1. Introduction
Human epidermal growth factor receptor 2-positive (HER2+) breast cancer is characterized by the overexpression of the HER2/neu receptor and represents 15–20% of annual breast cancer cases [
1]. Trastuzumab, a monoclonal antibody targeted to the HER2 receptor, is used clinically in both neoadjuvant and adjuvant standard-of-care treatment, and is often combined with radiation in the adjuvant setting to eliminate residual disease and prevent recurrence [
2,
3,
4]. Preclinical studies have shown that the overexpression of HER2 is a contributing factor to radiation resistance, and treatment with anti-HER2 therapy could potentially act as a radiosensitizer [
5,
6,
7,
8,
9]. However, HER2+ patients still have a greater chance of recurrence than patients with HER2- status [
10,
11]. Thus, there is a need to investigate the optimization of trastuzumab and radiation in a combination treatment regimen.
One mechanism by which HER2 overexpression promotes tumorigenesis is through the inhibition of apoptosis by the upregulation of the phosphatidylinositol 3’-kinase–protein kinase B–mammalian target of rapamycin (PI3K/AKT/mTOR) pathway. Trastuzumab blocks PI3K/AKT/mTOR pathway signaling, causing cell cycle arrest and inducing cancer cell death [
12,
13]. Studies by Guo et al. and Liang et al. found that, when used in combination, trastuzumab sensitizes HER2+ breast cancer cells to radiation therapy in vitro, and that the upregulation of the PI3K/AKT pathway may be specifically involved in radioresistance in HER2+ breast cancer [
5,
7]. Additionally, breast tumors can become hypoxic due to the irregular and tortuous vasculature, which increases radioresistance [
14,
15,
16]. Previous results in HER2+ breast cancer revealed that treatment with trastuzumab could increase tumor oxygenation in a pre-clinical mouse model through the stabilization of tumor blood vessels [
17]. Although there has been investigation into the potential ability of trastuzumab to radiosensitize breast cancer, studies lack longitudinal data quantifying sensitization over time, reducing its clinical relevance. Furthermore, the effects of the order of therapies have not been systematically investigated [
18].
Common in vitro approaches for evaluating cell responses to radiation therapy include end-point analyses, such as the 3-[4,5-dimethylthiazolyl-2]-2,5-diphenyltetrazolium bromide (MTT) assay or the clonogenic assay. Furthermore, the results evaluating cell viability after treatment can largely vary depending on the end time-point chosen [
19]. Additionally, these methods lack the ability to longitudinally observe cell response, which is critical in determining underlying changes in cell physiology during therapy [
20].
This contribution has two goals: (1) to investigate and quantify the effects of radiation and trastuzumab longitudinally in vitro, and (2) to test the hypothesis that trastuzumab sensitizes HER2+ breast cancer to radiation therapy in an in vivo model of HER2+ breast cancer. This study uses the IncuCyte Live-Cell Analysis System (Essen Bioscience, Ann Arbor, MI, USA), which delivers real-time data of cellular responses and has been shown to be accurate in analyzing changes in breast cancer cell proliferation over time [
21]. Quantifying the synergy between trastuzumab and radiation over time has the potential to elucidate optimal combination regimens and decrease the amount of therapy needed to achieve tumor control or eradication.
2. Materials and Methods
2.1. Cell Culture
BT474, SKBR3, and MDA-MB-231 cell lines were acquired from ATCC. BT474 cells were maintained in improved minimal essential medium (IMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS, 1% penicillin/streptomycin, and 20 μg/mL of insulin. SKBR3 cells were cultured in McCoy’s 5A medium (ATCC, Manassas, VA, USA) supplemented with 10% FBS and 1% penicillin/streptomycin. MDA-MB-231 cells were maintained in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 5% FBS and 200 μg/mL of G418. All cells were grown at 37 °C with 5% CO2. The cells were cultured to 70–80% confluency and the cell counts were determined with a Countess II FL automated cell counter (Thermo Fisher Scientific Inc., Waltham, MA, USA). All used cell lines were acquired from ATCC.
2.2. Western Blot Evaluation of HER2 Expression and Quantification
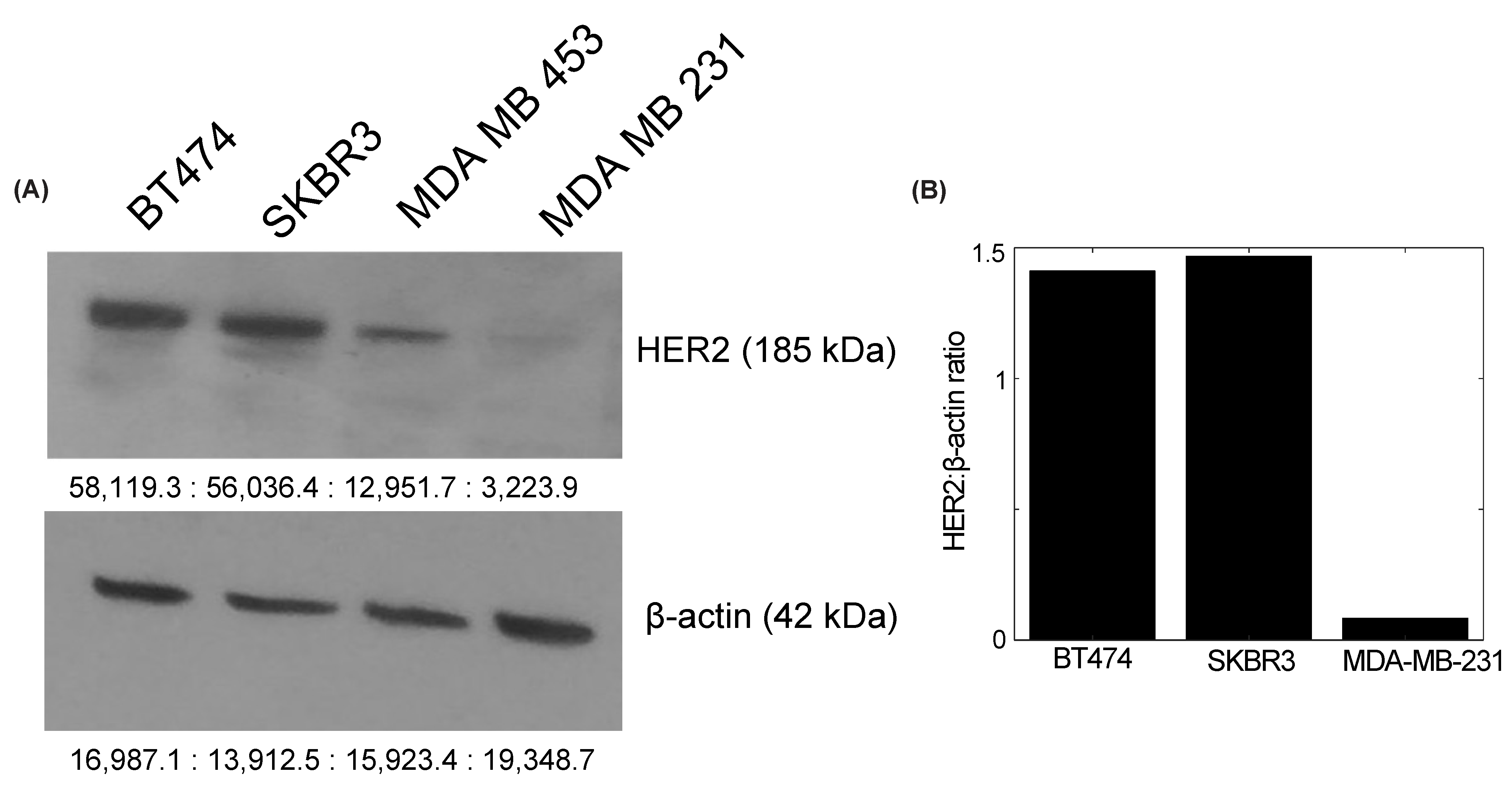
BT474, SKBR3, and MDA-MB-231 breast cancer cells were washed with ice-cold phosphate-buffered saline and lysed for 10 min on ice with radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor (Roche Applied Science, Indianapolis, IN, USA). The lysates were centrifuged at 13,400× g for 20 min at 4 °C and collected to undergo the bicinchoninic acid (BCA) protein quantification assay using a Nanodrop 2000 c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). For each cell line, 20 μg of protein was prepared in a solution of sodium dodecyl sulfate (SDS) and β-mercaptoethanol, and boiled for 5 min at 95° C. The samples were run on NuPAGE Bis-Tris gel and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked in 5% dry milk in tris-buffered saline (TBST), probed with horseradish peroxidase (HRP)-conjugated mouse anti-human β-actin overnight at 4 °C, and developed with an Amersham ECL western blot detection system (GE Healthcare, Buckinghamshire, UK) for one minute at room temperature. Membranes were developed in the absence of light and visualized with an SRX-101A Medical Film Processor (Konica Minolta Medical and Graphic, Inc., Shanghai, China). The membrane was incubated in stripping buffer for 15 min, washed with TBST, and probed with Rabbit anti-human HER2/ErbB2 primary antibody (Cell Signaling Technology, Danvers, MA, USA) for 2 h at room temperature. The membrane was washed and incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody (Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature. After a final wash, the membrane was redeveloped and visualized for HER2 expression. The quantification of bands was conducted with the Image Studio Lite program, version 5.0 (LI-COR Biosciences, Lincoln, NE, USA). HER2 expression was normalized to β-actin expression for quantification.
2.3. Transfection of Breast Cancer Cell Lines
BT474, SKBR3, and MDA-MB-231 cells were transfected to express enhanced green fluorescent protein (EGFP) through the Sleeping Beauty Transposon System. An EGFP plasmid was obtained and cloned into a Sleeping Beauty-compatible vector, psDBbi-Neo (Addgene, Cambridge, MA, USA plasmid #60525). Lipofectamine LTX (Thermo Fisher Scientific, Waltham, MA, USA) was used to co-transfect the psDBbi-Neo vector and the pCMV (CAT) T7-SB100 (Addgene, Cambridge, MA, USA, plasmid #34879) Sleeping Beauty transposase. Following transfection, cells were cultured in their respective media supplemented with 200 μg/mL G418 for four weeks to select for positively transfected cells. Fluorescence-activated cell sorting was used to separate the cells, and the 25% highest intensity EGFP-expressing cells were used in the experiments. The pCMV (CAT)T7-SB100 plasmid was a gift from Dr. Zsuzsanna Izsvak [
22], and the pSBbi-Neo was a gift from Dr. Eric Kowarz [
23].
2.4. In Vitro Treatment Experiments
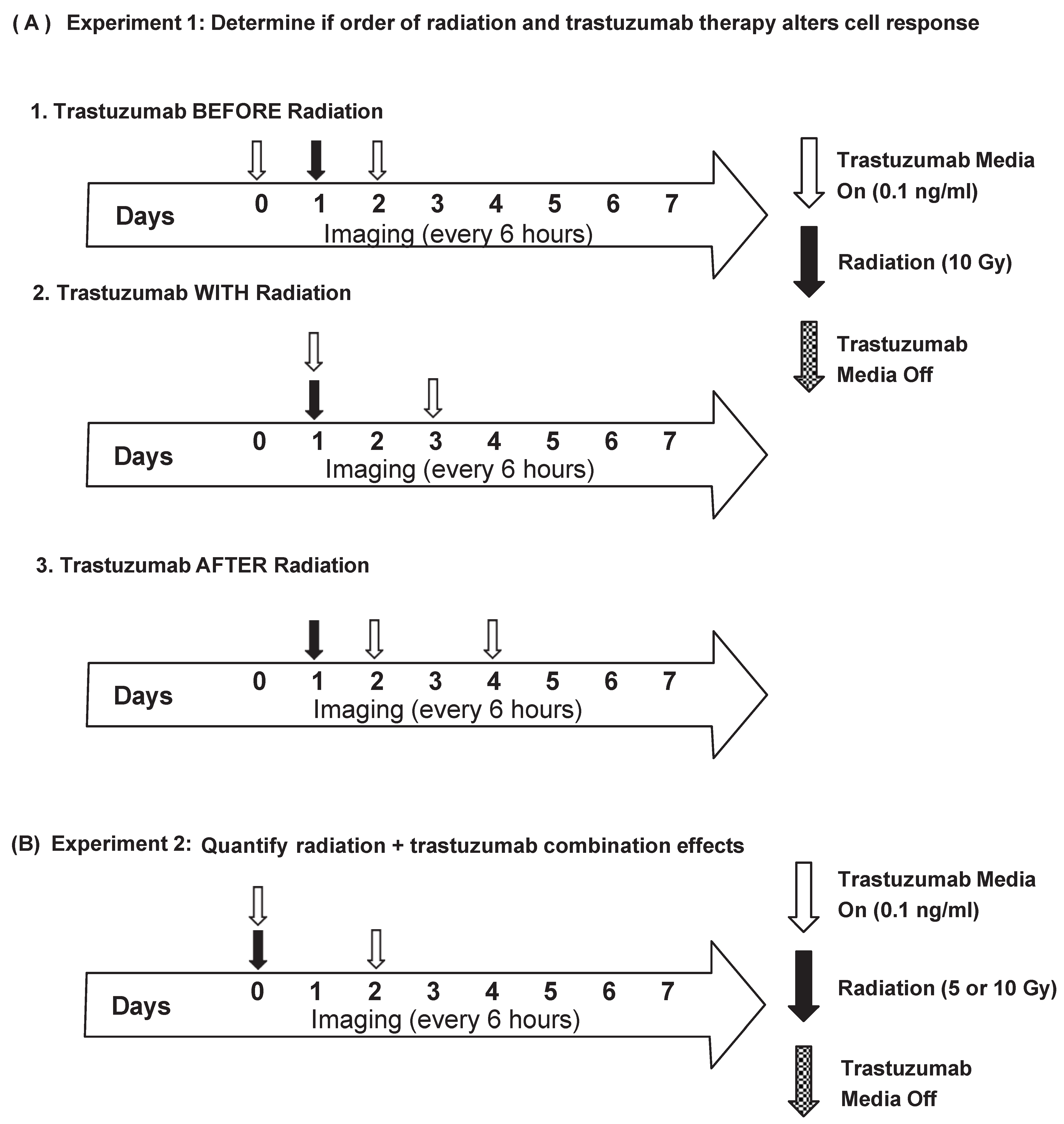
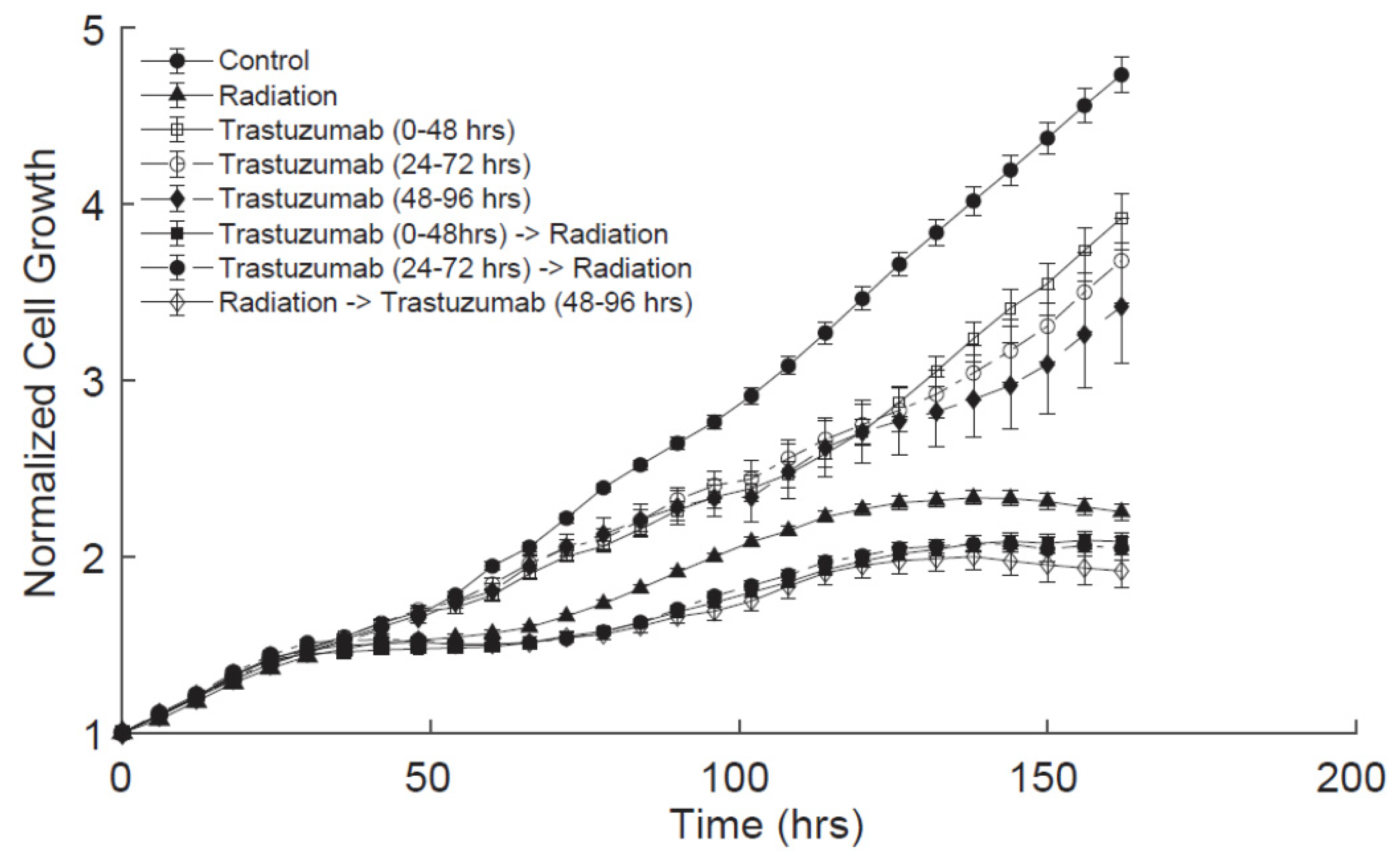
2.4.1. Experiment 1: Order of Dosing
BT474-GFP cells were plated in 96-well plates at 5000 cells/well. Twenty-four hours later (Day 0), the plates were placed in an IncuCyte S3 Live-Cell Analysis System (Essen BioScience, Ann Arbor, MI, USA) and whole-well imaged every six hours with the phase contrast and green channels (excitation 440–480, emission 504–544) for one week. Single-agent trastuzumab treatment groups were treated with trastuzumab (0.1 ng/mL) from 0 to 48 h (group 1), 24–72 h (group 2), or 48–96 h (group 3). For the single-agent radiation treatment groups, cells were treated with radiation (10 Gy) at 24 h with a CellRad Dedicated Benchtop Cell Irradiator (Faxitron, Tucson, AZ, USA). For the combination treatment groups, the cells received trastuzumab (0.1 ng/mL) before, with, or after radiation treatment (10 Gy), as detailed in
Figure 1A. Each treatment group had 24 replicates per experiment. The experiments were performed three times.
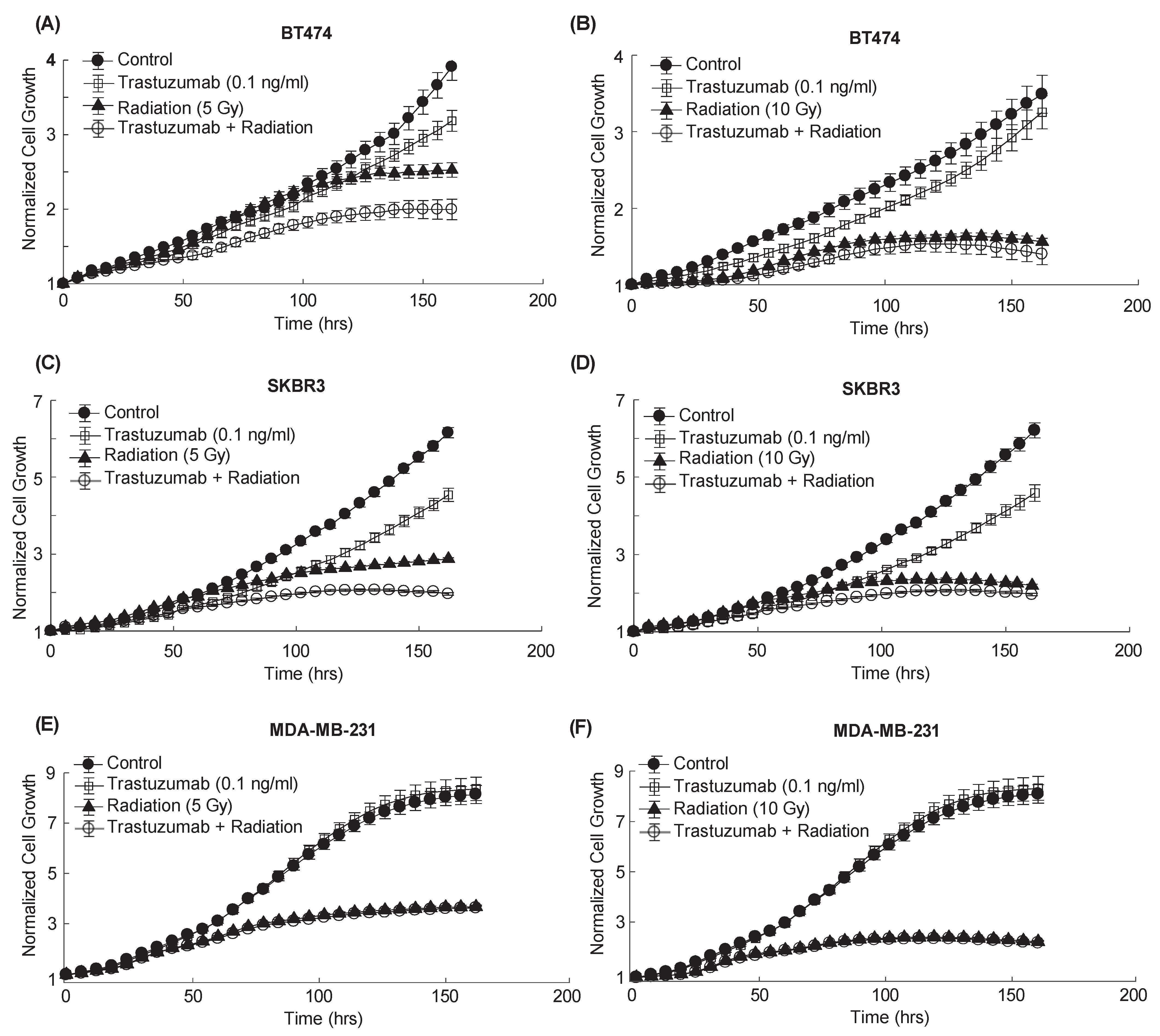
2.4.2. Experiment 2: Quantifying Radiation + Trastuzumab Combination Effects
BT474-GFP, SKBR3-GFP, or MDA-MB-231-GFP cells were plated in 96-well plates at 5000 cells/well. Twenty-four hours later (Day 0), the cells were treated with either 0.1% saline, trastuzumab (0.1 ng/mL), or radiation (5 or 10 Gy) for the control and single-agent treatment groups. Combination groups received both trastuzumab (0.1 ng/mL) and radiation (5 or 10 Gy) on Day 0 (
Figure 1B). The plates were placed in an IncuCyte S3 Live-Cell Analysis System (Essen BioScience, Ann Arbor, MI, USA) and whole-well imaged (4×, 2.82 μm/pixel) every six hours with the phase contrast and green channels (excitation 440–480, emission 504–544) for one week. Each treatment group had 30 replicates per experiment. The experiments were performed three times.
2.5. Image Analysis
Images were analyzed using an IncuCyte S3 Live-Cell Analysis System (Essen BioScience, Ann Arbor, MI, USA) to segment discrete green fluorescent objects (cell counts). The background signal was estimated in 100 μm segments and subtracted from the image. The edges of clumped cells were determined to be the dimmest point between two objects. Objects greater than 1000 μm2 were filtered out of the analysis. Cell growth was normalized to the initial seeding density (0 h).
2.6. Interaction Index Calculation
The interaction index [
24,
25] based on the Bliss independence was used to determine the effects of combined radiation and trastuzumab treatment in vitro. To calculate the effects of the radiation (
A), trastuzumab (
B), and combination (
AB) treatments compared with the control (
C), let (
f) represent the difference in normalized cell growth (
N):
The interaction index (
I) can be calculated by Equation (2):
and the value of
I determines the effect of the combination therapy:
2.7. Animal Procedures
Female nude athymic mice (NU/J) (N = 47) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) at 3–4 weeks of age with an average weight of 12–15 g and acclimated for one week in housing with a normal light cycle, sterile water and food, and microisolation cages. The mice were subcutaneously implanted with a 0.72 mg, 60-day release, 17β-estradiol pellet (Innovative Research of America, Sarasota, FL, USA) in the nape of the neck. One day later, 107 BT474 cells were implanted subcutaneously in 100 μL of serum-free IMEM media with 30% growth-factor-reduced Matrigel. The tumor volume was calculated using the formula: (4/3) × Π × (transverse tumor diameter/2) × (longitudinal tumor diameter/2) × (average of (transverse × longitudinal tumor diameter/2)). The tumors were grown to approximately 250 mm3 (8–10 weeks) and entered into the study. The animals were randomly sorted into treatment groups: group (1) control: 100 μL of saline on Days 0 and 3 (N = 6), group (2) trastuzumab alone: 10 mg/kg on Days 0 and 3 (N = 7), group (3) 5 Gy of radiation alone on Day 0 (N = 6) and 100 μL of saline on Day 3, group (4) 10 Gy of radiation alone on Day 0 (N = 5) and 100 μL of saline on Day 3, group (5) Radiation 5 Gy on Day 0 + trastuzumab 10 mg/kg on Days 0, 3 (N = 5), group (6) 10 Gy of radiation on Day 0 and 10 mg/kg of trastuzumab on Days 0 and 3 (N = 5). trastuzumab and saline were administered via intraperitoneal (IP) injection. Radiation treatments (~4 Gy/min, 225 kV, 17.8 mA) were administered using a MultiRad 350 Irradiation System (Faxitron, Tucson, AZ, USA). Tumor measurements were taken three times per week using calipers for four weeks, at which point the mice were humanely euthanized via sustained isofluorane exposure (5% isofluorane for 5 min), followed by cervical dislocation. Mice in the immunohistochemistry cohort were divided into three treatment groups: (1) 5 Gy of radiation on Day 0 (N = 4) and 100 μL of saline on Day 3, (2) 10 mg/kg of trastuzumab on Days 0 and 3 (N = 4), and (3) 5 Gy of radiation on Day 0 and 10 mg/kg of trastuzumab on Days 0 and 3 (N = 5). Tumor growth was measured three times with calipers for one week, at which point the mice were euthanized and tumors were extracted. One hour prior to sacrifice, the mice were intravenously injected with 60 mg/kg of pimonidazole (Hypoxyprobe, Inc., Burlington, MA, USA) in 100 μL of saline. Tumors were cut across the longest cross-section and half was fixed in 10% neutral-buffered formalin (Fisher Scientific International Inc., Pittsburgh, PA, USA) for 48 h and then placed in 70% ethanol. The normalized tumor volume was calculated with the formula: ((X1 − X0)/X0) × 100, where X0 and X1 represent the tumor volume at baseline and tumor volume at subsequent timepoints, respectively.
2.8. Immunohistochemistry
Formalin-fixed tumor sections were embedded in paraffin and sliced into four-micron sections. The sections were stained for hematoxylin and eosin (H&E), mouse anti-CD31, mouse anti-α-smooth muscle actin (α-SMA, Abcam, Cambridge, UK), mouse anti-CD45 (Invitrogen, Carlsbad, CA, USA), and anti-pimonidazole (Hypoxyprobe, Inc., Burlington, MA, USA). The immuno-stained slides were scanned (20×, 0.495 μm/pixel) with an Aperio ScanScope (Leica Microsystems, Wetzlar, Germany). Regions of necrosis were determined using manual segmentation. All other stains were quantitatively segmented based on the color thresholds determined from positive and negative controls following in-house MATLAB (MathWorks Inc., Natick, MA, USA) routines. The images were converted to grayscale for registration to the corresponding tumor H&E. Then, transformations consisting of translation, rotation, and scale (similarity) based on intensity were applied. A viable tissue mask was defined as the total tumor area minus the necrotic area. Inflammation (CD45), hypoxia (pimonidazole), and apoptosis (caspase3) were defined as the percent of positive stain per viable tissue area. The microvessel density (CD31) and vascular smooth muscle coverage (α-SMA) were calculated as the number of vessels per square millimeter of tumor tissue. The vessel maturation index was evaluated as the ratio of α-SMA coverage to microvessel density.
2.9. Statistical Analysis
Statistical analysis was conducted using MATLAB (MathWorks Inc., Natick, MA, USA). A two-way analysis of variance (ANOVA), adjusting for multiple comparisons with Dunn’s, was used to determine longitudinal differences in the order of dosing experiments. All in vitro data are presented as the mean ±95% confidence interval, with p < 0.05 indicating significance. Statistical differences between in vivo tumor growth at each time point and ex vivo immunohistochemistry samples were determined using a nonparametric Wilcoxon rank sum test. All in vivo and ex vivo data are presented as the mean ± standard error, with p < 0.05 indicating significance. The Friedman’s test adjusting for multiple comparisons with Dunn’s was used to assess longitudinal changes in tumor growth between treatment groups.
4. Discussion
Trastuzumab and radiation are commonly used as adjuvant therapies in the clinical treatment of HER2+ breast cancer. Radiation therapy can reduce recurrence rates in patients by eradicating the remaining cancer cells after the surgical removal of tumors; however, HER2+ patients have greater chances of recurrence than HER2- patients [
11,
26]. In vitro evidence suggests that trastuzumab can sensitize HER2+ breast cancer cells to radiation through the inhibition of signaling pathways involved in DNA repair mechanisms [
7,
27,
28], although, systematic evaluations of the order of therapies and longitudinal data of cell responses are limited. This study demonstrates that the order of therapy does not alter cell proliferation after treatment, and longitudinal treatment effects are additive in vitro. Furthermore, in vivo, tumors regress faster when treated with combination therapy than either single-agent therapy, and there is evidence that immune modulation may impact the treatment response.
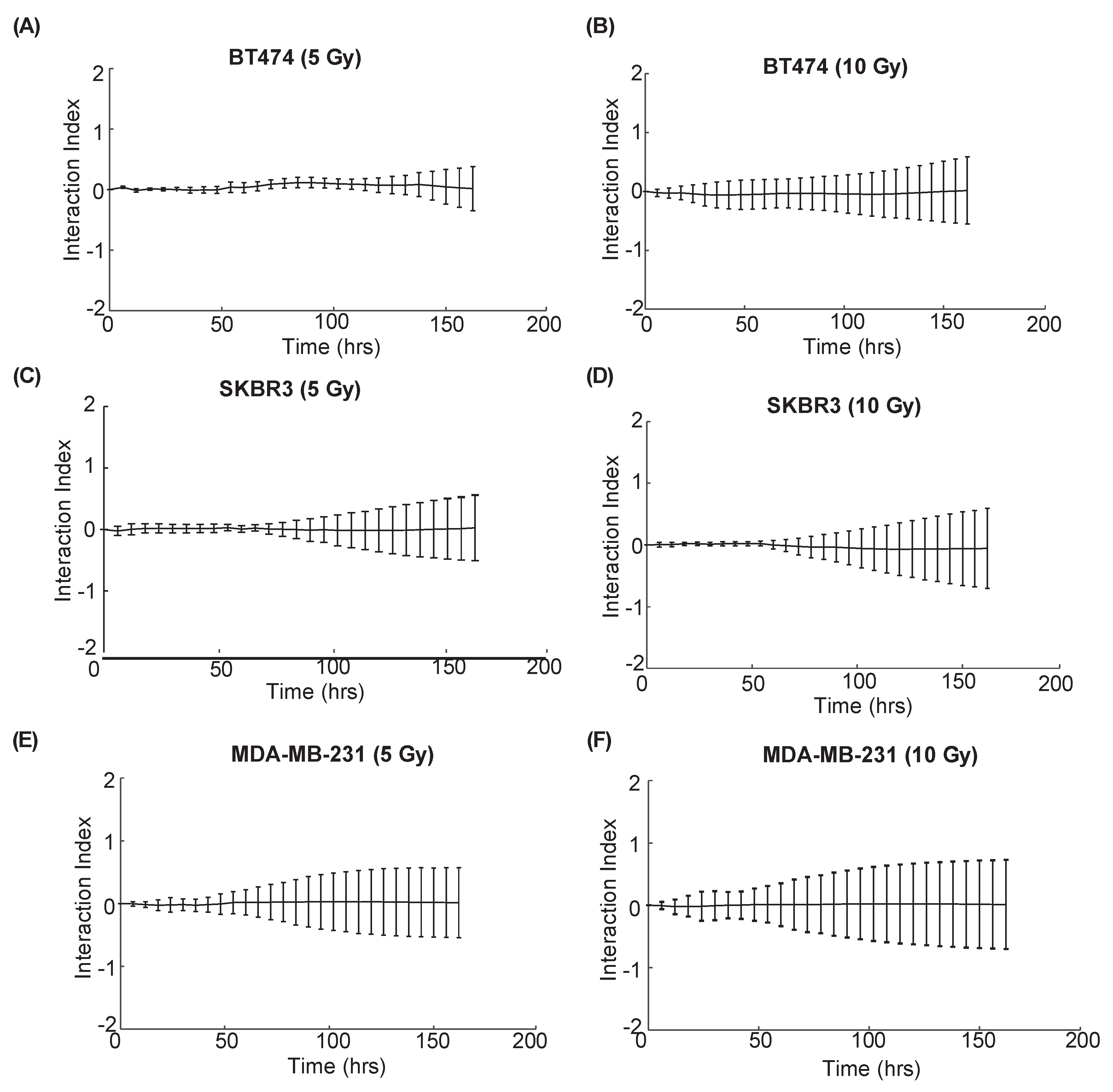
The interaction index calculations revealed additive effects of combination radiation and trastuzumab treatment across all HER2+ breast cancer cells evaluated. This contrasts with the findings of others who have evaluated cell responses to combination radiation and trastuzumab using end-point assays [
7,
9]. For example, Liang et al. found a synergistic effect of trastuzumab and radiation treatment with BT474 and SKBR3 cells 24 h after therapy using an ELISA to evaluate apoptosis (cytoplasmic histone-associated DNA fragments) [
7]. In their study, cells were pre-incubated with trastuzumab for 16 h before radiation treatment; however, our results did not indicate that pre-treatment with trastuzumab altered cell death under radiation. Rao et al. found a similar synergistic effect using SUM-149PT cells treated with a HER2 tyrosine–kinase inhibitor in combination with radiation therapy; however, this study used a longer pre-incubation time with trastuzumab (7 days) and evaluated cell growth two weeks after radiation treatment using a clonogenic assay [
9]. The differences in the results could have arisen from the alternate methods used to detect cellular responses to treatment, different dosing strategies, and differences in the timing of evaluation after treatment. One important aspect of our study is that we have quantified longitudinal cell growth after variations in combination treatments, thereby providing a better understanding of the cellular dynamics after combination treatment compared with traditional end-point assays. This provides the opportunity to assess the long-term effects of the order of sequencing of therapies and variations in dosing strategies.
In vivo tumor growth data show significant differences in the longitudinal tumor response between groups that were treated with 5 Gy and 10 Gy of radiation; however, no significant difference in the longitudinal tumor response was observed between the doses of radiation when trastuzumab was added to the treatment regimen. This finding suggests that a smaller dose of radiation could be used in combination with trastuzumab without decreasing efficacy. Additionally, the results show a faster rate of tumor regression in groups treated with combination therapy compared with either single therapy. The results of this research provide a framework to examine treatment interactions between radiation therapy and targeted treatments, and can be used to guide combination therapies to enhance treatment efficacy while minimizing off-target side effects. This approach will be particularly important, as there are increased individualized medicine plans and de-escalated radiation treatments.
To evaluate if microenvironmental alterations could be impacting treatment responses, immune infiltration, hypoxia, and the vascular maturation index were evaluated in tumors treated with 5 Gy radiation, trastuzumab, and 5 Gy radiation in combination with trastuzumab seven days after treatment. CD45 histology staining revealed an increase in immune infiltration in treatment groups receiving trastuzumab. Previously collected data revealed that trastuzumab has the potential to reprogram the immunosuppressive components of the tumor microenvironment and could contribute to anti-tumor responses. The evaluation of immune cell characterization and impact on tumor regression in this treatment regimen is needed to further elucidate the mechanisms contributing to enhanced tumor regression in the combination treatment group [
29]. Although no significant differences were shown in hypoxia and the vascular maturation index, previous studies using longitudinal imaging have revealed that trastuzumab can reduce hypoxia and increase vascular perfusion [
17,
30]. It is possible that, by employing longitudinal imaging methods, windows of heightened oxygenation could be revealed, and an alternate order of dosing could enhance the treatment response in vivo.
The limitations of this study include the lack of longitudinal data evaluating microenvironmental changes corresponding with tumor growth dynamics. Immuno–PET imaging is an alternative method that could longitudinally evaluate immune infiltration after treatment [
31].
18F-Fluoromisonidazole positron emission tomography is one method used to monitor the tumor oxygenation levels, and dynamic contrast-enhanced magnetic resonance imaging is an alternative method to quantitatively assess vascularity [
27,
30]. Neither of these methods would allow for histological validation at every time point; however, they would offer finely time-resolved data of tumor status over the course of treatment. This study used a single dose of radiation and provided preliminary data showing that a lesser dose of radiation could be used in combination with trastuzumab to achieve the same therapeutic efficacy as a higher dose of radiation. Further studies should be conducted to evaluate efficacy with a clinical treatment regimen that uses fractionated dosing. Additional trastuzumab treatment doses were investigated for the in vitro experiments in this study. Doses higher than 0.01 ng/mL exhibited greater decreases in cell proliferation in the single-agent treatment groups; however, they did not affect the combination treatment response. Doses lower than 0.01 ng/mL had no significant effect on HER2+ cell growth, which is not biologically observed in vivo; therefore, a sub-therapeutic dose of trastuzumab (0.01 ng/mL) was chosen as the appropriate treatment condition to assess synergy. It is possible that using a higher seeding density would allow for the evaluation of cell proliferation with a higher trastuzumab dose, although, when cells become confluent, the IncuCyte system is less accurate in quantifying cell numbers [
21].