Lung Cancer Recurrence Risk Prediction through Integrated Deep Learning Evaluation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
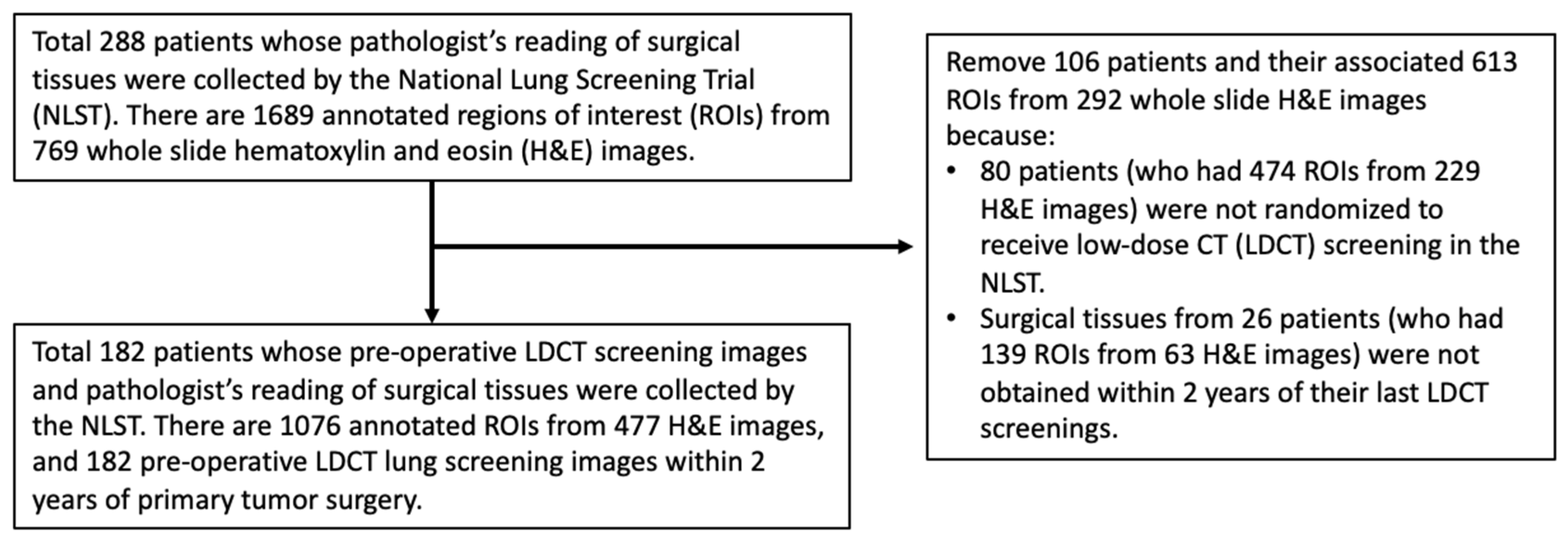
2.1. Study Sample
2.2. Tissue Image Feature Extraction
2.3. Preoperative CT Image Feature Extraction
(0,0,1), (0,1,1), (0,−1,1), (−1,0,1), (−1,1,1), (−1,−1,1).
2.4. Prediction Algorithm Development
2.5. Statistical Methods
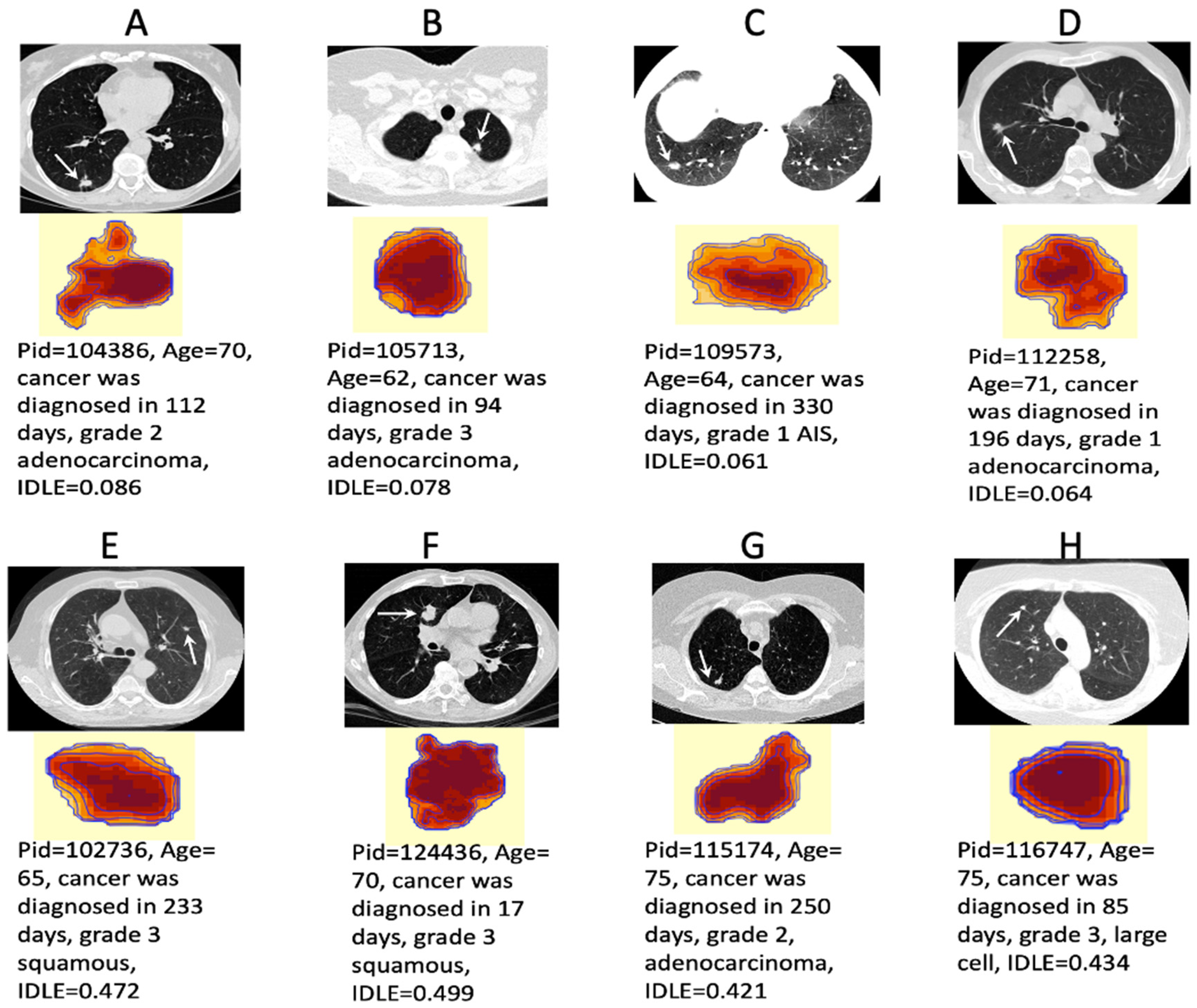
2.6. Decoding the Deep Learning Black Box
3. Results
3.1. Study Sample Characteristics
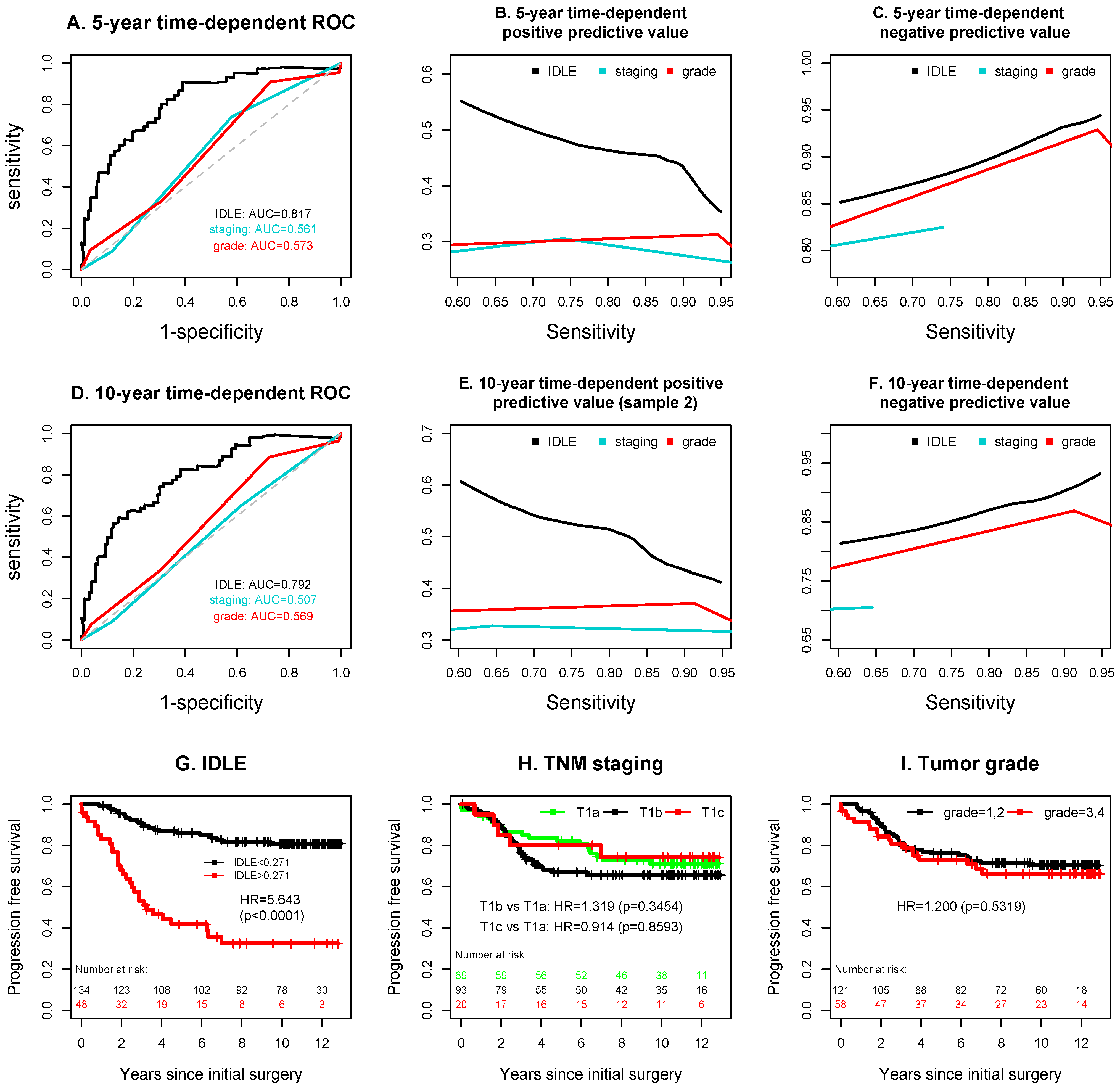
3.2. Added Values of the IDLE Scores to TNM Staging and Tumor Grade
3.3. Synergy inside the Deep Learning Network Black Box
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, J.K.; Duffy, S.W.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Green, B.A.; Holemans, J.A.; Kavanagh, T.; Kerr, K.M.; et al. The UK Lung Cancer Screening Trial: A pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol. Assess. 2016, 20, 1–146. [Google Scholar] [CrossRef] [PubMed]
- Tammemagi, M.C.; Schmidt, H.; Martel, S.; McWilliams, A.; Goffin, J.R.; Johnston, M.R.; Nicholas, G.; Tremblay, A.; Bhatia, R.; Liu, G.; et al. PanCan Study T. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): A single-arm, prospective study. Lancet Oncol. 2017, 18, 1523–1531. [Google Scholar] [CrossRef]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Crowley, J.J.; Goldstraw, P. The revised TNM staging system for lung cancer. Ann. Thorac. Cardiovasc. Surg. 2009, 15, 4–9. [Google Scholar]
- Wang, C.; Wu, Y.; Shao, J.; Liu, D.; Li, W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer 2020, 20, 150. [Google Scholar] [CrossRef]
- Ujiie, H.; Kadota, K.; Chaft, J.E.; Buitrago, D.; Sima, C.S.; Lee, M.C.; Huang, J.; Travis, W.D.; Rizk, N.P.; Rudin, C.M.; et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J. Clin. Oncol. 2015, 33, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Scott, W.J.; Allen, M.S.; Darling, G.E.; Decker, P.A.; McKenna, R.J.; Meyers, B.F. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J. Thorac. Cardiovasc. Surg. 2014, 147, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Saji, H.; Yoshida, K.; Kakihana, M.; Honda, H.; Nomura, M.; Usuda, J.; Kajiwara, N.; Ohira, T.; Ikeda, N. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 2013, 143, 1626–1634. [Google Scholar] [CrossRef]
- Hung, J.J.; Jeng, W.J.; Hsu, W.H.; Wu, K.J.; Chou, T.Y.; Hsieh, C.C.; Huang, M.H.; Liu, J.S.; Wu, Y.C. Prognostic factors of postrecurrence survival in completely resected stage I non-small cell lung cancer with distant metastasis. Thorax 2010, 65, 241–245. [Google Scholar] [CrossRef]
- Hung, J.J.; Hsu, W.H.; Hsieh, C.C.; Huang, B.S.; Huang, M.H.; Liu, J.S.; Wu, Y.C. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009, 64, 192–196. [Google Scholar] [CrossRef]
- Nakagawa, T.; Okumura, N.; Ohata, K.; Igai, H.; Matsuoka, T.; Kameyama, K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2008, 34, 499–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csoszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; He, L.; Chen, X.; Pan, D.; Ma, Z.; Liang, C.; Tian, J.; Liang, C. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology 2016, 281, 947–957. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Patz, E.F., Jr.; Caporaso, N.E.; Dubinett, S.M.; Massion, P.P.; Hirsch, F.R.; Minna, J.D.; Gatsonis, C.; Duan, F.; Adams, A.; Apgar, C.; et al. National Lung Cancer Screening Trial American College of Radiology Imaging Network Specimen Biorepository originating from the Contemporary Screening for the Detection of Lung Cancer Trial (NLST, ACRIN 6654): Design, intent, and availability of specimens for validation of lung cancer biomarkers. J. Thorac. Oncol. 2010, 5, 1502–1506. [Google Scholar] [CrossRef]
- Huang, P.; Park, S.; Yan, R.; Lee, J.; Chu, L.C.; Lin, C.T.; Hussien, A.; Rathmell, J.; Thomas, B.; Chen, C.; et al. Added Value of Computer-aided CT Image Features for Early Lung Cancer Diagnosis with Small Pulmonary Nodules: A Matched Case-Control Study. Radiology 2017, 286, 286–295. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.M.; Majid, M.; Qayyum, A.; Awais, M.; Alnowami, M.; Khan, M.K. Medical Image Analysis using Convolutional Neural Networks: A Review. J. Med. Syst. 2018, 42, 226. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Sohail, A.; Zahoora, U.; Qureshi, A.S. A survey of the recent architectures of deep convolutional neural networks. Artif. Intell. Rev. 2020, 53, 5455–5516. [Google Scholar] [CrossRef]
- Lin, W.; Hasenstab, K.; Moura Cunha, G.; Schwartzman, A. Comparison of handcrafted features and convolutional neural networks for liver MR image adequacy assessment. Sci. Rep. 2020, 10, 20336. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lin, C.T.; Li, Y.; Tammemagi, C.M.; Brock, M.; Atkar-Khattra, S.; Xu, Y.; Hu, P.; Mayo, J.; Schmidt, H.; et al. Prediction of lung cancer risk at follow-up screening with low-dose CT: A training and validation study of a deep learning method. Lancet Digit. Health 2019, 1, e353–e362. [Google Scholar] [CrossRef]
- Passiglia, F.; Bronte, G.; Castiglia, M.; Listi, A.; Calo, V.; Toia, F.; Cicero, G.; Fanale, D.; Rizzo, S.; Bazan, V.; et al. Prognostic and predictive biomarkers for targeted therapy in NSCLC: For whom the bell tolls? Expert Opin. Biol. Ther. 2015, 15, 1553–1566. [Google Scholar] [CrossRef]
- Dejima, H.; Iinuma, H.; Kanaoka, R.; Matsutani, N.; Kawamura, M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol. Lett. 2017, 13, 1256–1263. [Google Scholar] [CrossRef]
- Tang, Y.; Qiao, G.; Xu, E.; Xuan, Y.; Liao, M.; Yin, G. Biomarkers for early diagnosis, prognosis, prediction, and recurrence monitoring of non-small cell lung cancer. Onco Targets Ther. 2017, 10, 4527–4534. [Google Scholar] [CrossRef]
- Kanaoka, R.; Iinuma, H.; Dejima, H.; Sakai, T.; Uehara, H.; Matsutani, N.; Kawamura, M. Usefulness of Plasma Exosomal MicroRNA-451a as a Noninvasive Biomarker for Early Prediction of Recurrence and Prognosis of Non-Small Cell Lung Cancer. Oncology 2018, 94, 311–323. [Google Scholar] [CrossRef]
- Camidge, D.R.; Doebele, R.C.; Kerr, K.M. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat. Rev. Clin. Oncol. 2019, 16, 341–355. [Google Scholar] [CrossRef]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Akinci D’Antonoli, T.; Farchione, A.; Lenkowicz, J.; Chiappetta, M.; Cicchetti, G.; Martino, A.; Ottavianelli, A.; Manfredi, R.; Margaritora, S.; Bonomo, L.; et al. CT Radiomics Signature of Tumor and Peritumoral Lung Parenchyma to Predict Nonsmall Cell Lung Cancer Postsurgical Recurrence Risk. Acad. Radiol. 2020, 27, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Janowczyk, A.; Zhou, Y.; Thawani, R.; Fu, P.; Schalper, K.; Velcheti, V.; Madabhushi, A. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci. Rep. 2017, 7, 13543. [Google Scholar] [CrossRef] [PubMed]
- Corredor, G.; Wang, X.; Zhou, Y.; Lu, C.; Fu, P.; Syrigos, K.; Rimm, D.L.; Yang, M.; Romero, E.; Schalper, K.A.; et al. Spatial Architecture and Arrangement of Tumor-Infiltrating Lymphocytes for Predicting Likelihood of Recurrence in Early-Stage Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ma, K.; Yang, X.; Li, M.; Shi, Y.; Zhan, C.; Jiang, W.; Wang, Q. A nomogram to predict prognosis after surgery in early stage non-small cell lung cancer in elderly patients. Int. J. Surg. 2017, 42, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Khullar, O.V.; Liu, Y.; Gillespie, T.; Higgins, K.A.; Ramalingam, S.; Lipscomb, J.; Fernandez, F.G. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J. Thorac. Oncol. 2015, 10, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Kumar, A.; Klapper, J.A.; Hartwig, M.G.; Tong, B.C.; Harpole, D.H., Jr.; Berry, M.F.; D’Amico, T.A. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann. Surg. 2019, 269, 163–171. [Google Scholar] [CrossRef]
- Ijsseldijk, M.A.; Shoni, M.; Siegert, C.; Seegers, J.; van Engelenburg, A.K.C.; Tsai, T.C.; Lebenthal, A.; Ten Broek, R.P.G. Oncological Outcomes of Lobar Resection, Segmentectomy, and Wedge Resection for T1a Non-Small-Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 582–590. [Google Scholar] [CrossRef]
- Winckelmans, T.; Decaluwe, H.; De Leyn, P.; Van Raemdonck, D. Segmentectomy or lobectomy for early-stage non-small-cell lung cancer: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2020, 57, 1051–1060. [Google Scholar] [CrossRef]
- Chan, M.V.; Huo, Y.R.; Cao, C.; Ridley, L. Survival outcomes for surgical resection versus CT-guided percutaneous ablation for stage I non-small cell lung cancer (NSCLC): A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 5421–5433. [Google Scholar] [CrossRef]

| No Progression N = 128 | Progression N = 54 | p1 | ||
|---|---|---|---|---|
| Cancers diagnosed 6 months after the last LDCT screening date | 27 | 21 | 0.0167 | |
| Lung cancer-related death | 0 | 45 | ||
| Age at surgery | 64.7 ± 4.9 | 65.9 ± 4.8 | 0.1067 | |
| Female, N (%) | 58 (45%) | 24 (44%) | 1.0 | |
| Smoke pack-years | 66 ± 29 | 72 ± 41 | 0.2889 | |
| Days from the last LDCT screening to the date of lung surgery | 177 ± 210 | 267 ± 299 | 0.0468 | |
| Surgery type | Sublobar resection Lobectomy | 21 107 | 8 46 | 1.0 |
| Lymphadenectomy | N (%) | 115 (90%) | 49 (91%) | 1.0 |
| Residual disease after surgery | R0 | 124 | 53 | 1.0 |
| R1 | 4 | 1 | ||
| Surgically removed lesion size (mm) | 19.7 ± 13.9 | 20.6 ± 12.6 | 0.6713 | |
| Largest invasive tumor size (mm) | 11.4 ± 6.8 | 12.9 ± 6.4 | 0.1604 | |
| Pathological cancer stage (TNM, 8th edition) | IA1 (T1a) | 50 | 19 | |
| IA1 (T1b) | 63 | 30 | 1.0 | |
| IA1 (T1c) | 15 | 5 | ||
| Highest tumor grade from all the ROIs | 1 = well-differentiated | 34 | 4 | 0.0163 |
| 2 = moderately differentiated | 53 | 30 | ||
| 3 = poorly differentiated | 35 | 14 | ||
| 4 = undifferentiated | 5 | 4 | ||
| Undetermined (GX) | 1 | 2 |
| HR | 95% CI | p | |
|---|---|---|---|
| IDLE high | 5.6708 | (3.1650, 10.1605) | <0.0001 |
| T1b 1 | 0.8665 | (0.4667, 1.6087) | 0.6499 |
| T1c 1 | 0.7708 | (0.2620, 2.2680) | 0.6364 |
| High grade | 0.9818 | (0.5440, 1.7720) | 0.9513 |
| Age at surgery | 1.0318 | (0.9738, 1.0934) | 0.2888 |
| Chemotherapy | 0.6700 | (0.2806, 1.5996) | 0.3671 |
| Radiotherapy | 1.3959 | (0.4064, 4.7945) | 0.5963 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.; Illei, P.B.; Franklin, W.; Wu, P.-H.; Forde, P.M.; Ashrafinia, S.; Hu, C.; Khan, H.; Vadvala, H.V.; Shih, I.-M.; et al. Lung Cancer Recurrence Risk Prediction through Integrated Deep Learning Evaluation. Cancers 2022, 14, 4150. https://doi.org/10.3390/cancers14174150
Huang P, Illei PB, Franklin W, Wu P-H, Forde PM, Ashrafinia S, Hu C, Khan H, Vadvala HV, Shih I-M, et al. Lung Cancer Recurrence Risk Prediction through Integrated Deep Learning Evaluation. Cancers. 2022; 14(17):4150. https://doi.org/10.3390/cancers14174150
Chicago/Turabian StyleHuang, Peng, Peter B. Illei, Wilbur Franklin, Pei-Hsun Wu, Patrick M. Forde, Saeed Ashrafinia, Chen Hu, Hamza Khan, Harshna V. Vadvala, Ie-Ming Shih, and et al. 2022. "Lung Cancer Recurrence Risk Prediction through Integrated Deep Learning Evaluation" Cancers 14, no. 17: 4150. https://doi.org/10.3390/cancers14174150
APA StyleHuang, P., Illei, P. B., Franklin, W., Wu, P.-H., Forde, P. M., Ashrafinia, S., Hu, C., Khan, H., Vadvala, H. V., Shih, I.-M., Battafarano, R. J., Jacobs, M. A., Kong, X., Lewis, J., Yan, R., Chen, Y., Housseau, F., Rahmim, A., Fishman, E. K., ... Gabrielson, E. (2022). Lung Cancer Recurrence Risk Prediction through Integrated Deep Learning Evaluation. Cancers, 14(17), 4150. https://doi.org/10.3390/cancers14174150






