The Therapeutic Role of Plastic and Reconstructive Surgery in the Interdisciplinary Treatment of Soft-Tissue Sarcomas in Germany—Cross-Sectional Results of a Prospective Nationwide Observational Study (PROSa)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
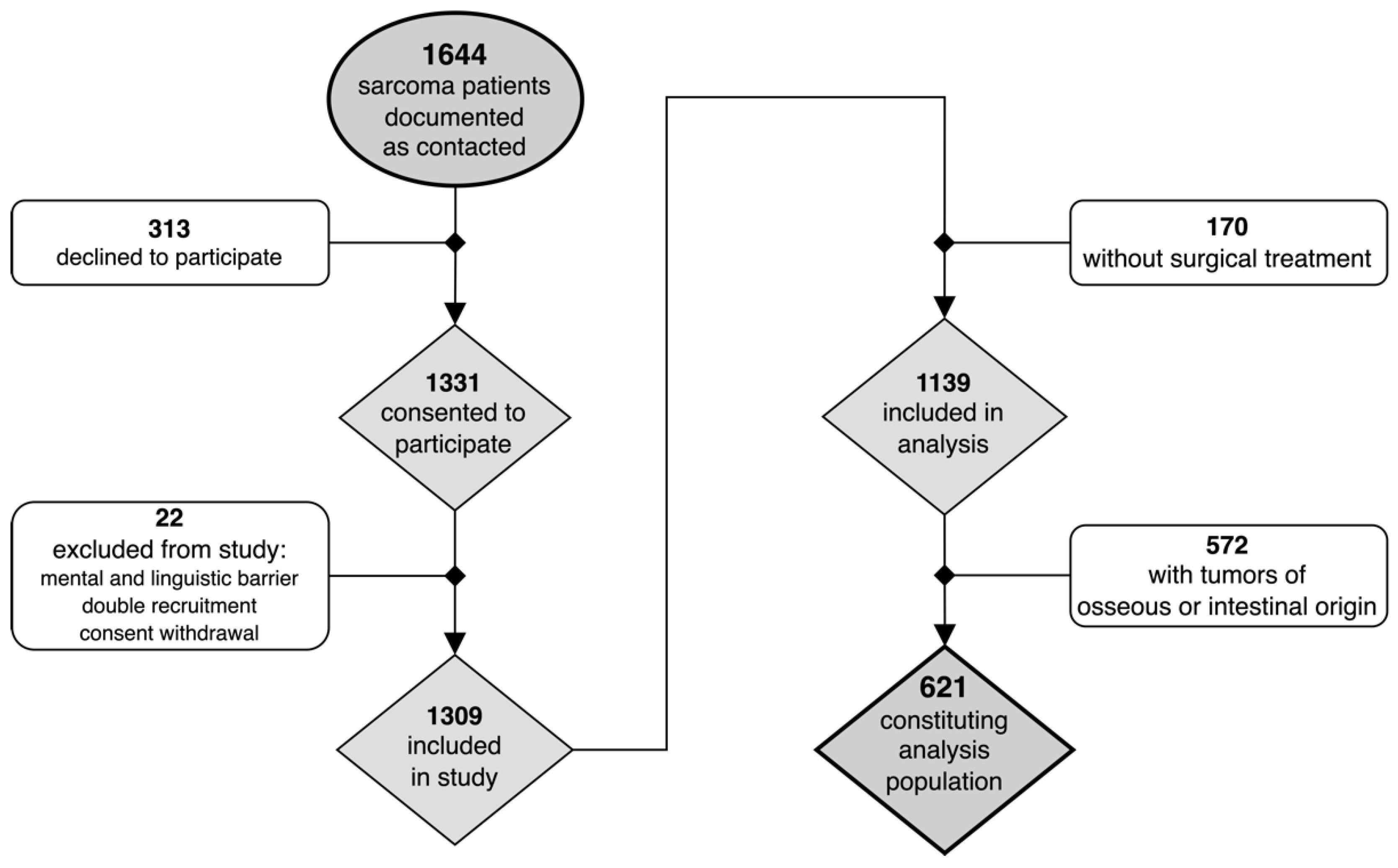
2.1. Study Population
2.2. Explored Variables
2.3. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Treatment Details and Oncological Outcomes
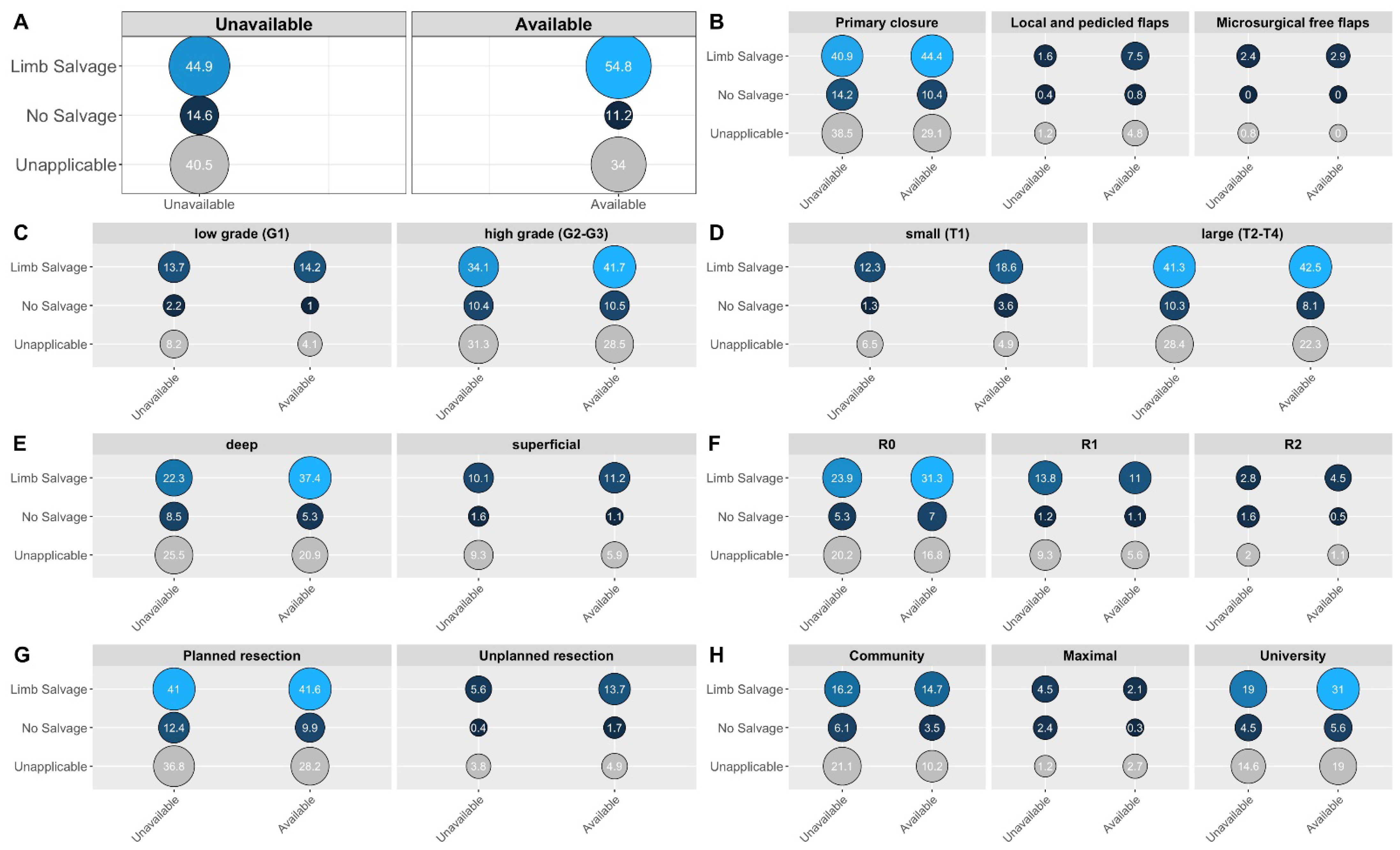
3.3. Univariate Comparisons Regarding Defect Reconstruction
3.4. Multifactorial Logistic Regression Regarding Defect Reconstruction
3.5. Exploratory Subgroup Analyses
4. Discussion
4.1. Main Findings Put in Context
4.2. Further Results Put in Context
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive Epidemiology of Sarcomas in Europe: Report from the RARECARE Project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and Centralised Treatment in Europe of Rare Tumours: Results of RARECAREnet—A Population-Based Study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft Tissue and Visceral Sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Ressing, M.; Wardelmann, E.; Hohenberger, P.; Jakob, J.; Kasper, B.; Emrich, K.; Eberle, A.; Blettner, M.; Zeissig, S.R. Strengthening Health Data on a Rare and Heterogeneous Disease: Sarcoma Incidence and Histological Subtypes in Germany. BMC Public Health 2018, 18, 235. [Google Scholar] [CrossRef] [PubMed]
- Saltus, C.W.; Calingaert, B.; Candrilli, S.; Lorenzo, M.; D’yachkova, Y.; Otto, T.; Wagner, U.; Kaye, J.A. Epidemiology of Adult Soft-Tissue Sarcomas in Germany. Sarcoma 2018, 2018, 5671926. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons Learned from the Study of 10,000 Patients with Soft Tissue Sarcoma. Ann. Surg. 2014, 260, 416–422. [Google Scholar] [CrossRef]
- Clark, M.A.; Fisher, C.; Judson, I.; Thomas, J.M. Soft-Tissue Sarcomas in Adults. N. Engl. J. Med. 2005, 353, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, A.; Leung, D.H.Y.; Hoos, A.; Jaques, D.P.; Lewis, J.J.; Brennan, M.F. Analysis of the Prognostic Significance of Microscopic Margins in 2,084 Localized Primary Adult Soft Tissue Sarcomas. Ann. Surg. 2002, 235, 424–434. [Google Scholar] [CrossRef]
- Byerly, S.; Chopra, S.; Nassif, N.A.; Chen, P.; Sener, S.F.; Eisenberg, B.L.; Tseng, W.W. The Role of Margins in Extremity Soft Tissue Sarcoma. J. Surg. Oncol. 2016, 113, 333–338. [Google Scholar] [CrossRef]
- Bowden, L.; Boohrer, R.J. The Principles and Technique of Resection of Soft Parts for Sarcoma. Surgery 1958, 44, 963–977. [Google Scholar] [CrossRef]
- Cantin, J.; McNeer, G.P.; Chu, F.C.; Booher, R.J. The Problem of Local Recurrence after Treatment of Soft Tissue Sarcoma. Ann. Surg. 1968, 168, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Suit, H.D.; Russell, W.O.; Martin, R.G. Sarcoma of Soft Tissue: Clinical and Histopathologic Parameters and Response to Treatment. Cancer 1975, 35, 1478–1483. [Google Scholar] [CrossRef]
- Abbas, J.S. The Surgical Treatment and Outcome of Soft-Tissue Sarcoma. Arch. Surg. 1981, 116, 765. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennan, M.; DeMoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The Treatment of Soft-Tissue Sarcomas of the Extremities: Prospective Randomized Evaluations of (1) Limb-Sparing Surgery plus Radiation Therapy Compared with Amputation and (2) the Role of Adjuvant Chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Steinau, H.U.; Biemer, E. Resection Method and Functional Reconstructive Surgery of Malignant Soft Tissue Tumors of the Extremities. Langenbeck’s Arch. Surg. 1990, 375, 239–245. [Google Scholar] [CrossRef]
- Williard, W.C.; Hajduu, S.I.; Casper, E.S.; Brennan, M.F. Comparison of Amputation with Limb-Sparing Operations for Adult Soft Tissue Sarcoma of the Extremity. Ann. Surg. 1992, 215, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; DeLaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized Prospective Study of the Benefit of Adjuvant Radiation Therapy in the Treatment of Soft Tissue Sarcomas of the Extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef]
- Pisters, P.W.T. Combined Modality Treatment of Extremity Soft Tissue Sarcomas. Ann. Surg. Oncol. 1998, 5, 464–472. [Google Scholar] [CrossRef]
- Ferraro, P.; Cugno, S.; Liberman, M.; Danino, M.A.; Harris, P.G. Principles of Chest Wall Resection and Reconstruction. Thorac. Surg. Clin. 2010, 20, 465–473. [Google Scholar] [CrossRef]
- Harati, K.; Kolbenschlag, J.; Behr, B.; Goertz, O.; Hirsch, T.; Kapalschinski, N.; Ring, A.; Lehnhardt, M.; Daigeler, A. Thoracic Wall Reconstruction after Tumor Resection. Front. Oncol. 2015, 5, 247. [Google Scholar] [CrossRef]
- Johansen, R.; Nielsen, O.S.; Keller, J. Functional Outcome in Sarcomas Treated with Limb-Salvage Surgery or Amputation. Sarcoma 1998, 2, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, L.H.; Bauer, H.C.F.; Jebsen, N.L.; Follerås, G.; Allert, C.; Haugen, G.S.; Hall, K.S. Limb-Sparing Surgery Preserves More Function than Amputation. J. Bone Joint. Surg. Br. 2008, 90, 786–794. [Google Scholar] [CrossRef]
- Skibber, J.M.; Lotze, M.T.; Seipp, C.A.; Salcedo, R.; Rosenberg, S.A. Limb-Sparing Surgery for Soft Tissue Sarcomas: Wound Related Morbidity in Patients Undergoing Wide Local Excision. Surgery 1987, 102, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.A.; Carrasquillo, I. The Treatment of Lower Extremity Sarcomas with Wide Excision, Radiotherapy, and Free-Flap Reconstruction. Plast. Reconstr. Surg. 1992, 89, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.G.; Pairolero, P.C. Chest-Wall Reconstruction: An Account of 500 Consecutive Patients. Plast. Reconstr. Surg. 1996, 98, 804–810. [Google Scholar] [CrossRef]
- Lohman, R.F.; Nabawi, A.S.; Reece, G.P.; Pollock, R.E.; Evans, G.R.D. Soft Tissue Sarcoma of the Upper Extremity: A 5-Year Experience at Two Institutions Emphasizing the Role of Soft Tissue Flap Reconstruction. Cancer 2002, 94, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Subramanian, V.; Yousef, A.; Rogers, B.A.; Robb, G.L.; Chang, D.W. Upper Extremity Limb Salvage with Microvascular Reconstruction in Patients with Advanced Sarcoma. Plast. Reconstr. Surg. 2004, 114, 400–408. [Google Scholar] [CrossRef]
- Agrawal, N.; Wan, D.; Bryan, Z.; Boehmler, J.; Miller, M.; Tiwari, P. Outcomes Analysis of the Role of Plastic Surgery in Extremity Sarcoma Treatment. J. Reconstr. Microsurg. 2013, 29, 107–112. [Google Scholar] [CrossRef]
- Usui, M.; Ishii, S.; Yamamura, M.; Minami, A.; Sakuma, T. Microsurgical Reconstructive Surgery Following Wide Resection of Bone and Soft Tissue Sarcomas in the Upper Extremities. J. Reconstr. Microsurg. 1986, 2, 77–84. [Google Scholar] [CrossRef]
- Krag, D.N.; Klein, H.; Schneider, P.D.; Goodnight, J.E. Composite Tissue Transfer in Limb-Salvage Surgery. Arch. Surg. 1991, 126, 639. [Google Scholar] [CrossRef]
- Steinau, H.-U.; Daigeler, A.; Langer, S.; Steinsträsser, L.; Hauser, J.; Goertz, O.; Lehnhardt, M. Limb Salvage in Malignant Tumors. Semin. Plast. Surg. 2010, 24, 018–033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barwick, W.J.; Goldberg, J.A.; Scully, S.P.; Harrelson, J.M. Vascularized Tissue Transfer for Closure of Irradiated Wounds after Soft Tissue Sarcoma Resection. Ann. Surg. 1992, 216, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Saddegh, M.K.; Bauer, H.C.F. Wound Complication in Surgery of Soft Tissue Sarcoma: Analysis of 103 Consecutive Patients Managed without Adjuvant Therapy. Clin. Orthop. Relat. Res. 1993, 289, 247–253. [Google Scholar] [CrossRef]
- Penna, V.; Iblher, N.; Momeni, A.; Stark, G.B.; Bannasch, H. Free Tissue Transfer in Reconstruction Following Soft Tissue Sarcoma Resection. Microsurgery 2011, 31, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Viñals, J.; Rodrigues, T.; Sildenikova, D.; Payro, J.; Porté, J.; Suñe, C.; Ojeda, A.; Vidal, J.; Dewever, M.; Lopez, C.; et al. Indications of Microsurgery in Soft Tissue Sarcomas. J. Reconstr. Microsurg. 2012, 28, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Abouarab, M.H.; Salem, I.L.; Degheidy, M.M.; Henn, D.; Hirche, C.; Eweida, A.; Uhl, M.; Kneser, U.; Kremer, T. Therapeutic Options and Postoperative Wound Complications after Extremity Soft Tissue Sarcoma Resection and Postoperative External Beam Radiotherapy. Int. Wound J. 2018, 15, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Losken, A.; Thourani, V.H.; Carlson, G.W.; Jones, G.E.; Culbertson, J.H.; Miller, J.I.; Mansour, K.A. A Reconstructive Algorithm for Plastic Surgery Following Extensive Chest Wall Resection. Br. J. Plast. Surg. 2004, 57, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Netscher, D.T.; Baumholtz, M.A. Chest Reconstruction: I. Anterior and Anterolateral Chest Wall and Wounds Affecting Respiratory Function. Plast. Reconstr. Surg. 2009, 124, 240–252. [Google Scholar] [CrossRef]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, A. S3-Leitlinie Adulte Weichgewebesarkome, Langversion Version 1.1, 2022, AWMF-Registernummer: 032/044OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/adulte-weichgewebesarkome/ (accessed on 30 August 2022).
- Soomers, V.L.M.N.; van der Graaf, W.T.A.; Zaidi, S.; Kaal, S.E.J.; Hayes, A.J.; Schreuder, B.H.W.B.; Jones, R.L.; Desar, I.M.E.; Husson, O. The Route to Diagnosis of Sarcoma Patients: Results from an Interview Study in the Netherlands and the United Kingdom. PLoS ONE 2020, 15, e0243439. [Google Scholar] [CrossRef]
- Soomers, V.; Husson, O.; Young, R.; Desar, I.; Van Der Graaf, W. The Sarcoma Diagnostic Interval: A Systematic Review on Length, Contributing Factors and Patient Outcomes. ESMO Open 2020, 5, e000592. [Google Scholar] [CrossRef]
- Marré, D.; Buendía, J.; Hontanilla, B. Complications Following Reconstruction of Soft-Tissue Sarcoma: Importance of Early Participation of the Plastic Surgeon. Ann. Plast. Surg. 2012, 69, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; Jakob, J.; Singer, S.; Grützmann, R.; et al. The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of a Nationwide Observational Study (PROSa). Cancers 2020, 12, 3590. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Andreou, D.; Golcher, H.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Pink, D.; Jakob, J.; Ashmawy, H.; et al. Utilization of Interdisciplinary Tumor Boards for Sarcoma Care in Germany: Results from the PROSa Study. Oncol. Res. Treat. 2021, 44, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Singer, S.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; Jakob, J.; Grützmann, R.; et al. The Association of Health-Related Quality of Life and 1-Year-Survival in Sarcoma Patients—Results of a Nationwide Observational Study (PROSa). Br. J. Cancer 2022, 126, 1346–1354. [Google Scholar] [CrossRef]
- Slump, J.; Hofer, S.O.P.; Ferguson, P.C.; Wunder, J.S.; Griffin, A.M.; Hoekstra, H.J.; Bastiaannet, E.; O’Neill, A.C. Flap Reconstruction Does Not Increase Complication Rates Following Surgical Resection of Extremity Soft Tissue Sarcoma. Eur. J. Surg. Oncol. 2018, 44, 251–259. [Google Scholar] [CrossRef]
- Götzl, R.; Sterzinger, S.; Arkudas, A.; Boos, A.M.; Semrau, S.; Vassos, N.; Grützmann, R.; Agaimy, A.; Hohenberger, W.; Horch, R.E.; et al. The Role of Plastic Reconstructive Surgery in Surgical Therapy of Soft Tissue Sarcomas. Cancers 2020, 12, 3534. [Google Scholar] [CrossRef]
- Dadras, M.; Koepp, P.; Wallner, C.; Wagner, J.M.; Sogorski, A.; Lehnhardt, M.; Harati, K.; Behr, B. Predictors of Oncologic Outcome in Patients with and without Flap Reconstruction after Extremity and Truncal Soft Tissue Sarcomas. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1239–1252. [Google Scholar] [CrossRef]
- Dadras, M.; Koepp, P.; Wallner, C.; Wagner, J.M.; Sogorski, A.; Lehnhardt, M.; Harati, K.; Behr, B. Wound Complications Are a Predictor of Worse Oncologic Outcome in Extremity Soft Tissue Sarcomas. Surg. Oncol. 2020, 33, 126–134. [Google Scholar] [CrossRef]
- Althubaiti, G.; Butler, C.E. Abdominal Wall and Chest Wall Reconstruction. Plast. Reconstr. Surg. 2014, 133, 688–701. [Google Scholar] [CrossRef]
- Momeni, A.; Kovach, S.J. Important Considerations in Chest Wall Reconstruction. J. Surg. Oncol. 2016, 113, 913–922. [Google Scholar] [CrossRef]
- Salo, J.T.K.; Tukiainen, E.J. Oncologic Resection and Reconstruction of the Chest Wall: A 19-Year Experience in a Single Center. Plast. Reconstr. Surg. 2018, 142, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Slump, J.; Bastiaannet, E.; Halka, A.; Hoekstra, H.J.; Ferguson, P.C.; Wunder, J.S.; Hofer, S.O.P.; O’Neill, A.C. Risk Factors for Postoperative Wound Complications after Extremity Soft Tissue Sarcoma Resection: A Systematic Review and Meta-Analyses. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.K.; Adams, S.C.; Pitcher, D.J.; Temple, T.H. Local Recurrence of Disease after Unplanned Excisions of High-Grade Soft Tissue Sarcomas. Clin. Orthop. Relat. Res. 2008, 466, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Tunn, P.-U.; Goldenitsch, E.; Posch, F.; Szkandera, J.; Bergovec, M.; Liegl-Atzwanger, B.; Leithner, A. The Prognostic Impact of Unplanned Excisions in a Cohort of 728 Soft Tissue Sarcoma Patients: A Multicentre Study. Ann. Surg. Oncol. 2017, 24, 1596–1605. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ogura, K.; Healey, J. Greater Travel Distance to Specialized Facilities Is Associated with Higher Survival for Patients with Soft-Tissue Sarcoma: US Nationwide Patterns. PLoS ONE 2021, 16, e0252381. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.; Alvegård, T.A.; Berlin, Ö.; Ranstam, J.; Rydholm, A.; Rööser, B.; Stener, B. Surgical Margin in Soft Tissue Sarcoma The Scandinavian Sarcoma Group Experience. Acta Orthop. Scand. 1989, 60, 687–692. [Google Scholar] [CrossRef]
- Trovik, C.; Bauer, H.C.F.; Styring, E.; Sundby Hall, K.; Vult Von Steyern, F.; Eriksson, S.; Johansson, I.; Sampo, M.; Laitinen, M.; Kalén, A.; et al. The Scandinavian Sarcoma Group Central Register: 6,000 Patients after 25 Years of Monitoring of Referral and Treatment of Extremity and Trunk Wall Soft-Tissue Sarcoma. Acta Orthop. 2017, 88, 341–347. [Google Scholar] [CrossRef] [PubMed]
| Sarcoma Subtypes | Frequency | Percentage |
|---|---|---|
| Liposarcoma | n = 163 | 26.2% |
| Fibroblastic, myofibroblastic, fibrohistiocytic sarcoma | n = 123 | 19.8% |
| Leiomyosarcoma | n = 72 | 11.6% |
| Synovial sarcoma | n = 40 | 6.4% |
| Vascular tumors | n = 15 | 2.4% |
| Nerve-sheath tumors | n = 15 | 2.4% |
| Tumors of uncertain differentiation | n = 15 | 2.4% |
| Skeletal muscle tumors | n = 11 | 1.8% |
| Extraosseous chondrosarcoma | n = 11 | 1.8% |
| Extraosseous ewing sarcoma | n = 6 | 1.0% |
| Extraosseous osteosarcoma | n = 4 | 0.6% |
| Stroma sarcoma | n = 1 | 0.2% |
| Unclassified sarcoma | n = 145 | 23.3% |
| Flap Types | Frequency | Percentage |
|---|---|---|
| Local and pedicled flaps (LPFs): | n = 52 | 68.4% |
| Local muscle flap | n = 21 | 40.4% |
| Local skin flap | n = 19 | 36.5% |
| Pedicled Latissimus dorsi muscle flap | n = 4 | 7.7% |
| Pedicled ALT flap | n = 2 | 3.8% |
| Pedicled Sartorius muscle flap | n = 2 | 3.8% |
| Pedicled Gluteus muscle flap | n = 2 | 3.8% |
| Pedicled Pectoralis muscle flap | n = 1 | 1.9% |
| Pedicled freestyle perforator flap | n = 1 | 1.9% |
| Free microsurgical flaps (FFs): | n = 24 | 31.6% |
| Free Latissimus dorsi muscle flap | n = 9 | 37.5% |
| Free ALT flap | n = 6 | 25.0% |
| Free PSC flap | n = 5 | 20.8% |
| Free VRAM muscle flap | n = 1 | 4.2% |
| Free TAP flap | n = 1 | 4.2% |
| Free TFL muscle flap | n = 1 | 4.2% |
| Free upper arm flap | n = 1 | 4.2% |
| Entire Cohort | Defect Reconstruction | Primary Closure | Univariate Odds Ratios | Univariate PError Robability | |
|---|---|---|---|---|---|
| 621 (100%) | 76 (12.2%) | 545 (87.8%) | |||
| Tumor characteristics | |||||
| Tumor grading (n = 477 §): | 0.077 | ||||
| High grade (G2–3) | 376 (78.8%) | 55 (87.3%) | 321 (77.5%) | Reference | |
| Low grade (G1) | 101 (21.2%) | 8 (12.7%) | 93 (22.5%) | 0.51 (0.22; 1.06) | |
| Unclassified § | 144 § (23.2%) | 13 (17.1%) | 131 (24.0%) | ||
| Tumor size (n = 402 $): | 0.028 | ||||
| Large (T2–4) | 304 (75.6%) | 36 (63.2%) | 268 (77.7%) | Reference | |
| Small (T1) | 98 (24.4%) | 21 (36.8%) | 77 (22.3%) | 2.03 (1.10; 3.67) | |
| Unclassified $ | 219 $ (35.3%) | 19 (25.0%) | 200 (36.7%) | ||
| Tumor location (n = 621): | 0.069 | ||||
| Trunk | 212 (34.1%) | 17 (22.4%) | 195 (35.8%) | Reference | |
| Pelvis and groin | 77 (12.4%) | 7 (9.2%) | 70 (12.8%) | ||
| Abdominal wall, lower back, retroperitoneum | 72 (11.6%) | 1 (1.3%) | 71 (13.0%) | ||
| Thoracic wall and upper back | 63 (10.1%) | 9 (11.8%) | 54 (9.9%) | ||
| Extremities | 366 (58.9%) | 53 (69.7%) | 313 (57.4%) | 1.93 (1.11; 3.53) | |
| Lower Extremity | 308 (49.8%) | 44 (57.9%) | 264 (48.6%) | 1.76 (1.02; 3.14) | |
| Thigh | 233 (37.6%) | 24 (31.6%) | 209 (38.5%) | ||
| Lower leg | 55 (8.9%) | 14 (18.4%) | 41 (7.6%) | ||
| Foot and ankle | 20 (3.2%) | 6 (7.9%) | 14 (2.6%) | ||
| Upper Extremity | 58 (9.4%) | 9 (11.8%) | 49 (9.0%) | 1.96 (0.80; 4.48) | |
| Upper arm | 33 (5.3%) | 6 (7.9%) | 27 (5.0%) | ||
| Forearm | 18 (2.9%) | 1 (1.3%) | 17 (3.1%) | ||
| Hand | 7 (1.1%) | 2 (2.6%) | 5 (0.9%) | ||
| Other incl. head and neck | 43 (6.9%) | 6 (7.9%) | 37 (6.8%) | 1.88 (0.63; 4.92) | |
| Tumor depth (n = 497 †): | 0.177 | ||||
| Deep | 377 (75.9%) | 43 (68.3%) | 334 (77.0%) | Reference | |
| Superficial | 120 (24.1%) | 20 (31.7%) | 100 (23.0%) | 1.56 (0.86; 2.74) | |
| Unclassified † | 124 † (20.0%) | 13 (17.1%) | 111 (20.4%) | ||
| Patient characteristics | |||||
| Gender (n = 621): | 0.575 | ||||
| Female | 276 (44.4%) | 31 (40.8%) | 245 (45.0%) | Reference | |
| Male | 345 (55.6%) | 45 (59.2%) | 300 (55.0%) | 1.18 (0.73; 1.94) | |
| Age at diagnosis (years) (n = 621): | 54.0 (15.1) | 57.5 (14.6) | 53.5 (15.2) | 1.02 (1.00; 1.04) | 0.027 |
| Age at baseline (years) (n = 621): | 58.2 (14.8) | 62.5 (14.8) | 57.6 (14.7) | 1.02 (1.01; 1.04) | 0.008 |
| Year of first resection (scaled) (n = 621): | 0.00 (4.87) | 0.11 (4.61) | −0.78 (6.41) | 0.97 (0.93; 1.01) | 0.246 |
| Disease status at baseline (n = 621): | 0.001 | ||||
| In remission | 335 (53.9%) | 51 (67.1%) | 284 (52.1%) | Reference | |
| Stable disease | 147 (23.7%) | 7 (9.2%) | 140 (25.7%) | 0.28 (0.11; 0.61) | |
| Progression | 95 (15.3%) | 8 (10.5%) | 87 (16.0%) | 0.52 (0.22; 1.09) | |
| Unclear | 44 (7.1%) | 10 (13.2%) | 34 (6.2%) | ||
| Treatment characteristics | |||||
| Treatment intention at baseline (n = 621): | 0.013 | ||||
| Curative | 486 (78.3%) | 69 (90.8%) | 417 (76.5%) | Reference | |
| Palliative | 127 (20.5%) | 7 (9.2%) | 120 (22.0%) | 0.36 (0.15; 0.75) | |
| Unclear | 8 (1.3%) | 0 (0.00%) | 8 (1.5%) | ||
| Treatment status at baseline (n = 621): | 0.503 | ||||
| Completed | 409 (65.9%) | 54 (71.1%) | 355 (65.1%) | Reference | |
| Active | 145 (23.3%) | 18 (23.7%) | 127 (23.3%) | 0.94 (0.52; 1.63) | |
| Planned | 44 (7.1%) | 3 (4.0%) | 41 (7.5%) | 0.50 (0.11; 1.46) | |
| Paused | 23 (3.7%) | 1 (1.3%) | 22 (4.0%) | 0.34 (0.01; 1.67) | |
| Type of first treatment (n = 621): | 0.882 | ||||
| Surgery | 492 (79.2%) | 63 (82.9%) | 429 (78.7%) | Reference | |
| Chemotherapy | 79 (12.7%) | 8 (10.5%) | 71 (13.0%) | 0.78 (0.33; 1.61) | |
| Radiotherapy | 42 (6.8%) | 4 (5.3%) | 38 (7.0%) | 0.74 (0.21; 1.93) | |
| Unknown or unclear | 8 (1.3%) | 1 (1.3%) | 7 (1.3%) |
| Entire Cohort | Defect Reconstruction | Primary Closure | Univariate Odds Ratios | Univariate Error Probability | |
|---|---|---|---|---|---|
| 621 (100%) | 76 (12.2%) | 545 (87.8%) | |||
| Details of surgical treatment | |||||
| Facility type upon first resection (n = 621): | 0.056 | ||||
| University hospital | 302 (48.6%) | 28 (36.8%) | 274 (50.3%) | Reference | |
| Hospital of maximum care | 39 (6.3%) | 4 (5.3%) | 35 (6.4%) | 1.15 (0.32; 3.18) | |
| Community hospital | 213 (34.3%) | 33 (43.4%) | 180 (33.0%) | 1.79 (1.04; 3.09) | |
| Private practice | 33 (5.3%) | 8 (10.5%) | 25 (4.6%) | 3.15 (1.22; 7.46) | |
| Unknown or unclear | 34 (5.5%) | 3 (4.0%) | 31 (5.7%) | ||
| Nature of first resection (n = 621): | 0.009 | ||||
| Planned | 485 (78.1%) | 50 (65.8%) | 435 (79.8%) | Reference | |
| Unplanned | 136 (21.9%) | 26 (34.2%) | 110 (20.2%) | 2.06 (1.21; 3.44) | |
| Margins achieved in first resection (n = 621): | 0.041 | ||||
| R0 | 328 (52.8%) | 34 (44.7%) | 294 (53.9%) | Reference | |
| R1 | 126 (20.3%) | 26 (34.2%) | 100 (18.3%) | 2.25 (1.27; 3.93) | |
| R2 | 39 (6.3%) | 5 (6.6%) | 34 (6.2%) | 1.30 (0.42; 3.31) | |
| Rx | 38 (6.1%) | 3 (4.0%) | 35 (6.4%) | 0.77 (0.17; 2.32) | |
| Unknown or unclear | 90 (14.5%) | 8 (10.5%) | 82 (15.0%) | ||
| Limb-salvage rates (at t2) (n = 621): | 0.047 | ||||
| LSS | 316 (50.9%) | 49 (64.5%) | 267 (49.0%) | Reference | |
| Amputations | 26 (4.2%) | 2 (2.6%) | 24 (4.4%) | 0.49 (0.07; 1.72) | |
| Unapplicable | 227 (36.6%) | 23 (30.3%) | 204 (37.4%) | 0.62 (0.36; 1.04) | |
| Unknown or unclear | 52 (8.4%) | 2 (2.6%) | 50 (9.2%) | ||
| Availability of reconstructive surgery (n = 621): | 0.001 | ||||
| Available | 374 (60.2%) | 60 (78.9%) | 314 (57.6%) | Reference | |
| Unavailable | 247 (39.8%) | 16 (21.1%) | 231 (42.4%) | 0.37 (0.20; 0.64) | |
| Details of adjuvant treatment | |||||
| Radiotherapy (diagnosis–t2) (n = 621): | 0.688 | ||||
| No radiotherapy | 301 (48.5%) | 36 (47.4%) | 265 (48.6%) | Reference | |
| Neoadjuvant | 79 (12.7%) | 12 (15.8%) | 67 (12.3%) | 1.33 (0.63; 2.63) | |
| Adjuvant | 241 (38.8%) | 28 (36.8%) | 213 (39.1%) | 0.97 (0.57; 1.64) | |
| Chemotherapy (diagnosis–t2) (n = 621): | 0.872 | ||||
| No chemotherapy | 430 (69.2%) | 53 (69.7%) | 377 (69.2%) | Reference | |
| Neoadjuvant | 101 (16.3%) | 11 (14.5%) | 90 (16.5%) | 0.88 (0.42; 1.70) | |
| Adjuvant | 90 (14.5%) | 12 (15.8%) | 78 (14.3%) | 1.10 (0.54; 2.10) | |
| Oncological Outcomes | |||||
| Overall mortality (t0–t2) ‡ (n = 621): | 0.155 | ||||
| Alive | 555 (89.4%) | 72 (94.7%) | 483 (88.6%) | Reference | |
| Deceased | 66 (10.6%) | 4 (5.3%) | 62 (11.4%) | 0.45 (0.13; 1.13) | |
| Local recurrences (t0–t2) ‡ (n = 621): | 0.517 | ||||
| None | 433 (69.7%) | 49 (64.5%) | 384 (70.5%) | Reference | |
| Recurrences | 185 (29.8%) | 27 (35.5%) | 158 (29.0%) | 1.34 (0.80; 2.21) | |
| Unknown or unclear | 3 (0.5%) | 0 (0.00%) | 3 (0.6%) | ||
| Systemic lesions (t0–t2) ‡ (n = 621): | 0.070 | ||||
| None | 439 (70.7%) | 60 (78.9%) | 379 (69.5%) | Reference | |
| Metastases | 173 (27.9%) | 14 (18.4%) | 159 (29.2%) | 0.56 (0.29; 1.01) | |
| Unknown or unclear | 9 (1.5%) | 2 (2.6%) | 7 (1.3%) |
| Entire Cohort | Defect Reconstruction | Primary Closure | Adjusted Odds Ratios | Adjusted Error Probability | |
|---|---|---|---|---|---|
| 621 (100%) | 76 (12.2%) | 545 (87.8%) | |||
| Tumor characteristics | |||||
| Tumor grading: | |||||
| High grade (G2–3) | 376 (78.8%) | 55 (87.3%) | 321 (77.5%) | 1.98 (0.86; 4.52) | 0.10 |
| Tumor size: | |||||
| Small (T1) | 98 (24.4%) | 21 (36.8%) | 77 (22.3%) | 1.45 (0.75; 2. 97) | 0.27 |
| Tumor location: | |||||
| Extremities | 366 (58.9%) | 53 (69.7%) | 313 (57.4%) | 1.47 (0.81; 2.67) | 0.20 |
| Treatment characteristics | |||||
| Treatment intention at baseline: | |||||
| Curative | 486 (78.3%) | 69 (90.8%) | 417 (76.5%) | 1.94 (0.83; 4.58) | 0.13 |
| Details of surgical treatment | |||||
| Margins at first ablative surgery: | |||||
| R1 | 141 (16.9%) | 27 (28.4%) | 114 (15.5%) | 2.28 (1.17; 4.44) | 0.015 |
| Availability of reconstructive surgery: | |||||
| Available | 374 (60.2%) | 60 (78.9%) | 314 (57.6%) | 2.95 (1.58; 5.50) | <0.001 |
| Facility type upon first resection | |||||
| University hospital | 302 (48.6%) | 28 (36.8%) | 274 (50.3%) | 0.47 (0.24; 0.84) | 0.015 |
| Community hospital | 213 (34.3%) | 33 (43.4%) | 180 (33.0%) | 2.24 (1.19; 4.23) | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, B.; Bigdeli, A.K.; Nolte, S.; Gazyakan, E.; Harhaus, L.; Bischel, O.; Lehner, B.; Egerer, G.; Mechtersheimer, G.; Hohenberger, P.; et al. The Therapeutic Role of Plastic and Reconstructive Surgery in the Interdisciplinary Treatment of Soft-Tissue Sarcomas in Germany—Cross-Sectional Results of a Prospective Nationwide Observational Study (PROSa). Cancers 2022, 14, 4312. https://doi.org/10.3390/cancers14174312
Thomas B, Bigdeli AK, Nolte S, Gazyakan E, Harhaus L, Bischel O, Lehner B, Egerer G, Mechtersheimer G, Hohenberger P, et al. The Therapeutic Role of Plastic and Reconstructive Surgery in the Interdisciplinary Treatment of Soft-Tissue Sarcomas in Germany—Cross-Sectional Results of a Prospective Nationwide Observational Study (PROSa). Cancers. 2022; 14(17):4312. https://doi.org/10.3390/cancers14174312
Chicago/Turabian StyleThomas, Benjamin, Amir K. Bigdeli, Steffen Nolte, Emre Gazyakan, Leila Harhaus, Oliver Bischel, Burkhard Lehner, Gerlinde Egerer, Gunhild Mechtersheimer, Peter Hohenberger, and et al. 2022. "The Therapeutic Role of Plastic and Reconstructive Surgery in the Interdisciplinary Treatment of Soft-Tissue Sarcomas in Germany—Cross-Sectional Results of a Prospective Nationwide Observational Study (PROSa)" Cancers 14, no. 17: 4312. https://doi.org/10.3390/cancers14174312
APA StyleThomas, B., Bigdeli, A. K., Nolte, S., Gazyakan, E., Harhaus, L., Bischel, O., Lehner, B., Egerer, G., Mechtersheimer, G., Hohenberger, P., Horch, R. E., Andreou, D., Schmitt, J., Schuler, M. K., Eichler, M., & Kneser, U. (2022). The Therapeutic Role of Plastic and Reconstructive Surgery in the Interdisciplinary Treatment of Soft-Tissue Sarcomas in Germany—Cross-Sectional Results of a Prospective Nationwide Observational Study (PROSa). Cancers, 14(17), 4312. https://doi.org/10.3390/cancers14174312










