1. Introduction
Cutaneous T-cell lymphoma (CTCL) is a rare and diverse group of non-Hodgkin’s lymphomas defined by the proliferation of skin-homing T-cells [
1,
2,
3]. Mycosis fungoides (MF), Sézary syndrome (SS), and primary cutaneous anaplastic large cell lymphoma are the most frequent types of CTCL, accounting for roughly 80% of all CTCLs [
4].
Between 1970 and 2000, previous analysis based on the Surveillance, Epidemiology, and End Results (SEER) databases indicated an approximate threefold rise in CTCL incidence in the United States [
5]. Based on historical demographics, CTCL more frequently affects elderly white individuals. However, a recent analysis of a more diverse population in the United States revealed that CTCL in the African American population manifests at a younger age, presents at more aggressive stages, and is associated with a worse prognosis [
6].
Comprehensive demographic analysis and spatial clustering of CTCL cases have been established in a limited number of geographic regions: Texas, Georgia, Pittsburgh, Pennsylvania, Sweden, and Canada [
7,
8,
9,
10,
11,
12,
13]. Thus, while advancements in CTCL diagnosis may contribute to higher case reporting, environmental exposure appears to factor in the constant increase in CTCL incidence. Additionally, evidence of regional clustering supports the existence of potentially preventable risk factors for this uncommon skin cancer.
In this report, we compare the CTCL patient population from the Arkansas Clinical Data Repository (AR-CDR) at the University of Arkansas for Medical Sciences Hospital to the SEER database from the National Cancer Institute to better understand the prevalence and incidence trends that underpin the disparities. Additionally, we employ the AR-CDR to highlight the regional clustering of CTCL cases across Arkansas and the link between spatial clustering and harmful environmental exposures. Finally, our data provide an updated and thorough demographic analysis for CTCL in Arkansas that has never been published previously.
4. Discussion
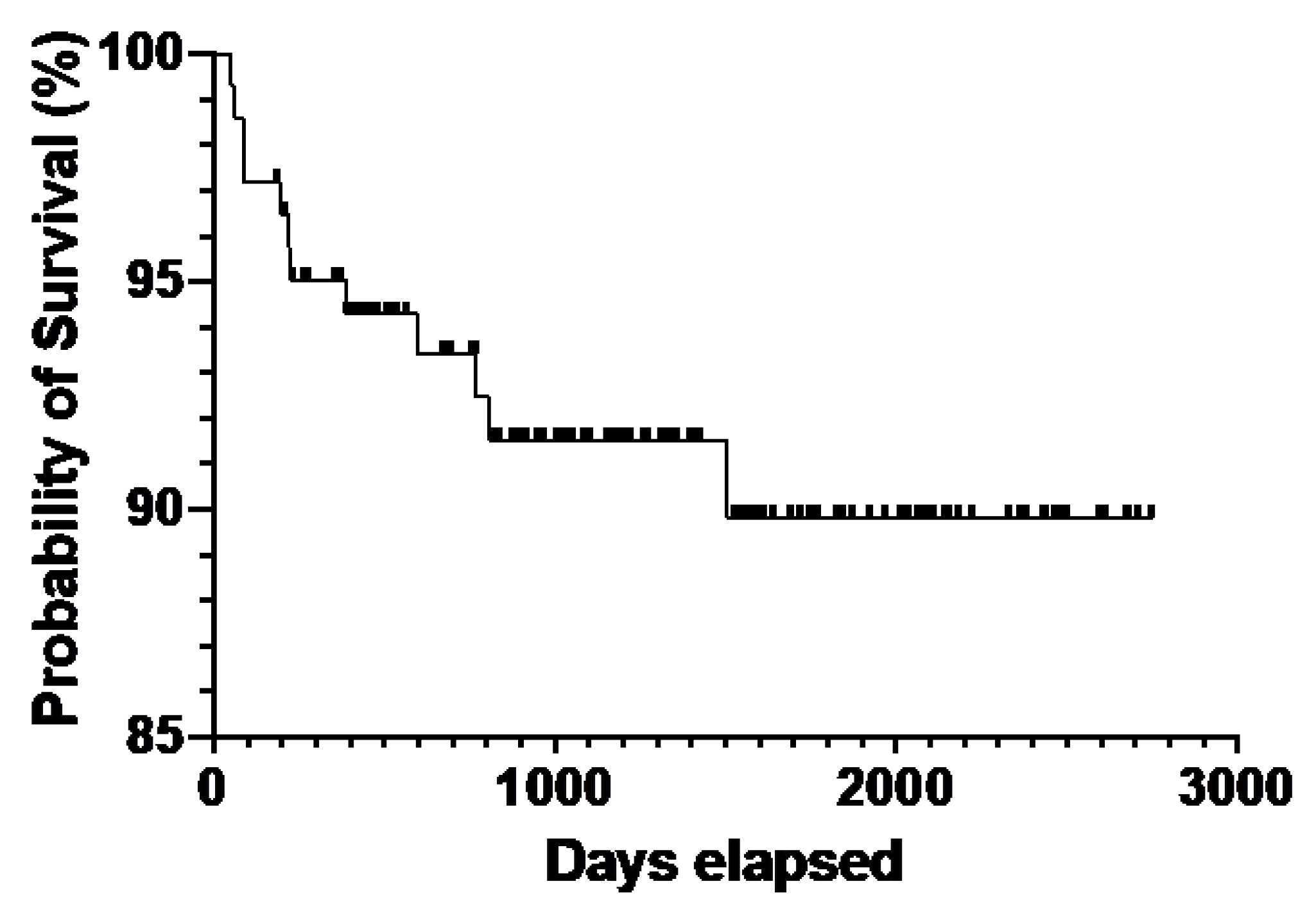
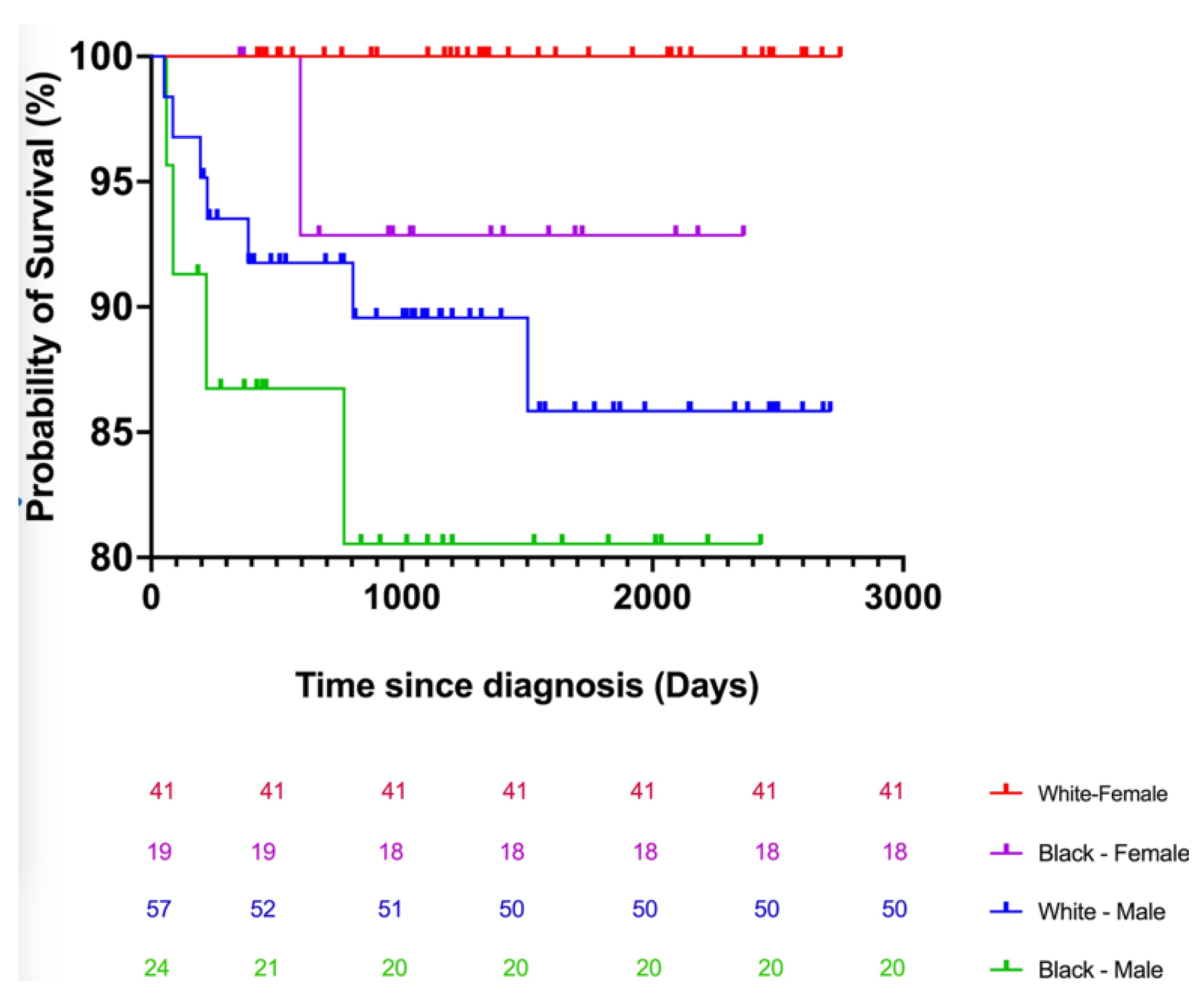
CTCL is an uncommon and clinically heterogeneous cancer with an unclear etiology. An epidemiologic investigation of demographic data and environmental exposure assessment may be essential for gaining insight into developmental risk factors and improving diagnosis and treatment. Additionally, comprehensive demographic studies elucidate a more accurate demographic distribution of the incidence and environmental variables, which will benefit patient diagnosis and treatment. Although most patients in our study sample were white, our results demonstrate that the adjusted incidence of CTCL, particularly MF, is higher in black individuals than in white individuals [
24]. According to a national study by Iman et al., black patients were diagnosed with CTCL at a younger age, presented with more advanced stages of illness, and had a worse survival rate than white patients [
25]. Using the Kaplan–Meier analysis, we estimated the odds of a CTCL patient in Arkansas surviving at least 6 years following diagnosis to be 50% of the probability expected for the general population. In Arkansas, the median age of death for CTCL patients was 70. According to the SEER data, the mortality rate for patients with CTCL has increased significantly in the United States. Most CTCL patients who die are white men over the age of 60. However, black men die from CTCL at an even greater rate than white males. In our Arkansas cohort, African American patients acquired CTCL at a younger age. There is a nearly 10-year difference (10.3 years) between the mean age of white patients (65 years) and African American patients (56 years) in our research sample. This study demonstrates the differential impact of CTCL on young black patients. At UAMS, the mean age of the black male CTCL patient population was the lowest (56.2 years).
Additionally, black patients were diagnosed with CTCL at a younger age than white patients in Arkansas (
Table 3). The stages (IA-IVB) were assigned according to TNMB criteria, with stages IIB-IVB being considered advanced [
26]. In our research sample, black patients in the early stages of sickness were on average 8.3 years younger than white patients. The average age in advanced stages is 58.7 years for black patients compared to 56 in white patients.
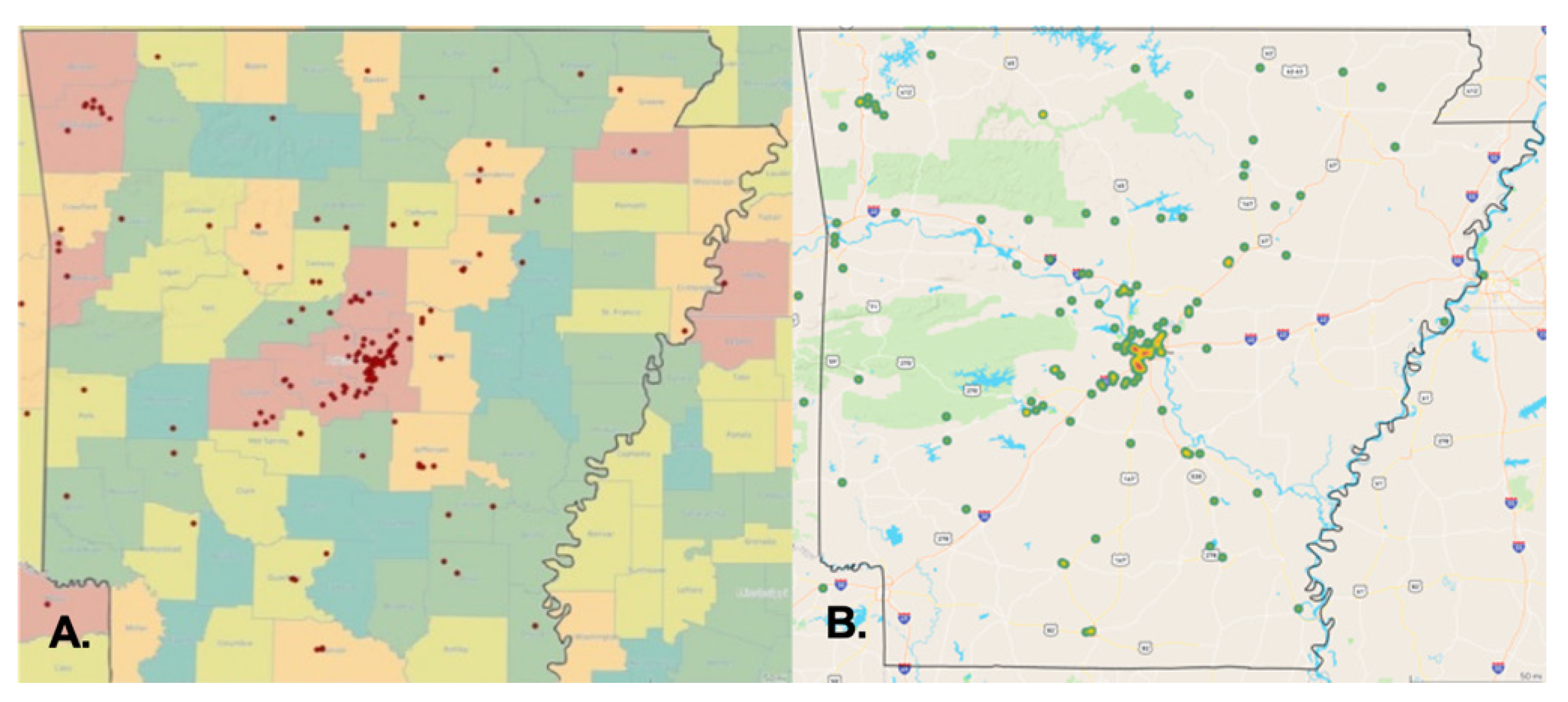
Our findings indicate considerable discrepancies in the demographic characteristics of CTCL patients in Arkansas. We also presented a previously unknown geographical clustering of CTCL instances, including industrial and federal entities that release dangerous chemicals. There is a substantial concentration of instances in central and northwest Arkansas. Specifically, 35% of patients in our research sample live in Pulaski County, Arkansas’s most populated county. We observed local spatial clustering inside Pulaski County, Arkansas’s most densely populated county, next to major interstates and roadways, most notably I-30 and I-430, while mapping patients’ addresses (
Figure 3B)). Local geographic clustering was previously documented in Texas, where clusters of CTCL patients were detected in three distinct locations in the greater Houston region, with CTCL incidence rates ranging from 5 to 20 times the anticipated rate [
7,
8]. As with our results, there have been instances in Houston where patients resided near the same freeway or waterway. In 2005, the same Houston researchers documented a significant sickness epidemic in the Spring, Katy, and Houston Memorial districts linked to an external etiologic agent [
7,
8]. Since the AR-CDR database was developed in 2014, no patient data before 2014 could be analyzed to demonstrate a similar increase. By and large, our geographic clustering of cases supports the hypothesis that unknown external or environmental exposures cause CTCL and other types of CTCL.
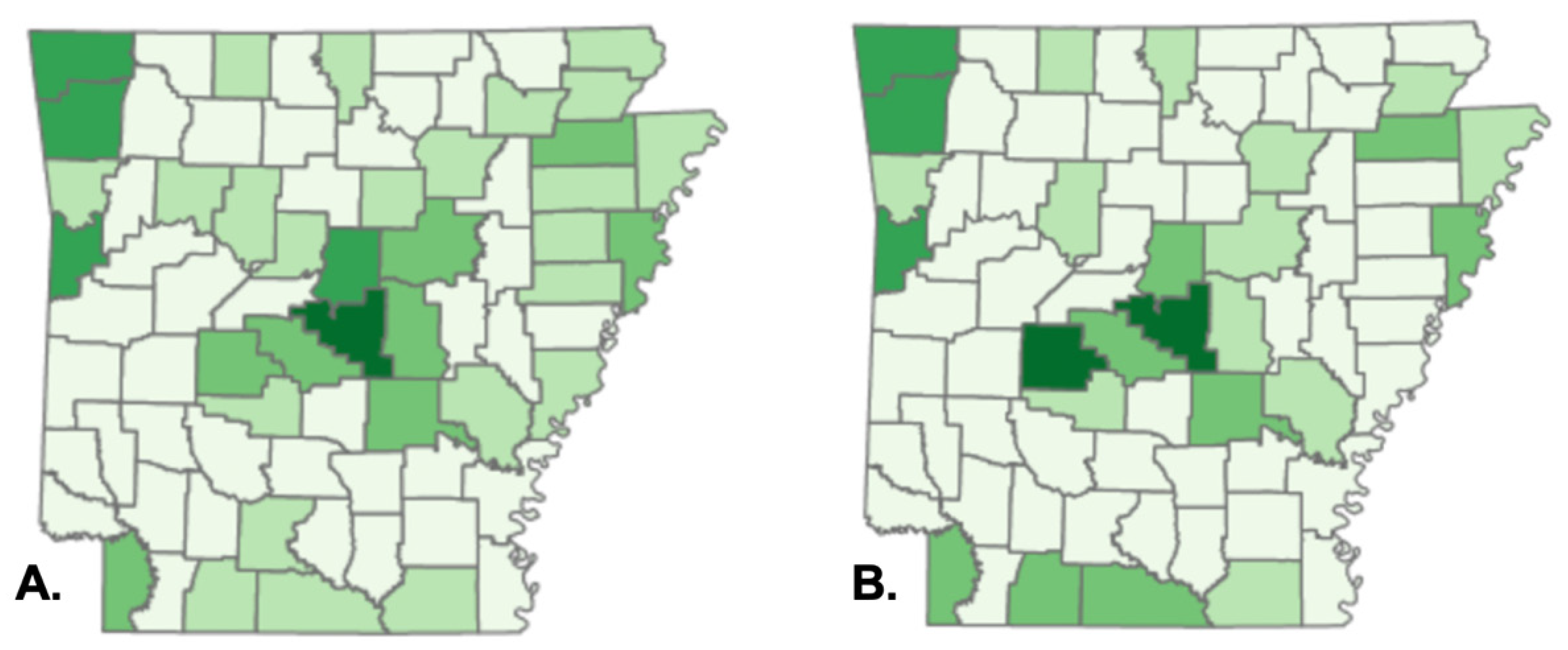
The geographic clustering of our research sample corresponded to a concentration of TRI facilities in central and northwest Arkansas, implying a probable relationship between CTCL cases and proximity to large chemical-emitting plants. The Toxics Release Inventory (TRI) is a program designed by the U.S. Environmental Protection Agency to track the management of hazardous compounds that are potentially damaging to human health and the environment [
27]. The TRI program requires establishments in various industry sectors in the United States to report the quantity of each chemical released or handled by each industry. TRI discloses substances known to cause cancer, have chronic adverse human health effects and severe adverse acute human health effects, or have significant environmental repercussions. TRI facilities include manufacturing, metal mining, electric power generation, chemical manufacturing, and hazardous waste treatment [
28]. Arkansas is ranked 19th in terms of total chemical discharges per square mile out of 56 states and territories [
29]. According to the FDA’s 2019 TRI Factsheet, styrene and creosote are the two most frequently released chemicals in Pulaski County, Arkansas [
30]. Both chemicals are classed as Group 2A carcinogens, or "probably carcinogenic to humans" by the International Agency for Research on Cancer [
31,
32,
33]. Additional research is needed to evaluate whether there is a relationship between exposure to styrene and creosote and CTCL.
In January 2020, clustering of CTCL was associated with increased levels of the environmental contaminants benzene and TCE in Georgia [
9]. The effect of benzene and TCE concentrations on the SIR of CTCL in Arkansas was determined using a simple linear regression analysis. Additional research on the exposure levels of our patient group is necessary to determine a probable link between CTCL and benzene and TCE in Arkansas patients.
The limitations of our study include the retrospective nature of the chart review, which depended on the data entered in the UAMS and SEER clinical databases. In addition, there is variability in how clinical providers labeled CTCL diagnoses and CTCL subtypes with ICD codes; for example, the subtype of CTCL was not available for some patients. However, the majority of the patients in our cohort were coded as mycosis fungoides, which aligns with the most common subtype seen in the national SEER database.