Management of Cancer-Associated Thrombosis in France: A National Survey among Vascular Disease and Supportive Care Specialists
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Objective
2.2. Survey Design
2.3. Survey Population and Survey Administration
2.4. Data Analysis
3. Results
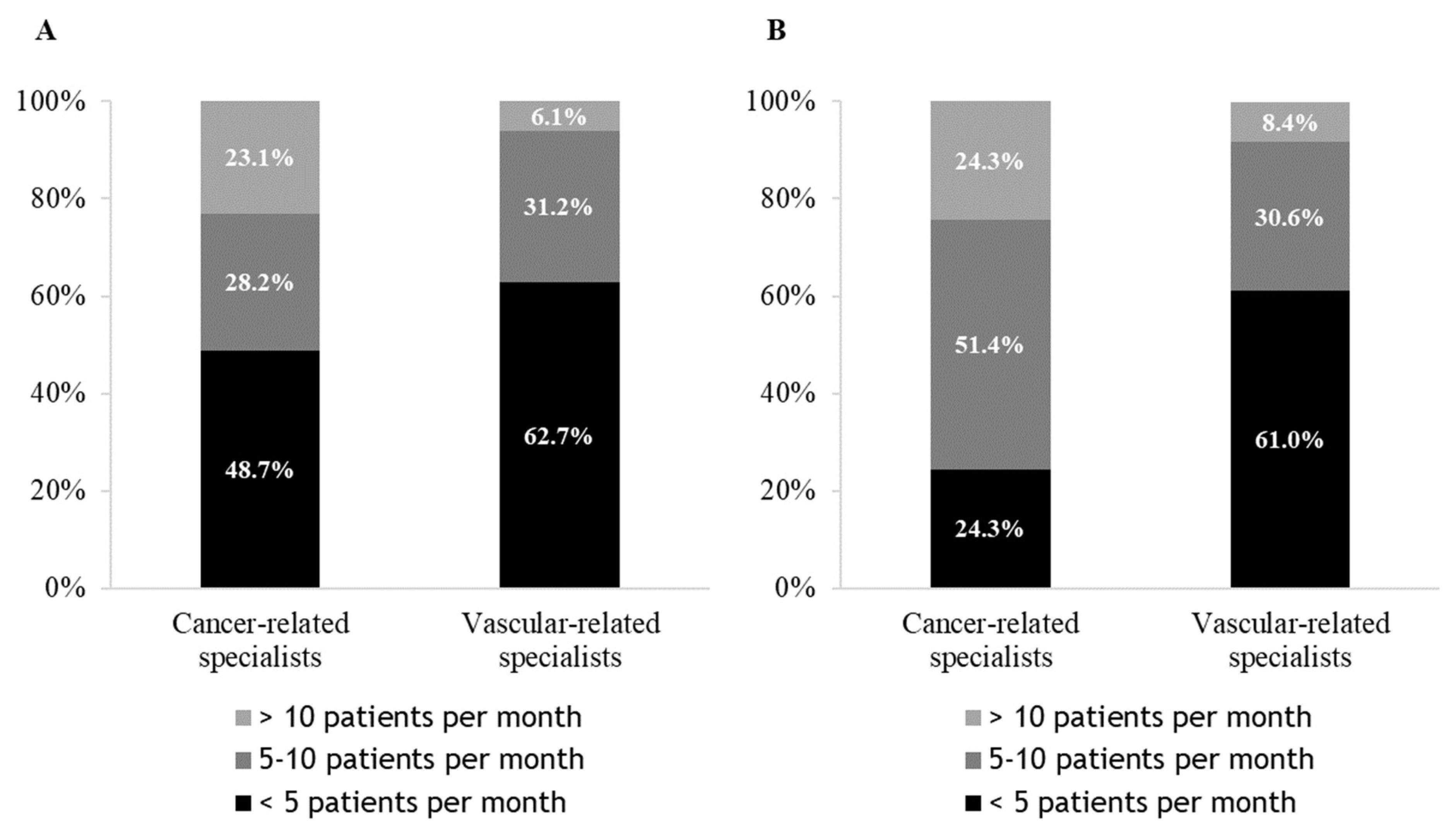
3.1. Management of a CAT, Choice of Anticoagulant Therapy and Tumour Site
3.2. CAT Treatment, Reasons for the Choice
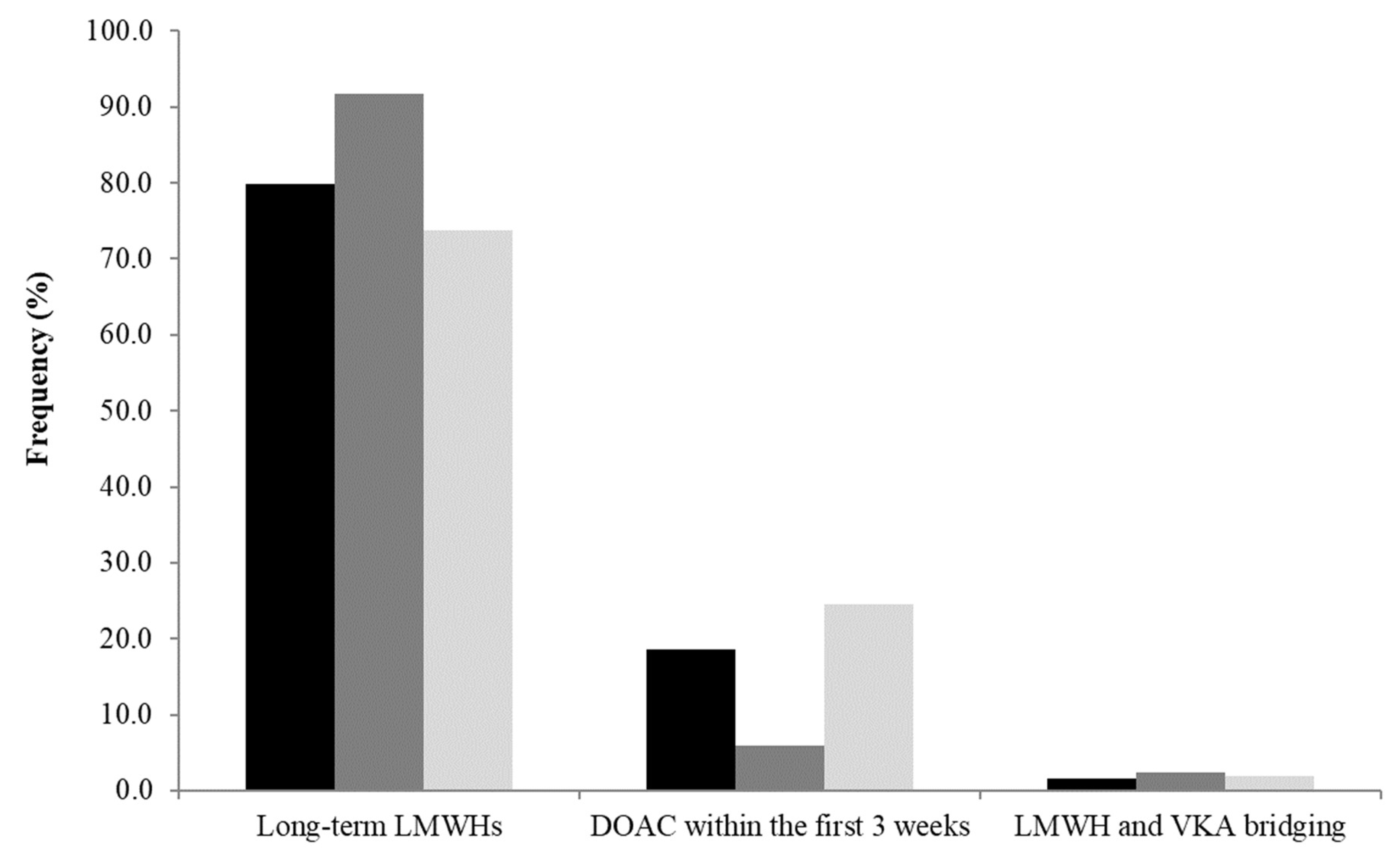
3.2.1. Initial Treatment
3.2.2. After Initial Treatment with LMWHs: Switch to DOACs?
3.3. Results: CAT and Specific Situations
Management of Patients with VTE Recurrence during Anticoagulant Treatment
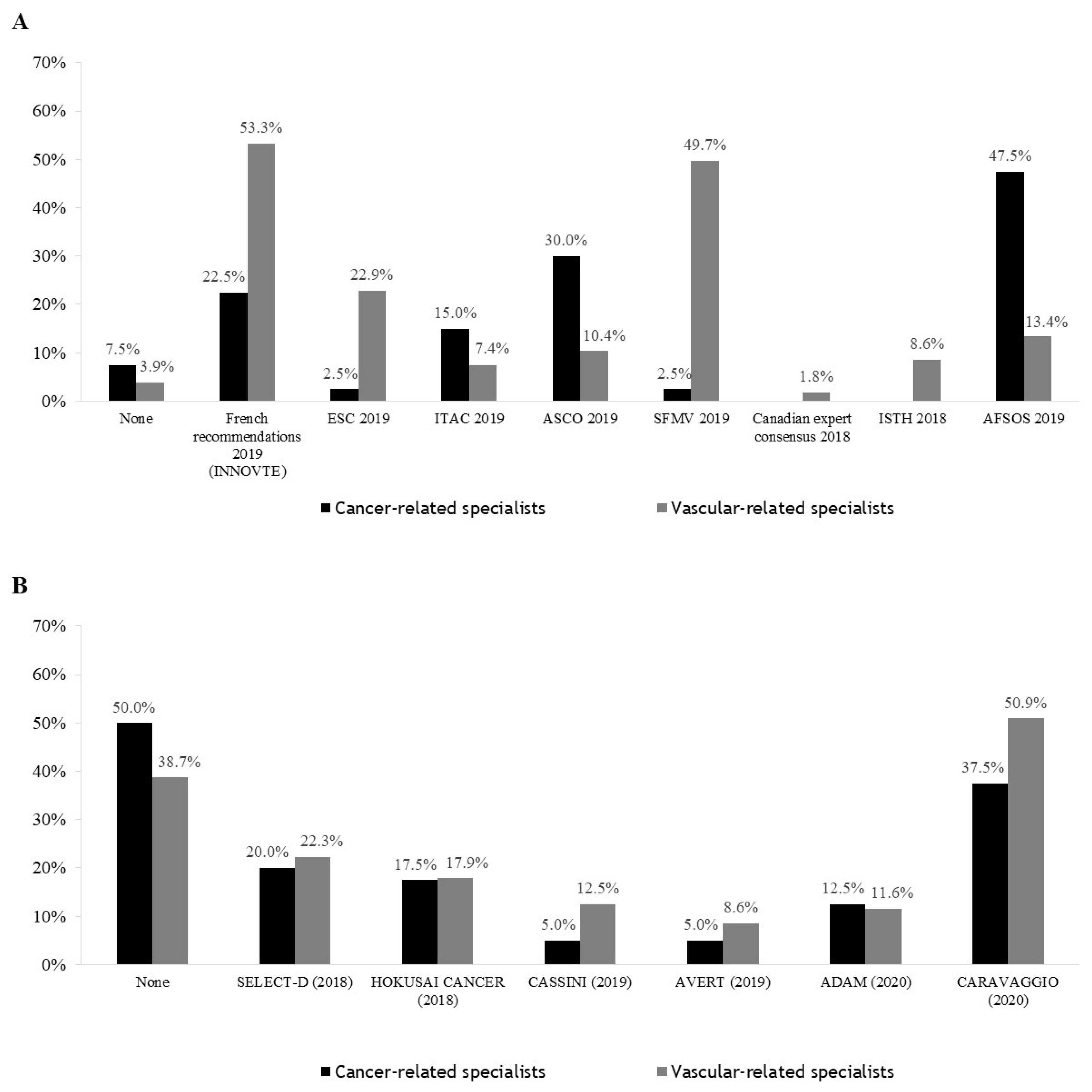
3.4. Guidelines Followed by Practitioners and Studies That Have Influenced Practices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulder, F.I.; Horváth-Puhó, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Büller, H.R.; Sørensen, H.T. Venous Thromboembolism in Cancer Patients: A Population-Based Cohort Study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Brunson, A.; Adesina, O.; Keegan, T.H.M.; Wun, T. The Incidence of Cancer-Associated Thrombosis Is Increasing over Time. Blood Adv. 2022, 6, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Posch, F.; Königsbrügge, O.; Zielinski, C.; Pabinger, I.; Ay, C. Treatment of Venous Thromboembolism in Patients with Cancer: A Network Meta-Analysis Comparing Efficacy and Safety of Anticoagulants. Thromb. Res. 2015, 136, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N.; et al. Rivaroxaban vs Dalteparin in Cancer-Associated Thromboembolism: A Randomized Trial. Chest 2022, 161, 781–790. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor with Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 International Clinical Practice Guidelines for the Treatment and Prophylaxis of Venous Thromboembolism in Patients with Cancer, Including Patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 Guidelines for Management of Venous Thromboembolism: Prevention and Treatment in Patients with Cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 1181–1201. [Google Scholar] [CrossRef]
- Mahé, I.; Meyer, G.; Girard, P.; Bertoletti, L.; Laporte, S.; Couturaud, F.; Mismetti, P.; Sanchez, O. Traitement de la maladie veineuse thromboembolique au cours du cancer. Mise à jour mars 2021. Rev. Mal. Respir. 2021, 38, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kraaijpoel, N.; Di Nisio, M.; Mulder, F.I.; van Es, N.; Beyer-Westendorf, J.; Carrier, M.; Garcia, D.; Grosso, M.; Kakkar, A.K.; Mercuri, M.F.; et al. Clinical Impact of Bleeding in Cancer-Associated Venous Thromboembolism: Results from the Hokusai VTE Cancer Study. Thromb. Haemost. 2018, 118, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Riess, H.; Verhamme, P.; Weitz, J.I.; Young, A.; Bauersachs, R.; Beyer-Westendorf, J.; Crowther, M.; Maraveyas, A. Treatment of Cancer-Associated Thrombosis: The Evolution of Anticoagulant Choice and Clinical Insights into Practical Management. Crit. Rev. Oncol. Hematol 2021, 157, 103125. [Google Scholar] [CrossRef] [PubMed]
- Lafaie, L.; Hodin, S.; Saïb, S.; Bin, V.; Bertoletti, L.; Delavenne, X. Tyrosine Kinase Inhibitors and Direct Oral Anticoagulants: In Vitro Evaluation of Drug-Drug Interaction Mediated by P-Glycoprotein. Fundam. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-F.; Baumann Kreuziger, L.; Leader, A.; Spectre, G.; Lim, M.Y.; Gahagan, A.; Gangaraju, R.; Sanfilippo, K.M.; Mallick, R.; Zwicker, J.I.; et al. Characteristics and Outcomes of Patients on Concurrent Direct Oral Anticoagulants and Targeted Anticancer Therapies-TacDOAC Registry: Communication from the ISTH SSC Subcommittee on Hemostasis and Malignancy. J. Thromb. Haemost. 2021, 19, 2068–2081. [Google Scholar] [CrossRef]
- Wang, C.-L.; Wu, V.C.-C.; Tu, H.-T.; Huang, Y.-T.; Chen, S.-W.; Chu, P.-H.; Wen, M.-S.; Huang, H.-L.; Chang, S.-H. Risk of Major Bleeding Associated with Concomitant Use of Anticancer Drugs and Direct Oral Anticoagulant in Patients with Cancer and Atrial Fibrillation. J. Thromb. Thrombolysis 2022, 53, 633–645. [Google Scholar] [CrossRef]
- Wang, T.-F.; Billett, H.H.; Connors, J.M.; Soff, G.A. Approach to Cancer-Associated Thrombosis: Challenging Situations and Knowledge Gaps. Oncologist 2021, 26, e17–e23. [Google Scholar] [CrossRef]
- Mahé, I.; Scotté, F.; Rey, J.-B.; Elalamy, I.; Lamblin, A.; Mayeur, D.; Pernod, G.; AFSOS (French-Speaking Association for Supportive Care in Oncology); SFMV (French Society for Vascular Medicine). Prevention and Treatment of Cancer-Associated Thrombosis in France: A National Survey among Vascular Disease and Supportive Care Specialists. J. Med. Vasc. 2017, 42, 255–262. [Google Scholar] [CrossRef]
- Kaliel, H.; Mior, M.; Quan, S.; Ghosh, S.; Wu, C.; Bungard, T.J. Retrospective Review of Prescribing Patterns in Cancer-Associated Thrombosis: A Single Center Experience in Edmonton, Alberta, Canada. Clin. Appl. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Maraveyas, A.; Beyer-Westendorf, J.; Lee, A.Y.; Mantovani, L.G.; De Sanctis, Y.; Abdelgawwad, K.; Fatoba, S.; Bach, M.; Cohen, A.T. Cancer-Associated ThrOmboSIs—Patient-Reported OutcoMes With RivarOxaban (COSIMO)—Baseline characteristics and clinical outcomes. Res Pract Thromb Haemost. 2021, 5, e12604. [Google Scholar] [CrossRef]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb Res 2019, 173, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Muñoz, A.; Franco, L.; Mahé, I.; Brenner, B.; Connors, J.M.; Gussoni, G.; Hamulyak, E.N.; Lambert, C.; Suero, M.R.; et al. Apixaban and Dalteparin for the Treatment of Venous Thromboembolism in Patients with Different Sites of Cancer. Thromb Haemost. 2022, 122, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Le Gal, G.; Cho, R.; Tierney, S.; Rodger, M.; Lee, A.Y. Dose Escalation of Low Molecular Weight Heparin to Manage Recurrent Venous Thromboembolic Events despite Systemic Anticoagulation in Cancer Patients. J. Thromb. Haemost. 2009, 7, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Ihaddadene, R.; Le Gal, G.; Delluc, A.; Carrier, M. Dose Escalation of Low Molecular Weight Heparin in Patients with Recurrent Cancer-Associated Thrombosis. Thromb. Res. 2014, 134, 93–95. [Google Scholar] [CrossRef]
- Spyropoulos, J.; Padbury, C. Uncovering Clinical Gaps in Treatment of Cancer-Associated Thrombosis: A Clinical Practice Assessment. ISTH Congress Abstracts. Res. Pract. Thromb. Haemost. 2020, 4 (Suppl. S1). Available online: https://abstracts.isth.org/abstract/uncovering-clinical-gaps-in-treatment-of-cancer-associated-thrombosis-a-clinical-practice-assessment/ (accessed on 7 July 2022).
| Cancer-Related Specialists (N = 40) | Vascular-Related Specialists (N = 336) | Total (N = 376) | |
|---|---|---|---|
| Age | |||
| Less than 40 years | 23 (57.5%) | 66 (19.6%) | 89 (23.7%) |
| 40–49 years | 7 (17.5%) | 69 (20.5%) | 76 (20.2%) |
| 50–59 years | 4 (10.0%) | 118 (35.1%) | 122 (32.4%) |
| 60 years and more | 6 (15.0%) | 83 (24.7%) | 89 (23.7%) |
| Mode of practice | |||
| Public hospital | 22 (55.0%) | 123 (36.6%) | 145 (38.6%) |
| Private | 18 (45.0%) | 213 (63.4%) | 231 (61.4%) |
| Initial Treatment | Consider Switching to DOAC * (N = 376) | |||
|---|---|---|---|---|
| Cancer Specialists (N = 40) | Vascular Specialists (N = 336) | Total (N = 376) | ||
| - | - | - | 334 (88.8% [85.6–92.0]) | |
| Stage and/or evolution of the cancer | 19 (47.5% [32.0–63.0]) | 130 (38.7% [33.5–43.9]) | 149 (39.6% [34.7–44.6]) | 130 (38.9% [33.7–44.1]) |
| Site of cancer | 13 (32.5% [18.0–47.0]) | 126 (37.5% [32.3–42.7]) | 139 (37.0% [32.1–41.8]) | 134 (40.1% [34.9–45.4]) |
| Patient comorbidities/additional risk factors | 15 (37.5% [22.5–52.5]) | 109 (32.4% [27.4–37.4]) | 124 (33.0% [28.2–37.7]) | 91 (27.2% [22.5–32.0]) |
| Risk of drug interaction | 16 (40.0% [24.8–55.2]) | 103 (30.7% [25.7–35.6]) | 119 (31.6% [26.9–36.3]) | 75 (22.5% [18.0–26.9]) |
| Anticancer treatment | 8 (20.0% [7.6–32.4]) | 56 (16.7% [12.7–20.6]) | 64 (17.0% [13.2–20.8]) | 57 (17.1% [13.0–21.1]) |
| Type of index event | 2 (5.0% [0.0–11.7]) | 55 (16.4% [12.4–20.3]) | 57 (15.2% [11.5–18.8]) | 23 (6.9% [4.2–9.6]) |
| Patient preferences | 0 (0.0%) | 40 (11.9% [8.4–15.4]) | 40 (10.6% [7.5–13.7]) | 128 (38.3% [33.1–43.5]) |
| Some anticancer treatments are contraindications to DOACs | 26 (65.0% [50.2–79.8]) | 204 (60.7% [55.5–65.9]) | 230 (61.2% [56.2–66.1]) | |
| Main reason | ||||
| Haemorrhagic risk | 21 (80.8% [65.6–95.9]) | 120 (58.8% [52.1–65.6]) | 141 (61.3% [55.0–67.6]) | |
| Thromboembolic risk | 0 (0.0%) | 53 (26.0% [20.0–32.0]) | 53 (23.0% [17.6–28.5]) | |
| Toxicity of antitumour treatment | 2 (7.7% [0.0–17.9]) | 23 (11.3% [6.9–15.6]) | 25 (10.9% [6.8–14.9]) | |
| Inefficacy of anticancer treatment | 3 (11.5% [0.0–23.8]) | 8 (3.9% [1.3–6.6]) | 11 (4.8% [2.0–7.5]) | |
| LMWH | DOAC | ||
| Thrombocytopenia < 50 G/L | Thrombocytopenia ≤ 75 G/L | Thrombocytopenia < 50 G/L | |
| Continue the ongoing anticoagulant treatment | 30 (8.0% [5.2–10.7]) | 211 (56.1% [51.1–61.1]) | 72 (19.1% [15.2–23.1]) |
| Reduce the anticoagulant dosage by 50% | 86 (22.9% [18.6–27.1]) | 18 (4.8% [2.6–6.9]) | 49 (13.0% [9.6–16.4]) |
| Switch to a prophylactic dose of LMWH | 46 (12.2% [8.9–15.5]) | 6 (1.6% [0.3–2.9]) | 33 (8.8% [5.9–11.6]) |
| Switch to DOAC (if LMWH)/LMWH (if DOAC) | 105 (27.9% [23.3–32.5]) | 85 (22.6% [18.4–26.8]) | 51 (13.6% [10.1–17.0]) |
| Switch to a reduced dose of DOAC | 20 (5.3% [3.0–7.6]) | 7 (1.9% [0.5–3.2]) | 39 (10.4% [7.3–13.4]) |
| Switch to intravenous UFH | 19 (5.1% [2.8–7.3]) | 11 (2.9% [1.2–4.6]) | 20 (5.3% [3.0–7.6]) |
| Switch to Fondaparinux | 70 (18.6% [14.7–22.5]) | 38 (10.1% [7.1–13.1]) | 38 (10.1% [7.1–13.1]) |
| Continue the ongoing treatment + platelet transfusion | - | - | 49 (13.0% [9.6–16.4]) |
| Inferior vena cava filter insertion | - | - | 25 (6.6% [4.1–9.2]) |
| First-Line Management: | LMWH * | DOAC ** |
|---|---|---|
| Increase the dosage of LMWH/ DOAC by 25% | 286 (76.1% [71.7–80.4]) | 1 (0.3% [0.0–0.8]) |
| Switch to a DOAC/ another DOAC | 37 (9.8% [6.8–12.8]) | 12 (3.2% [1.4–5.0]) |
| Switch to intravenous UFH | 16 (4.3% [2.2–6.3]) | 5 (1.3% [0.2–2.5]) |
| VKA bridging | 3 (0.8% [0.0–1.7]) | - |
| Switch to Fondaparinux | 4 (1.1% [0.0–2.1]) | 2 (0.5% [0.0–1.3]) |
| Inferior vena cava filter insertion | 30 (8.0% [5.2–10.7]) | 12 (3.2% [1.4–5.0]) |
| Switch to LMWH (bodyweight-adjusted dose) | - | 309 (82.2% [78.3–86.0]) |
| Switch to LMWH (125% of the bodyweight-adjusted dose) | - | 35 (9.3% [6.4–12.2]) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahé, I.; Chapelle, C.; Plaisance, L.; Bertoletti, L.; Mismetti, P.; Mayeur, D.; Mahé, G.; Couturaud, F., on behalf of AFSOS, SFMV and INNOVTE. Management of Cancer-Associated Thrombosis in France: A National Survey among Vascular Disease and Supportive Care Specialists. Cancers 2022, 14, 4143. https://doi.org/10.3390/cancers14174143
Mahé I, Chapelle C, Plaisance L, Bertoletti L, Mismetti P, Mayeur D, Mahé G, Couturaud F on behalf of AFSOS, SFMV and INNOVTE. Management of Cancer-Associated Thrombosis in France: A National Survey among Vascular Disease and Supportive Care Specialists. Cancers. 2022; 14(17):4143. https://doi.org/10.3390/cancers14174143
Chicago/Turabian StyleMahé, Isabelle, Céline Chapelle, Ludovic Plaisance, Laurent Bertoletti, Patrick Mismetti, Didier Mayeur, Guillaume Mahé, and Francis Couturaud on behalf of AFSOS, SFMV and INNOVTE. 2022. "Management of Cancer-Associated Thrombosis in France: A National Survey among Vascular Disease and Supportive Care Specialists" Cancers 14, no. 17: 4143. https://doi.org/10.3390/cancers14174143
APA StyleMahé, I., Chapelle, C., Plaisance, L., Bertoletti, L., Mismetti, P., Mayeur, D., Mahé, G., & Couturaud, F., on behalf of AFSOS, SFMV and INNOVTE. (2022). Management of Cancer-Associated Thrombosis in France: A National Survey among Vascular Disease and Supportive Care Specialists. Cancers, 14(17), 4143. https://doi.org/10.3390/cancers14174143






