A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Microbiome of Structures of the Aerodigestive System throughout the Development of HNSCC
2.1. The Healthy Microbiome of Structures Affected by HNSCC
2.2. The Microbiome Unique to Pre-Malignant Lesions in Environments Developing HNSCC
2.3. The Oral Microbiota Unique to HNSCC
3. The Role of the Microbiome in Sickness and Health
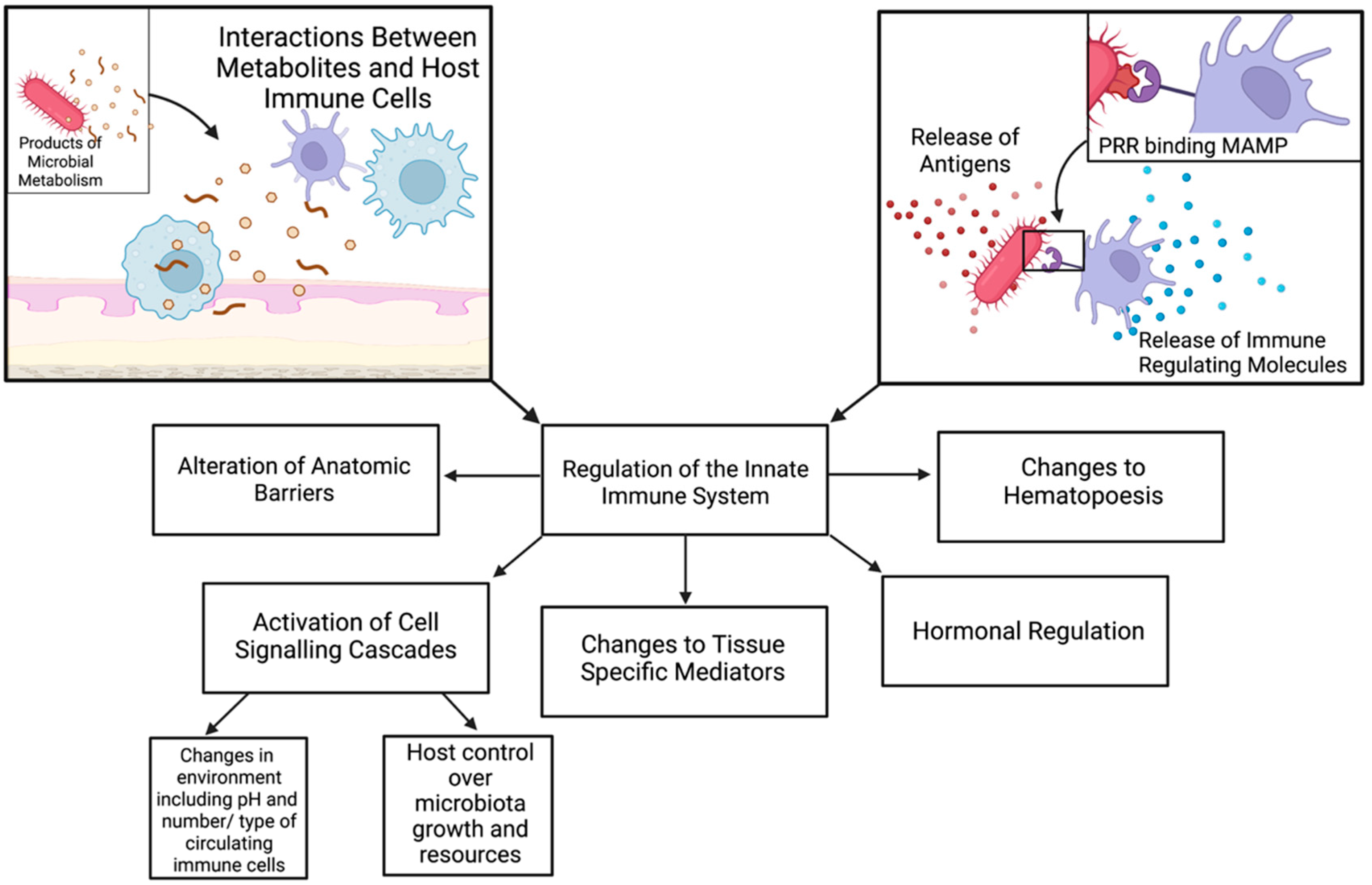
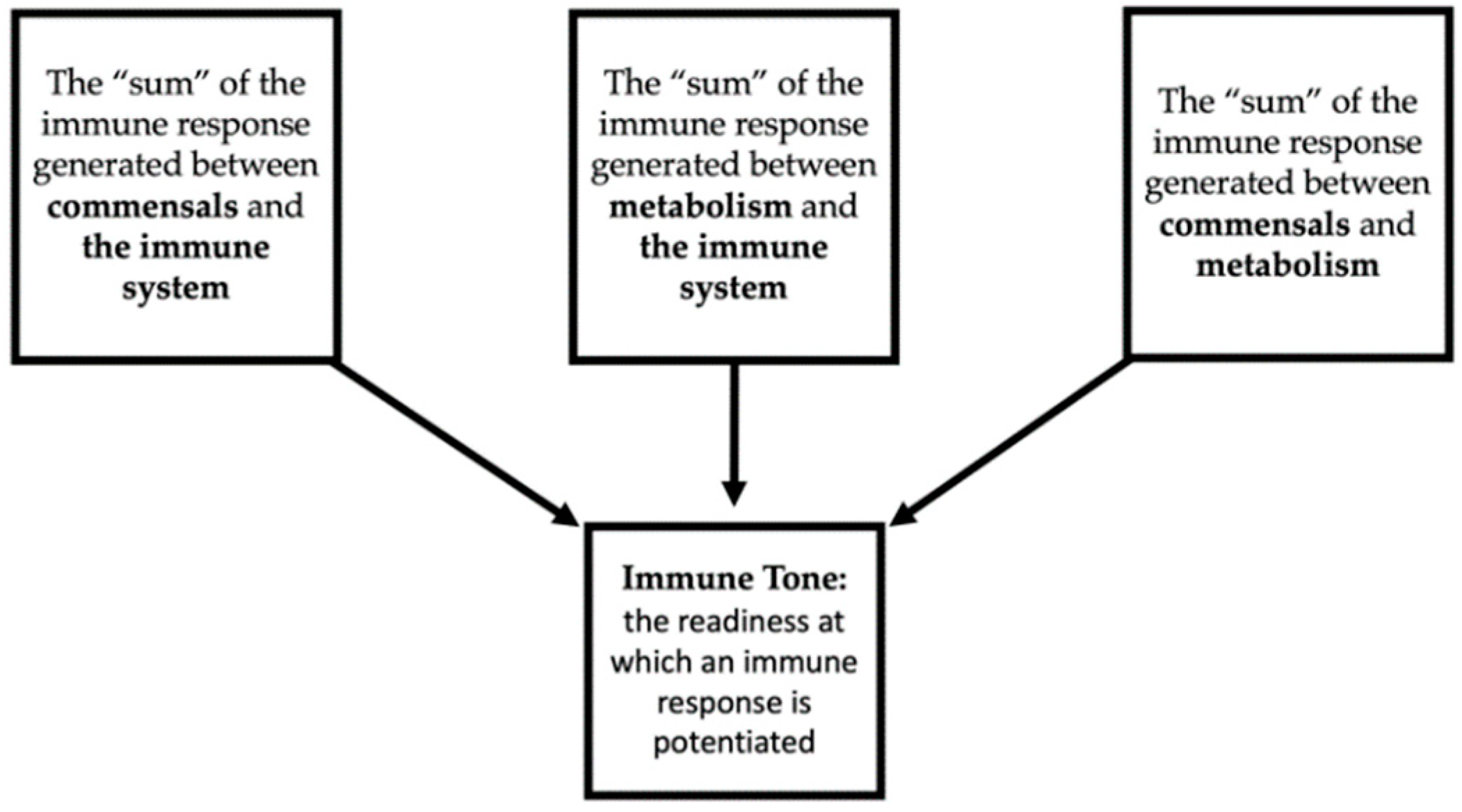
3.1. The Microbiome as a Modulator of Immunity
3.2. The Microbiome as a Modulator of Malignancy
4. The Microbiome and Treatment and Prognosis in HNSCC
4.1. Changes to the Oral Microbiome as a Response to Treatment of HNSCC
4.2. The Microbiome in HNSCC Outcomes
4.3. The Microbiome in HNSCC Treatment Toxicities
4.4. The Microbiome as a Therapeutic Instrument
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- García-Castillo, V.; Sanhueza, E.; McNerney, E.; Onate, S.A.; García, A. Microbiota dysbiosis: A new piece in the understanding of the carcinogenesis puzzle. J. Med. Microbiol. 2016, 65, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Garud, N.R.; Pollard, K.S. Population Genetics in the Human Microbiome. Trends Genet. 2020, 36, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.L.; Tomkovich, S.; Yang, Y.; Jobin, C. Microbiota as a mediator of cancer progression and therapy. Transl. Res. 2017, 179, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, X.-J.; Chao, Y.-L.; Zheng, H.-M.; Sheng, H.-F.; Liu, H.-Y.; He, Y.; Zhou, H.-W. The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. eBioMedicine 2017, 18, 23–31. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Daillère, R.; DeRosa, L.; Bonvalet, M.; Segata, N.; Routy, B.; Gariboldi, M.; Budinská, E.; De Vries, I.J.M.; Naccarati, A.G.; Zitvogel, V.; et al. Trial watch: The gut microbiota as a tool to boost the clinical efficacy of anticancer immunotherapy. OncoImmunology 2020, 9, 1774298. [Google Scholar] [CrossRef]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef]
- Strouse, C.; Mangalam, A.; Zhang, J. Bugs in the system: Bringing the human microbiome to bear in cancer immunotherapy. Gut Microbes 2019, 10, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.H.; Diven, M.A.; Huff, L.W.; Paulos, C.M. Harnessing the Microbiome to Enhance Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 368736. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Bashir, H.; Kumar, R. Emerging role of microbiota in immunomodulation and cancer immunotherapy. Semin. Cancer Biol. 2020, 70, 37–52. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kwon, E.J.; Yu, Y.; Kim, J.; Woo, S.-Y.; Choi, H.-S.; Kwon, M.; Jung, K.; Kim, H.-S.; Park, H.R.; et al. Microbial and molecular differences according to the location of head and neck cancers. Cancer Cell Int. 2022, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Vanhoecke, B.; De Ryck, T.; Stringer, A.; Van De Wiele, T.; Keefe, D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis. 2015, 21, 17–30. [Google Scholar] [CrossRef]
- Ertz-Archambault, N.; Keim, P.; Von Hoff, D. Microbiome and pancreatic cancer: A comprehensive topic review of literature. World J. Gastroenterol. 2017, 23, 1899–1908. [Google Scholar] [CrossRef]
- Morgan, X.C.; Huttenhower, C. Chapter 12: Human microbiome analysis. PLoS Comput. Biol. 2012, 8, e1002808. [Google Scholar] [CrossRef]
- Ewald, H.A.S.; Ewald, P.W. Integrating the microbiome into the barrier theory of cancer. Evol. Appl. 2020, 13, 1701–1707. [Google Scholar] [CrossRef]
- Rastogi, Y.R.; Saini, A.K.; Thakur, V.K.; Saini, R.V. New Insights into Molecular Links Between Microbiota and Gastrointestinal Cancers: A Literature Review. Int. J. Mol. Sci. 2020, 21, 3212. [Google Scholar] [CrossRef]
- Schmidt, B.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.S.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in Abundance of Oral Microbiota Associated with Oral Cancer. PLoS ONE 2014, 9, e106297. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, P.; Matamoros, S.; Montassier, E.; Le Vacon, F.; Potel, G.; Soueidan, A.; Jordana, F.; De La Cochetière, M.-F. The oral cavity microbiota: Between health, oral disease, and cancers of the aerodigestive tract. Can. J. Microbiol. 2017, 63, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Iacovelli, N.A.; Tombolini, V.; Rancati, T.; Polimeni, A.; De Cecco, L.; Valdagni, R.; De Felice, F. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019, 99, 104453. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Han, S.; Chen, Y.; Ji, Z. Variations of Tongue Coating Microbiota in Patients with Gastric Cancer. BioMed Res. Int. 2015, 2015, 173729. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef]
- Oliva, M.; Mulet-Margalef, N.; Ochoa-De-Olza, M.; Napoli, S.; Mas, J.; Laquente, B.; Alemany, L.; Duell, E.; Nuciforo, P.; Moreno, V. Tumor-Associated Microbiome: Where Do We Stand? Int. J. Mol. Sci. 2021, 22, 1446. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, B.; Hu, X.; Li, J.; Wu, M.; Yan, C.; Yang, Y.; Li, Y. Neisseria sicca and Corynebacterium matruchotii inhibit-ed oral squamous cell carcinomas by regulating genome stability. Bioengineered 2022, 13, 14094–14106. [Google Scholar] [CrossRef]
- Avila, M.; Ojcius, D.M.; Yilmaz, Ö. The Oral Microbiota: Living with a Permanent Guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Shin, H.; Pizoni, A.; Werlang, I.C.; Matte, U.; Goldani, M.Z.; Goldani, H.A.S.; Dominguez-Bello, M.G. Delivery Mode and the Transition of Pioneering Gut-Microbiota Structure, Composition and Predicted Metabolic Function. Genes 2017, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007, 5, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Snider, E.J.; Freedberg, D.E.; Abrams, J.A. Potential Role of the Microbiome in Barrett’s Esophagus and Esophageal Adenocarcinoma. Am. J. Dig. Dis. 2016, 61, 2217–2225. [Google Scholar] [CrossRef]
- Galvão-Moreira, L.V.; da Cruz, M.C.F.N. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016, 53, 17–19. [Google Scholar] [CrossRef]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef]
- Lee, W.-H.; Chen, H.-M.; Yang, S.-F.; Wen-Liang, C.; Peng, C.-Y.; Tzu-Ling, Y.; Tsai, L.-L.; Wu, B.-C.; Hsin, C.-H.; Huang, C.-N.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef]
- Ott, S.J.; El Mokhtari, N.E.; Musfeldt, M.; Hellmig, S.; Freitag, S.; Rehman, A.; Kühbacher, T.; Nikolaus, S.; Namsolleck, P.; Blaut, M.; et al. Detection of Diverse Bacterial Signatures in Atherosclerotic Lesions of Patients With Coronary Heart Disease. Circulation 2006, 113, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Mok, S.F.; Karuthan, C.; Cheah, Y.K.; Ngeow, W.C.; Rosnah, Z.; Yap, S.F.; Ong, H.K.A. The oral microbiome community variations associated with normal, potentially malignant disorders and malignant lesions of the oral cavity. Malays. J. Pathol. 2017, 39, 1–15. [Google Scholar] [PubMed]
- Wang, K.; Lu, W.; Tu, Q.; Ge, Y.; He, J.; Zhou, Y.; Gou, Y.; Van Nostrand, J.; Qin, Y.; Li, J.; et al. Preliminary analysis of salivary microbiome and their potential roles in oral lichen planus. Sci. Rep. 2016, 6, 22943. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Brazier, L.; Faugère, D.; Renaud, F.; Thomas, F.; Roche, B. Can intestinal microbiota be associated with non-intestinal cancers? Sci. Rep. 2017, 7, 112722. [Google Scholar] [CrossRef]
- Goedert, J.J.; Hua, X.; Bielecka, A.; Okayasu, I.; Milne, G.L.; Jones, G.; Fujiwara, M.; Sinha, R.; Wan, Y.; Xu, X.; et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-noncoated faecal microbiota. Br. J. Cancer 2018, 118, 471–479. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Zhou, X.; You, M.; Du, Q.; Yang, X.; He, J.; Zou, J.; Cheng, L.; Li, M.; et al. Oral Microbiota Distinguishes Acute Lymphoblastic Leukemia Pediatric Hosts from Healthy Populations. PLoS ONE 2014, 9, e102116. [Google Scholar] [CrossRef]
- Wang, H.; Funchain, P.; Bebek, G.; Altemus, J.; Zhang, H.; Niazi, F.; Peterson, C.; Lee, W.T.; Burkey, B.B.; Eng, C. Microbio-mic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017, 9, 14. [Google Scholar] [CrossRef]
- Takahashi, Y.; Park, J.; Hosomi, K.; Yamada, T.; Kobayashi, A.; Yamaguchi, Y.; Iketani, S.; Kunisawa, J.; Mizuguchi, K.; Maeda, N.; et al. Analysis of oral microbiota in Japanese oral cancer patients using 16S rRNA sequencing. J. Oral Biosci. 2019, 61, 120–128. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.; Perera, I.; Ipe, D.; Ulett, G.; Speicher, D.; Chen, T.; Johnson, N. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Z.; Qi, Y.; Wen, X.; Zhang, L. Metagenomic Analysis Reveals a Changing Microbiome Associated With the Depth of Invasion of Oral Squamous Cell Carcinoma. Front. Microbiol. 2022, 13, 795777. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Ma, C.; Wang, K.; Liu, S.; Sun, J.; Tan, W.; Neckenig, M.; Wang, Q.; Dong, Z.; Gao, W.; et al. Dysbiotic tumor micro-biota associates with head and neck squamous cell carcinoma outcomes. Oral. Oncol. 2022, 124, 105657. [Google Scholar] [CrossRef]
- Eun, Y.-G.; Lee, J.-W.; Kim, S.W.; Hyun, D.-W.; Bae, J.-W.; Lee, Y.C. Oral microbiome associated with lymph node metastasis in oral squamous cell carcinoma. Sci. Rep. 2021, 11, 23176. [Google Scholar] [CrossRef]
- Mager, D.; Haffajee, A.; Devlin, P.; Norris, C.; Posner, M.R.; Goodson, J. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; White, J.R.; Godoy-Vitorino, F.; Rodríguez-Hilario, A.; Navarro, K.; González, H.; Michailidi, C.; Jedlicka, A.; Canapp, S.; Bondy, J.; et al. High-resolution microbiome profiling uncovers Fusobacterium nucleatum, Lactobacillus gasseri/johnsonii, and Lactobacillus vaginalis associated to oral and oropharyngeal cancer in saliva from HPV positive and HPV negative patients treated with surgery and chemo-radiation. Oncotarget 2017, 8, 110931–110948. [Google Scholar] [CrossRef]
- Lim, Y.; Fukuma, N.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front. Cell. Infect. Microbiol. 2018, 8, 267. [Google Scholar] [CrossRef]
- Yang, S.; Lin, C.; Chuang, C.; Lee, Y.; Chung, W.; Lai, H.; Chang, L.; Su, S. Host Genetic Associations with Salivary Microbiome in Oral Cancer. J. Dent. Res. 2022, 101, 590–598. [Google Scholar] [CrossRef]
- Chen, Z.; Wong, P.Y.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Wong, S.H.; et al. The Intersection between Oral Microbiota, Host Gene Methylation and Patient Outcomes in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 3425. [Google Scholar] [CrossRef]
- Neuzillet, C.; Marchais, M.; Vacher, S.; Hilmi, M.; Schnitzler, A.; Meseure, D.; Leclere, R.; Lecerf, C.; Dubot, C.; Jeannot, E.; et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci. Rep. 2021, 11, 7870. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.E.; White, J.R.; Godoy-Vitorino, F.; Gonzalez, H.; Rodríguez-Hilario, A.; Navarro, K.; Miranda-Carboni, G.A.; Michailidi, C.; Jedlicka, A.; Hao, S.; et al. Abstract 1018: High-resolution microbiome profiling and genome wide arrays uncover bacteria driven alterations of oncogenic and immune pathways in head and neck cancer patients treated with surgery, chemo-radiation and PD-1 checkpoint blockade therapy. Cancer Res. 2017, 77, 1018. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Chen, T.; Li, Q.; Peng, W.; Li, H.; Tang, X.; Fu, X. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis in Mice via a Toll-Like Receptor 4/p21-Activated Kinase 1 Cascade. Am. J. Dig. Dis. 2018, 63, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, É.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. MBio 2022, 13, e03144-21. [Google Scholar] [CrossRef]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef]
- Giuffrè, M.; Campigotto, M.; Campisciano, G.; Comar, M.; Crocè, L.S. A story of liver and gut microbes: How does the intestinal flora affect liver disease? A review of the literature. Am. J. Physiol. Liver Physiol. 2020, 318, G889–G906. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Moutsopoulos, N.M. IL-17: Overview and role in oral immunity and microbiome. Oral Dis. 2017, 23, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol. 2000 2015, 69, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Kuipers, F.; de Boer, J.F.; Staels, B. Microbiome Modulation of the Host Adaptive Immunity through Bile Acid Modification. Cell Metab. 2020, 31, 445–447. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef]
- Hoeppli, R.E.; Wu, D.; Cook, L.; Levings, M.K. The Environment of Regulatory T Cell Biology: Cytokines, Metabolites, and the Microbiome. Front. Immunol. 2015, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Chervonsky A, V. Microbiota and autoimmunity. Cold Spring Harb. Perspect. Biol. 2013, 5, a007294. [Google Scholar] [CrossRef]
- Kroemer, G.; Senovilla, L.; Galluzzi, L.; Andre, F.; Zitvogel, L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 2015, 21, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef]
- Capurso, G.; Lahner, E. The interaction between smoking, alcohol and the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 579–588. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 2019, 176, 998–1013.e16. [Google Scholar] [CrossRef]
- Frank, D.N.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.M.; Robertson, C.E.; Liu, Y.; Wang, H.; Levens, C.L.; et al. A dysbiotic microbiome promotes head and neck squamous cell carcinoma. Oncogene 2022, 41, 1269–1280. [Google Scholar] [CrossRef]
- Zackular, J.P.; Baxter, N.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The Gut Microbiome Modulates Colon Tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.; Yao, L.; Maeda, K.; Rose, T.M.; Lewis, E.L.; Duman, M.; Lamont, R.J.; Ojcius, D.M. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell. Microbiol. 2008, 10, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Speicher, D.J.; Perera, I.; Johnson, N.W. Emerging role of bacteria in oral carcinogenesis: A review with special ref-erence to perio-pathogenic bacteria. J. Oral Microbiol. 2016, 8, 32762. [Google Scholar] [CrossRef]
- Vogelmann, R.; Amieva, M. The role of bacterial pathogens in cancer. Curr. Opin. Microbiol. 2007, 10, 76–81. [Google Scholar] [CrossRef]
- Zhang, G.; Ghosh, S. Molecular mechanisms of NF-κB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000, 6, 453–457. [Google Scholar] [CrossRef]
- Yost, S.; Stashenko, P.; Choi, Y.; Kukuruzinska, M.; Genco, C.A.; Salama, A.; Weinberg, E.; Kramer, C.D.; Frias-Lopez, J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int. J. Oral Sci. 2018, 10, 32. [Google Scholar] [CrossRef]
- Ohshima, J.; Wang, Q.; Fitzsimonds, Z.R.; Miller, D.P.; Sztukowska, M.N.; Jung, Y.-J.; Hayashi, M.; Whiteley, M.; Lamont, R.J. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc. Natl. Acad. Sci. USA 2019, 116, 8544–8553. [Google Scholar] [CrossRef] [Green Version]
- Abdulkareem, A.A.; Shelton, R.M.; Landini, G.; Cooper, P.R.; Milward, M.R. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adhes. Migr. 2018, 12, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Ojo, A.; Ahmed, S.; Carenbauer, A.L.; Wang, Q.; Shumway, B.; Jenkinson, H.F.; Wang, H.; Darling, D.S.; Lamont, R.J. Porphyromonas gingivalis initiates a mesenchymal-like transition through ZEB1 in gingival epithelial cells. Cell Microbiol. 2016, 18, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Liu, J.; Guo, Y.; Li, C.; Wang, H.; Wang, H.; Zhao, H.; Pan, Y. Persistent exposure to Porphyromonas gingivalis promotes proliferative and invasion capabili-ties, and tumorigenic properties of human immortalized oral epithelial cells. Front. Cell Infect. Microbiol. 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Jarzina, F.; Domann, E.; Meyle, J. Porphyromonas gingivalis activates NFκB and MAPK pathways in human oral epithelial cells. BMC Immunol. 2017, 18, 1. [Google Scholar] [CrossRef]
- Kumar, P.S.; Matthews, C.R.; Joshi, V.; de Jager, M.; Aspiras, M. Tobacco Smoking Affects Bacterial Acquisition and Colonization in Oral Biofilms. Infect. Immun. 2011, 79, 4730–4738. [Google Scholar] [CrossRef] [PubMed]
- Bagaitkar, J.; DeMuth, D.R.; Scott, D.A. Tobacco use increases susceptibility to bacterial infection. Tob. Induc. Dis. 2008, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Hernandez, D.Z.; Wong, Z.C.; Wandler, A.M.; Guillemin, K. The bacterial virulence factor CagA induces microbial dysbiosis that contributes to excessive epithelial cell proliferation in the Drosophila gut. PLoS Pathog. 2017, 13, e1006631. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2013, 491, 254–258. [Google Scholar] [CrossRef]
- Irrazábal, T.; Belcheva, A.; Girardin, S.E.; Martin, A.; Philpott, D.J. The Multifaceted Role of the Intestinal Microbiota in Colon Cancer. Mol. Cell 2014, 54, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.E.; Thiennimitr, P.; Winter, M.G.; Butler, B.P.; Huseby, D.L.; Crawford, R.W.; Russell, J.M.; Bevins, C.L.; Adams, L.G.; Tsolis, R.M.; et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 2010, 467, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Faber, F.; Bäumler, A.J. The impact of intestinal inflammation on the nutritional environment of the gut microbiota. Immunol. Lett. 2014, 162, 48–53. [Google Scholar] [CrossRef]
- Nuccio, S.-P.; Bäumler, A.J. Comparative Analysis of Salmonella Genomes Identifies a Metabolic Network for Escalating Growth in the Inflamed Gut. mBio 2014, 5, e00929-14. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chávez, F.; Winter, S.; Lopez, C.A.; Xavier, M.N.; Winter, M.G.; Nuccio, S.-P.; Russell, J.M.; Laughlin, R.; Lawhon, S.D.; Sterzenbach, T.; et al. Salmonella Uses Energy Taxis to Benefit from Intestinal Inflammation. PLoS Pathog. 2013, 9, e1003267. [Google Scholar] [CrossRef]
- Winter, E.S.; Bäumler, A.J. Dysbiosis in the inflamed intestine: Chance favors the prepared microbe. Gut Microbes 2014, 5, 71–73. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2014, 339, 708–711. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Winter, S.E.; Winter, M.G.; Xavier, M.N.; Tolstikov, V.; Huseby, D.L.; Sterzenbach, T.; Tsolis, R.M.; Roth, J.R.; Baumler, A.J. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 17480–17485. [Google Scholar] [CrossRef]
- Wiedemann, A.; Virlogeux-Payant, I.; Chaussã, A.-M.; Schikora, A.; Evelge, P. Interactions of Salmonella with animals and plants. Front. Microbiol. 2014, 5, 791. [Google Scholar] [CrossRef]
- Yao, á.L.; Jermanus, C.; Barbetta, B.; Choi, C.; Verbeke, P.; Ojcius, D.M.; Yilmaz, O. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010, 25, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Aymeric, L.; Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Martins, I.; Kroemer, G.; Smyth, M.J.; Zitvogel, L. Tumor Cell Death and ATP Release Prime Dendritic Cells and Efficient Anticancer Immunity. Cancer Res. 2010, 70, 855–858. [Google Scholar] [CrossRef]
- Almeida-Da-Silva, C.L.C.; Morandini, A.C.; Ulrich, H.; Ojcius, D.M.; Coutinho-Silva, R. Purinergic signaling during Porphyromonas gingivalis infection. Biomed. J. 2016, 39, 251–260. [Google Scholar] [CrossRef]
- Kadosh, E.; Snir-Alkalay, I.; Venkatachalam, A.; May, S.; Lasry, A.; Elyada, E.; Zinger, A.; Shaham, M.; Vaalani, G.; Mernberger, M.; et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 2020, 586, 133–138. [Google Scholar] [CrossRef]
- Gillenwater, A.; Zou, C.-P.; Zhong, M.; Lotan, R. Effects of sodium butyrate on growth, differentiation, and apoptosis in head and neck squamous carcinoma cell lines. Head Neck 2000, 22, 247–256. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Gillenwater, A.; Xu, X.; Estrov, Y.; Sacks, P.G.; Lotan, D.; Lotan, R. Modulation of galectin-1 content in human head and neck squamous carcinoma cells by sodium butyrate. Int. J. Cancer 1998, 75, 217–224. [Google Scholar] [CrossRef]
- Salaspuro, V.; Salaspuro, M. Synergistic effect of alcohol drinking and smoking onin vivo acetaldehyde concentration in saliva. Int. J. Cancer 2004, 111, 480–483. [Google Scholar] [CrossRef]
- Gaonkar, P.P.; Patankar, S.R.; Tripathi, N.; Sridharan, G. Oral bacterial flora and oral cancer: The possible link? J. Oral Maxillofac. Pathol. JOMFP 2018, 22, 234. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, S.; Garg, P.K.; Dubey, A.K.; Deo, S.V.S.; Verma, D. Comparative and analytical characterization of the oral bacteriome of smokeless tobacco users with oral squamous cell carcinoma. Appl. Microbiol. Biotechnol. 2022, 106, 4115–4128. [Google Scholar] [CrossRef]
- Hezel, M.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015, 21, 7–16. [Google Scholar] [CrossRef]
- Putze, J.; Hennequin, C.; Nougayrède, J.-P.; Zhang, W.; Homburg, S.; Karch, H.; Bringer, M.; Fayolle, C.; Carniel, E.; Rabsch, W. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun. 2009, 77, 4696–4703. [Google Scholar] [CrossRef] [PubMed]
- Thelestam, M. Cytolethal distending toxins. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 111–133. [Google Scholar]
- Banerjee, S.; Tian, T.; Wei, Z.; Peck, K.N.; Shih, N.; Chalian, A.A.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; Alwine, J.; et al. Microbial Signatures Associated with Oropharyngeal and Oral Squamous Cell Carcinomas. Sci. Rep. 2017, 7, 4036. [Google Scholar] [CrossRef] [PubMed]
- Ewald, P.W.; Ewald, H.A.S. Infection, mutation, and cancer evolution. Klin. Wochenschr. 2012, 90, 535–541. [Google Scholar] [CrossRef]
- Ewald, H.A.S.; Ewald, P.W. Natural selection, microbiomes, and public health. Yale J. Biol. Med. 2018, 91, 445–455. [Google Scholar]
- Cong, J.; Zhu, H.; Liu, D.; Li, T.; Zhang, C.; Zhu, J.; Lv, H.; Liu, K.; Hao, C.; Tian, Z.; et al. A Pilot Study: Changes of Gut Microbiota in Post-surgery Colorectal Cancer Patients. Front. Microbiol. 2018, 9, 2777. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Chan, J.Y.; Wong, B.; Wong, E.W.; Chan, P.; Chen, Z. Abstract A37: Restoration of oral microbiota dysbiosis in head and neck squamous cell carcinoma after surgery. Cancer Res. 2020, 80, A37. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Gao, L.; Hu, Y.; Wang, Y.; Jiang, W.; He, Z.; Zhu, C.; Ma, R.; Huang, Z. Exploring the variation of oral microbiota in supragingival plaque during and after head-and-neck radiotherapy using pyrosequencing. Arch. Oral Biol. 2015, 60, 1222–1230. [Google Scholar] [CrossRef]
- Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.; Langendijk, J.A.; van Winkelhoff, A.J.; Vissink, A.; Spijkervet, F.K. Head and neck intensity modulated radiation therapy leads to an increase of opportunistic oral pathogens. Oral Oncol. 2016, 58, 32–40. [Google Scholar] [CrossRef]
- Montassier, E.; Batard, E.; Massart, S.; Gastinne, T.; Carton, T.; Caillon, J.; Le Fresne, S.; Caroff, N.; Hardouin, J.B.; Moreau, P.; et al. 16S rRNA Gene Pyrosequencing Reveals Shift in Patient Faecal Microbiota During High-Dose Chemotherapy as Conditioning Regimen for Bone Marrow Transplantation. Microb. Ecol. 2014, 67, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Al-Dasooqi, N.; Bowen, J.M.; Tan, T.H.; Radzuan, M.; Logan, R.M.; Mayo, B.; Keefe, D.M.K.; Gibson, R.J. Biomarkers of chemotherapy-induced diarrhoea: A clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support. Care Cancer 2013, 21, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.J.; Tissing, W.J.E.; Dun, C.A.J.; Meessen, N.E.L.; Kamps, W.A.; de Bont, E.S.J.M.; Harmsen, H.J.M. Chemotherapy Treatment in Pediatric Patients with Acute Myeloid Leukemia Receiving Antimicrobial Prophylaxis Leads to a Relative Increase of Colonization with Potentially Pathogenic Bacteria in the Gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Smith, D.P.; Ms, P.S.; Ajami, N.J.; Ms, W.D.W.; Daver, N.G.; Chemaly, R.F.; Marsh, L.; Ghantoji, S.S.; Pemmaraju, N.; et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 2016, 122, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Tang, K.D.; Karpe, A.V.; Beale, D.J.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. Chemoradiation therapy changes oral microbiome and metabolomic profiles in patients with oral cavity cancer and oropharyngeal cancer. Head Neck 2021, 43, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Schneeberger, P.H.H.; Rey, V.; Cho, M.; Taylor, R.; Hansen, A.R.; Taylor, K.; Hosni, A.; Bayley, A.; Hope, A.J.; et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br. J. Cancer 2021, 124, 1543–1551. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Liu, T.-B.; Zheng, Z.-H.; Pan, J.; Pan, L.-L.; Chen, L.-H. Prognostic role of plasma Epstein-Barr virus DNA load for nasopharyngeal carcinoma: A meta-analysis. Clin. Investig. Med. 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Pflug, N.; Kluth, S.; Vehreschild, J.J.; Bahlo, J.; Tacke, D.; Biehl, L.; Eichhorst, B.; Fischer, K.; Cramer, P.; Fink, A.-M.; et al. Efficacy of antineoplastic treatment is associated with the use of antibiotics that modulate intestinal microbiota. OncoImmunology 2016, 5, e1150399. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2019, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Koprinarova, M.; Markovska, P.; Iliev, I.; Anachkova, B.; Russev, G. Sodium butyrate enhances the cytotoxic effect of cisplatin by abrogating the cisplatin imposed cell cycle arrest. BMC Mol. Biol. 2010, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Woo, B.H.; Lee, J.H.; Yoon, S.; Cho, Y.; Kim, Y.-D.; Park, H.R. Oral Administration of Porphyromonas gingivalis, a Major Pathogen of Chronic Periodontitis, Promotes Resistance to Paclitaxel in Mouse Xenografts of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2494. [Google Scholar] [CrossRef]
- Woo, B.H.; Kim, D.J.; Choi, J.I.; Kim, S.J.; Park, B.S.; Song, J.M.; Lee, J.H.; Park, H.R. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget 2017, 8, 46981–46992. [Google Scholar] [CrossRef]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-De-Gómez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2020, 130, 466–479. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Kaiser, J. Gut microbes shape response to cancer immunotherapy. Science 2017, 358, 573. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Harrington, K.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Vokes, E.; Gillison, M.; Even, C.; et al. Abstract CT022: Evaluation of oral microbiome profiling as a response biomarker in squamous cell carcinoma of the head and neck: Analyses from CheckMate 141. Cancer Res. 2017, 77, CT022. [Google Scholar] [CrossRef]
- Bernal, M.O.; Schneeberger, P.H.; Taylor, R.; Rey, V.; Hansen, A.R.; Taylor, K.; Bayley, A.; Hope, A.; Hosni, A.; Bratman, S.V.; et al. Role of the oral and gut microbiota as a biomarker in locoregionally advanced oropharyngeal squamous cell carcinoma (ROMA LA-OPSCC). J. Clin. Oncol. 2019, 37, 6045. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melano-ma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C. Gut microbiome modulates response to anti–PD-1 immunotherapy in mel-anoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Deng, W.-W.; Song, W.-F.; Wu, C.-C.; Liu, J.; Hong, S.; Zhuang, Z.-N.; Cheng, H.; Sun, Z.-J.; Zhang, X.-Z. Biomaterial-mediated modulation of oral microbiota synergizes with PD-1 blockade in mice with oral squamous cell carcinoma. Nat. Biomed. Eng. 2022, 6, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Vellanki, P.J.; Marur, S.; Bandaru, P.; Mishra-Kalyani, P.S.; By, K.; Girvin, A.; Chatterjee, S.; Singh, H.; Keegan, P.; Larkins, E.A.; et al. Evaluation of the correlation between antibiotic use and survival in patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) treated with immune checkpoint inhibitors (ICIs). J. Clin. Oncol. 2020, 38, 6509. [Google Scholar] [CrossRef]
- DrPH, C.C.R.; Wang, J.; Zhang, L.; Peterson, C.B.; Do, K.; Jenq, R.R.; Shelburne, S.; Shah, D.P.; Chambers, M.S.; Hanna, E.Y.; et al. Oral microbiome and onset of oral mucositis in patients with squamous cell carcinoma of the head and neck. Cancer 2020, 126, 5124–5136. [Google Scholar] [CrossRef]
- Shao, Z.-Y.; Tang, Z.-S.; Yan, C.; Jiang, Y.-T.; Ma, R.; Liu, Z.; Huang, Z.-W. Effects of Intensity-modulated Radiotherapy on Human Oral Microflora. J. Radiat. Res. 2011, 52, 834–839. [Google Scholar] [CrossRef]
- Almståhl, A.; Wikström, M.; Fagerberg-Mohlin, B. Microflora in oral ecosystems in subjects with radiation-induced hyposalivation. Oral Dis. 2008, 14, 541–549. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Wiemann, B.; Starnes, C.O. Coley's toxins, tumor necrosis factor and cancer research: A historical perspective. Pharmacol. Ther. 1994, 64, 529–564. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Hasanian, S.M.; Avan, A.; Yaghoubi, A.; Khazaei, M. Bacteriotherapy in gastrointestinal cancer. Life Sci. 2020, 254, 117754. [Google Scholar] [CrossRef]
- Zlotta, A.R.; Fleshner, N.E.; Jewett, M.A. The management of BCG failure in non-muscle-invasive bladder cancer: An update. Can. Urol. Assoc. J. 2009, 3, S199–S205. [Google Scholar] [CrossRef] [PubMed]
- Gontero, P.; Bohle, A.; Malmstrom, P.-U.; O’Donnell, M.A.; Oderda, M.; Sylvester, R.; Witjes, F. The Role of Bacillus Calmette-Guérin in the Treatment of Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2010, 57, 410–429. [Google Scholar] [CrossRef] [PubMed]
- Begde, D.; Bundale, S.; Mashitha, P.; Rudra, J.; Nashikkar, N.; Upadhyay, A. Immunomodulatory efficacy of nisin-a bacterial lantibiotic peptide. J. Pept. Sci. 2011, 17, 438–444. [Google Scholar] [CrossRef]
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC 1. Cancer Med. 2012, 1, 295–305. [Google Scholar] [CrossRef]
- Kamarajan, P.; Hayami, T.; Matte, B.; Liu, Y.; Danciu, T.; Ramamoorthy, A.; Worden, F.; Kapila, S.; Kapila, Y. Nisin ZP, a Bacteriocin and Food Preservative, Inhibits Head and Neck Cancer Tumorigenesis and Prolongs Survival. PLoS ONE 2015, 10, e0131008. [Google Scholar] [CrossRef]
- Thomas, S.M.; Zeng, Q.; Epperly, M.W.; Gooding, W.E.; Pastan, I.; Wang, Q.C.; Greenberger, J.; Grandis, J.R. Abrogation of Head and Neck Squamous Cell Carcinoma Growth by Epidermal Growth Factor Receptor Ligand Fused to Pseudomonas Exotoxin Transforming Growth Factor α-PE38. Clin. Cancer Res. 2004, 10, 7079–7087. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- Watson, G.; Oliva, M.; Chen, B.; Eliason, A.; Jennings, S.; Taylor, R.; Abdalaty, A.; Bayley, A.; Hope, A.; Bratman, S.; et al. 977TiP Role of microbiome as a biomarker in locoregionally-advanced oropharyngeal squamous cell carcinoma 2 (ROMA LA-OPSCC2). Ann. Oncol. 2020, 31, S685–S686. [Google Scholar] [CrossRef]
- Gavrielatou, N.; Doumas, S.; Economopoulou, P.; Foukas, P.G.; Psyrri, A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat. Rev. 2020, 84, 101977. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, S.; Chinello, P.; Rossi, L.; Zanesco, L. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharo-myces boulardii. Support. Care Cancer 2000, 8, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus Sepsis Associated With Probiotic Therapy. Pediatrics 2005, 115, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Oggioni, M.R.; Pozzi, G.; Valensin, P.E.; Galieni, P.; Bigazzi, C. Recurrent Septicemia in an Immunocompromised Patient Due to Probiotic Strains of Bacillus subtilis. J. Clin. Microbiol. 1998, 36, 325–326. [Google Scholar] [CrossRef]
- Koyama, S.; Fujita, H.; Shimosato, T.; Kamijo, A.; Ishiyama, Y.; Yamamoto, E.; Ishii, Y.; Hattori, Y.; Hagihara, M.; Yamazaki, E.; et al. Septicemia from Lactobacillus rhamnosus GG, from a Probiotic Enriched Yogurt, in a Patient with Autologous Stem Cell Transplantation. Probiotics Antimicrob. Proteins 2019, 11, 295–298. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Jenq, R.R.; Shelburne, S.A. Can Consideration of the Microbiome Improve Antimicrobial Utilization and Treatment Outcomes in the Oncology Patient? Clin. Cancer Res. 2017, 23, 3263–3268. [Google Scholar] [CrossRef]
- Çiltaş, A.; Yılmaz, F.T. Relationship Between Cancer and Microbiota. Int. J. Hum. Health Sci. (IJHHS) 2020, 4, 251–256. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J.; et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef]
- Thomsen, M.; Vitetta, L. Adjunctive Treatments for the Prevention of Chemotherapy- and Radiotherapy-Induced Mucositis. Integr. Cancer Ther. 2018, 17, 1027–1047. [Google Scholar] [CrossRef]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; Dupont, H.L.; Jiang, Z.-D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.-C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef] [PubMed]

| Healthy | |||
|---|---|---|---|
| Flora | Technique | Notes | Source |
| Firmicutes, Bacteroidetes, Proteobacteria, Fusobacteria, and Actinobacteria | 16S rDNA V4 sequencing of Isohelix SK-2 swabs | Firmicutes and Actinobacteria reduced in malignant tissues | [21] |
| Streptococcus, Haemophilus, Actinomyces, and Prevotella | 16S rRNA sequencing | Reportedly present in the healthy oral microbiome (generally) | [17,22] |
| Neisseria, Haemophilus, Fusobacterium, Porphyromonas | 16S rRNA V2-V4 sequencing of oral swabs | Reportedly present in the healthy oral microbiome based on healthy controls in a gastric cancer study | [24] |
| Streptococcus | 16S rRNA sequencing | Reportedly present in the healthy oral microbiome (generally) | [16] |
| Actinomyces | 16S rDNA sequencing of paired normal and tumor resections | Concentration of Parvimonas positively correlated to T-stage | [48] |
| Haemophilus, Corynebacterium, Paludibacter, Porphyromonas, and Capnocytophaga | 16S rRNA sequencing of oral rinse | Examiners were able to reliably predict the presence of oral cavitycancer and oropharyngeal cancers | [56] |
| Rothia and Haemophilus | 16S rRNA sequencing of salivary samples | More prevalent in control patients than patients with HNSCC | [49] |
| Premalignant | |||
| Flora | Technique | Notes | Source |
| Cloacibacillus, Gemmiger, Oscillospira, and Roseburia | 16S rDNA V4 sequencing of saliva samples | Also present in patients with confirmed malignancy, but statistically decreased in healthy controls | [39] |
| M. micronuciformis | 16S PCR V6-V9 sequencing of swabs | A partner of Fusobacterium in fostering the development of malignancy in the throat by changing the microenvironment and biofilm formation | [41] |
| Prevotella melaninogenica, Porphyromonas, and Solobacterium | 16S rRNA V4 sequencing of salivary samples | Lower abundance of Haemophilus, Corynebacterium, Cellulosimicrobium, and Campylobacter in oral microbiota in comparison to healthy controls | [42] |
| Malignant | |||
| Flora | Technique | Notes | Source |
| Bacillus, Enterococcus, Parvimonas, Peptostreptococcus, and Slackia | 16S rDNA V4 sequencing of saliva samples | Increased in cases of malignancy when compared to oral potentially malignant disorders | [39] |
| Parvimonas | 16S rDNA sequencing of paired normal and tumor resections | Concentration of Parvimonas positively correlated to T-stage | [48] |
| Peptostreptococcus, Fusobacterium, Alloprevotella, and Capnocytophaga | 16S rRNA sequencing of salivary samples | More abundant when comparing the microbiome of cancer patients to the control patients | [49] |
| Fusobacterium nucleatum, Pseudomonas aeruginosa, and Campylobacter | 16S rRNA V1-V3 s equencing of tissue samples | An overabundance of these microbiota were noted in tumor tissue when compared to healthy tissue | [50] |
| Fusobacterium nucleatum, Capnocytophaga sputigena, Porphyromonas endodontalis, and Gemella haemolysans | NGS of oral swabs | The relative concentration of P. endodontalis, Gemella morbillorum, and G. haemolysans related to increased depth of invaision | [51] |
| Schlegelella and Methyloversatilis | 16S rRNA sequencing | Relative abundance of these organisms related to worse prognosis | [52] |
| Prevotella, Stomatobaculum, and Bifidobacterium | 16S rRNA V1-V3 sequencing of salivary samples | With a relative loss of Fusobacterium | [53] |
| Capnocytophaga gingivalis, Prevotella melaninogenica, and Streptococcus mitis | NGS of salivary samples | Examiners were able to reliably predict the presence of malignancy based upon these organisms | [54] |
| Oribacterium | 16S rRNA sequencing of oral rinse | Examiners were able to reliably predict the presence of oral cavity cancer and oropharyngeal cancers based on the presence of Oribacterium | [56] |
| Intervention | Associated Impact | Microbiota | Source |
|---|---|---|---|
| Surgery | Increased Levels | Haemophilus, Neisseria, Aggregatibacter, Leptotrichia | [127] |
| Radiation | Decreased Levels | Gram-negative obligate anaerobes | [129] |
| Radiation | Increased Levels | Streptococcus mutans | [129] |
| Radiation | Increased levels | Actinomyces, Lactobacillus, Sreptococcus mutans | [22] |
| Radiation | Decreased Levels | Neisseria, Fusobacterium, Streptococcus sanguinis | [22] |
| Chemoradiation | Increased Levels | Gut-associated taxa | [137] |
| Radiation | Increased Levels | Candida, enteric rods, Staphylococci | [131] |
| Chemotherapy | Decreased levels | Oral Streptococci | [16] |
| Chemotherapy | Increased levels | Oral Gram-negative anaerobes | [16] |
| Microbiota | Associated Impact | Outcome | Source |
|---|---|---|---|
| Fusobacterium nucleatum | Improved | Recurrence rate, overall survival, relapse free survival, metastasis free survival | [59] |
| Porphyromonas gingivalis | Increased | Chemoresistance | [146] |
| Butyrate producing microbes | Decreased | Radiotherapy effectiveness | [147] |
| Akkermansia muciniphila | Increased | Response to immune checkpoint inhibitors | [149] |
| Peptostreptococcus | Increased | Overall survival | [156] |
| Bifidobacterium longum, Collinsella aerofaciens, Enterococcus faecium | Increased | Response to immune checkpoint inhibitors | [154] |
| Ruminococcaceae family | Increased | Response to immune checkpoint inhibitors | [155] |
| Normal gut flora | Increased | Overall survival | [157] |
| Microbiota | Associated Impact | Toxicity | Source |
|---|---|---|---|
| Prevotella, Fusobacterium, Streptococcus, Megasphaera, Cardiobacterium | Increased risk | Oral mucositis | [158] |
| Gram negative bacteria | Increased severity | Oral mucositis | [6] |
| Enterobacteriaceae, Candida | Increased severity | Oral mucositis | [129] |
| Lactobacillus | Increased risk | Dental Caries | [160] |
| Bacteriodetes | Decreased risk | Immune checkpoint inhibitor induced colitis | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burcher, K.M.; Burcher, J.T.; Inscore, L.; Bloomer, C.H.; Furdui, C.M.; Porosnicu, M. A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers. Cancers 2022, 14, 4116. https://doi.org/10.3390/cancers14174116
Burcher KM, Burcher JT, Inscore L, Bloomer CH, Furdui CM, Porosnicu M. A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers. Cancers. 2022; 14(17):4116. https://doi.org/10.3390/cancers14174116
Chicago/Turabian StyleBurcher, Kimberly M., Jack T. Burcher, Logan Inscore, Chance H. Bloomer, Cristina M. Furdui, and Mercedes Porosnicu. 2022. "A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers" Cancers 14, no. 17: 4116. https://doi.org/10.3390/cancers14174116
APA StyleBurcher, K. M., Burcher, J. T., Inscore, L., Bloomer, C. H., Furdui, C. M., & Porosnicu, M. (2022). A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers. Cancers, 14(17), 4116. https://doi.org/10.3390/cancers14174116







