK2 | 2.47 (2.02–3.05) | <0.001 |
| Drug | Indications [14,15,16,17] | Biomarker | Main Trial |
|---|---|---|---|
| Olaparib | Advanced epithelial ovarian * | BRCA1/2 | SOLO-1 [13] (2018) |
| Advanced epithelial ovarian * | BRCA1/2, or GIS | PAOLA-1 [18] (2020) | |
| Recurrent epithelial ovarian * | X | SOLO-2 [19] (2017), Study 19 [20] (2017) | |
| Advanced ovarian | gBRCA1/2 | NCT01078662 [21] (2014) | |
| Metastatic breast: HER2-negative | gBRCA1/2 | OlympiAD [12] (2018) | |
| Metastatic pancreatic adenocarcinoma | gBRCA1/2 | POLO [22] (2019) | |
| Metastatic prostate | ATM, BRCA1/2, BARD1, BRIP1, CDK12, CHEK1/2, FANCL, PALB2, RAD51, RAD54L | PROfound [23] (2020) | |
| Rucaparib | Recurrent epithelial ovarian * | X | ARIEL3 [24] (2018) |
| Epithelial ovarian * | BRCA1/2 | Study 10 and ARIEL2 [25] (2016) | |
| Metastatic prostate | BRCA1/2 | TRITON2 [26] (2020) | |
| Niraparib | Advanced epithelial ovarian * | X | PRIMA [27] (2020) |
| Recurrent epithelial ovarian * | X | NOVA [28] (2017) | |
| Advanced ovarian * | BRCA1/2 or GIS | QUADRA [29] (2019) | |
| Talazoparib | Metastatic/advanced breast: HER2-negative | gBRCA1/2 | EMBRACA [11] (2018) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, M.C.S.; Raheem, F.; Mina, L.; Ernst, B.; Batalini, F. PARP Inhibitors for Breast Cancer: Germline BRCA1/2 and Beyond. Cancers 2022, 14, 4332. https://doi.org/10.3390/cancers14174332
Menezes MCS, Raheem F, Mina L, Ernst B, Batalini F. PARP Inhibitors for Breast Cancer: Germline BRCA1/2 and Beyond. Cancers. 2022; 14(17):4332. https://doi.org/10.3390/cancers14174332
Chicago/Turabian StyleMenezes, Maria Clara Saad, Farah Raheem, Lida Mina, Brenda Ernst, and Felipe Batalini. 2022. "PARP Inhibitors for Breast Cancer: Germline BRCA1/2 and Beyond" Cancers 14, no. 17: 4332. https://doi.org/10.3390/cancers14174332
APA StyleMenezes, M. C. S., Raheem, F., Mina, L., Ernst, B., & Batalini, F. (2022). PARP Inhibitors for Breast Cancer: Germline BRCA1/2 and Beyond. Cancers, 14(17), 4332. https://doi.org/10.3390/cancers14174332






