Pathologic Implications of Radial Resection Margin and Perineural Invasion to Adjuvant Chemotherapy after Preoperative Chemoradiotherapy and Surgery for Rectal Cancer: A Multi-Institutional and Case-Matched Control Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatments
2.3. Pathologic Examination
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salama, E.; Holland, J.; Boutros, M. Surgical Principles of Rectal Cancer. Surg. Oncol. Clin. N. Am. 2022, 31, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Sho, S.; Yothers, G.; Colangelo, L.H.; Ganz, P.A.; O’Connell, M.J.; Beart, R.W., Jr.; Hemmelgarn, M.; Chen, F.C.; Ko, C.Y.; Russell, M.M. Assessing the Quality of Rectal Cancer Pathology Reports in National Surgical Adjuvant Breast and Bowel Project Protocol R-04/NRG Oncology. Dis. Colon Rectum 2020, 63, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.; Lang, R.; Arya, S.; Bates, D.D.B.; Chang, K.J.; Fraum, T.J.; Friedman, K.A.; Golia Pernicka, J.S.; Gollub, M.J.; Harisinghani, M.; et al. Update to the structured MRI report for primary staging of rectal cancer: Perspective from the SAR Disease Focused Panel on Rectal and Anal Cancer. Abdom. Radiol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Haddad, P.; Ghalehtaki, R.; Saeedian, A.; Farhan, F.; Babaei, M.; Aghili, M. Current approaches in intensification of long-course chemoradiotherapy in locally advanced rectal cancer: A review. Radiat. Oncol. J. 2021, 39, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/archive/csr/1975_2016/ (accessed on 28 July 2021).
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Song, J.H.; Jeong, J.U.; Lee, J.H.; Kim, S.H.; Cho, H.M.; Um, J.W.; Jang, H.S. Korean Clinical Practice Guideline for Colon and Rectal Cancer Committee. Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II-III resectable rectal cancer: A meta-analysis of randomized controlled trials. Radiat. Oncol. J. 2017, 35, 198–207. [Google Scholar] [CrossRef]
- Bosset, J.F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.J.; Bardet, E.; Beny, A.; Ollier, J.C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef]
- Quasar Collaborative, G.; Gray, R.; Barnwell, J.; McConkey, C.; Hills, R.K.; Williams, N.S.; Kerr, D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef]
- Sainato, A.; Cernusco Luna Nunzia, V.; Valentini, V.; De Paoli, A.; Maurizi, E.R.; Lupattelli, M.; Aristei, C.; Vidali, C.; Conti, M.; Galardi, A.; et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother. Oncol. 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Counsell, N.; Quirke, P.; Mortensen, N.; Maraveyas, A.; Meadows, H.M.; Ledermann, J.; Sebag-Montefiore, D. Chronicle: Results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann. Oncol. 2014, 25, 1356–1362. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Rectal Cancer (Version 1.2021). 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 27 July 2021).
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.K.; Berry, S.; Spithoff, K.; Simunovic, M.; Chan, K.; Agboola, O.; Dingle, B.; on behalf of the Gastrointestinal Cancer Disease Site Group. Preoperative or postoperative therapy for stage II or III rectal cancer: An updated practice guideline. Clin. Oncol. (R Coll Radiol.) 2010, 22, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Chablani, P.; Nguyen, P.; Pan, X.; Robinson, A.; Walston, S.; Wu, C.; Frankel, W.L.; Chen, W.; Bekaii-Saab, T.; Chakravarti, A.; et al. Perineural Invasion Predicts for Distant Metastasis in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiation and Surgery. Am. J. Clin. Oncol. 2017, 40, 561–568. [Google Scholar] [CrossRef]
- Guillem, J.G.; Chessin, D.B.; Cohen, A.M.; Shia, J.; Mazumdar, M.; Enker, W.; Paty, P.B.; Weiser, M.R.; Klimstra, D.; Saltz, L.; et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann. Surg. 2005, 241, 829–836. [Google Scholar] [CrossRef]
- Kim, C.H.; Yeom, S.S.; Lee, S.Y.; Kim, H.R.; Kim, Y.J.; Lee, K.H.; Lee, J.H. Prognostic Impact of Perineural Invasion in Rectal Cancer After Neoadjuvant Chemoradiotherapy. World J. Surg. 2019, 43, 260–272. [Google Scholar] [CrossRef]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Albo, D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 2009, 27, 5131–5137. [Google Scholar] [CrossRef]
- Stojkovic Lalosevic, M.; Milovanovic, T.; Micev, M.; Stojkovic, M.; Dragasevic, S.; Stulic, M.; Rankovic, I.; Dugalic, V.; Krivokapic, Z.; Pavlovic Markovic, A. Perineural invasion as a prognostic factor in patients with stage I-III rectal cancer—5-year follow up. World J. Gastrointest Oncol. 2020, 12, 592–600. [Google Scholar] [CrossRef]
- van Wyk, H.C.; Going, J.; Horgan, P.; McMillan, D.C. The role of perineural invasion in predicting survival in patients with primary operable colorectal cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2017, 112, 11–20. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Quirke, P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 2008, 26, 303–312. [Google Scholar] [CrossRef]
- Quirke, P.; Durdey, P.; Dixon, M.F.; Williams, N.S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986, 2, 996–999. [Google Scholar] [CrossRef]
- Galvez, A.; Biondo, S.; Trenti, L.; Espin-Basany, E.; Kraft, M.; Farres Coll, R.; Codina-Cazador, A.; Flor-Lorente, B.; Garcia-Granero, E.; Enriquez-Navascues, J.M.; et al. Prognostic Value of the Circumferential Resection Margin After Curative Surgery For Rectal Cancer: A Multicenter Propensity Score-Matched Analysis. Dis. Colon Rectum 2022. [Google Scholar] [CrossRef]
- Agger, E.; Jörgren, F.; Lydrup, M.L.; Buchwald, P. Circumferential Resection Margin is Associated with Distant Metastasis After Rectal Cancer Surgery: A Nation-Wide Population-Based Study Cohort. Ann. Surg. 2021. [Google Scholar] [CrossRef]
- College of American Pathologists. Protocol for the Examination of Specimens from Patients with Primary Carcinoma of the Colon and Rectum. Cancer Protocol Templates. Available online: http://www.cap.org/cancerprotocols (accessed on 28 July 2021).
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.H.; Kim, K.P.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef]
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, Å.; van den Broek, C.B.M.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.H.; Marijnen, C.A.M.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef]
- Cafiero, F.; Gipponi, M.; Lionetto, R.; Group, P.A.R.C.S. Randomised clinical trial of adjuvant postoperative RT vs. sequential postoperative RT plus 5-FU and levamisole in patients with stage II-III resectable rectal cancer: A final report. J. Surg. Oncol. 2003, 83, 140–146. [Google Scholar] [CrossRef]
- Glimelius, B.; Dahl, O.; Cedermark, B.; Jakobsen, A.; Bentzen, S.M.; Starkhammar, H.; Gronberg, H.; Hultborn, R.; Albertsson, M.; Pahlman, L.; et al. Adjuvant chemotherapy in colorectal cancer: A joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol. 2005, 44, 904–912. [Google Scholar] [CrossRef]
- Yokoyama, S.; Matsuda, K.; Watanabe, T.; Mitani, Y.; Ieda, J.; Iwamoto, H.; Hotta, T.; Takifuji, K.; Yamaue, H. Perineural Invasion Is Associated with Poor Survival after Preoperative Chemoradiation Therapy for Advanced Lower Rectal Cancer. Dig. Surg. 2017, 34, 387–393. [Google Scholar] [CrossRef]
- Cercek, A.; Dos Santos Fernandes, G.; Roxburgh, C.S.; Ganesh, K.; Ng, S.; Sanchez-Vega, F.; Yaeger, R.; Segal, N.H.; Reidy-Lagunes, D.L.; Varghese, A.M.; et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin. Cancer Res. 2020, 26, 3271–3279. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Yuki, S.; Bando, H.; Tsukada, Y.; Inamori, K.; Komatsu, Y.; Homma, S.; Uemura, M.; Kato, T.; Kotani, D.; Fukuoka, S.; et al. Short-term results of VOLTAGE-A: Nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. J. Clin. Oncol. 2020, 38, 4100. [Google Scholar] [CrossRef]


| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic-No. (%) | Low-Risk Group (n = 1335) | High-Risk Group (n = 464) | p-Value | SMD | Lowhigh-Risk Group (n = 464) | High-Risk Group (n = 464) | p-Value | SMD |
| Age | 0.21 | 0.07 | 1.00 | 0.01 | ||||
| ≤65 | 823 (61.6%) | 302 (65.1%) | 301 (64.9%) | 302 (65.1%) | ||||
| >65 | 512 (38.4%) | 162 (34.9%) | 163 (35.1%) | 162 (34.9%) | ||||
| Gender | 0.02 | 0.13 | 0.94 | 0.01 | ||||
| Male | 894 (67.0%) | 339 (73.1%) | 337 (72.6%) | 339 (73.1%) | ||||
| Female | 441 (33.0%) | 125 (26.9%) | 127 (27.4%) | 125 (26.9%) | ||||
| CEA, ng/mL | <0.01 | 0.31 | 0.26 | 0.08 | ||||
| ≤5 | 926 (69.4%) | 252 (54.3%) | 270 (58.2%) | 252 (54.3%) | ||||
| >5 | 409 (30.6%) | 212 (45.7%) | 194 (41.8%) | 212 (45.7%) | ||||
| Surgery | <0.01 | 0.19 | 0.38 | 0.07 | ||||
| LAR | 1229 (92.1%) | 400 (86.2%) | 410 (88.4%) | 400 (86.2%) | ||||
| APR | 106 (7.9%) | 64 (13.8%) | 54 (11.6%) | 64 (13.8%) | ||||
| Pathologic T | <0.01 | 0.96 | 1.00 | <0.001 | ||||
| pT1-2 | 715 (53.6%) | 60 (12.9%) | 60 (12.9%) | 60 (12.9%) | ||||
| pT3-4 | 620 (46.4%) | 404 (87.1%) | 404 (87.1%) | 404 (87.1%) | ||||
| Pathologic N | <0.01 | 0.56 | 0.56 | 0.04 | ||||
| cN0 | 1000 (74.9%) | 227 (48.9%) | 237 (51.1%) | 227 (48.9%) | ||||
| cN+ | 335 (25.1%) | 237 (51.1%) | 227 (48.9%) | 237 (51.1%) | ||||
| Histologic grade | 0.02 | 0.13 | 0.63 | 0.04 | ||||
| Low | 1263 (94.6%) | 424 (91.4%) | 429 (92.5%) | 424 (91.4%) | ||||
| High | 72 (5.4%) | 40 (8.6%) | 35 (7.5%) | 40 (8.6%) | ||||
| Surgical margin | <0.01 | 1.26 | <0.01 | 1.26 | ||||
| Negative | 1335 (100.0%) | 259 (55.8%) | 464 (100.0%) | 259 (55.8%) | ||||
| Positive | 0 (0.0%) | 205 (44.2%) | 0 (0.0%) | 205 (44.2%) | ||||
| Perineural invasion | <0.01 | 2.48 | <0.01 | 2.48 | ||||
| Negative | 1335 (100.0%) | 114 (24.6%) | 464 (100.0%) | 114 (24.6%) | ||||
| Positive | 0 (0.0%) | 350 (75.4%) | 0 (0.0%) | 350 (75.4%) | ||||
| Variables | High-Risk Group | Low-Risk Group | ||
|---|---|---|---|---|
| Univariate (p) Hazard Ratio (95% CI) | Multivariate (p) Hazard Ratio (95% CI) | Univariate (p) Hazard Ratio (95% CI) | Multivariate (p) Hazard Ratio (95% CI) | |
| Age, year | 0.02 | <0.01 | 0.03 | 0.04 |
| ≤65 | 1 | 1 | 1 | |
| >65 | 1.74 (1.22–2.49) | 1.87 (1.30–2.69) | 1.77 (1.04–2.99) | 1.73 (1.02–2.94) |
| Gender | 0.96 | 0.92 | ||
| Male | 1 | 1 | ||
| Female | 1.01 (0.68–1.49) | 0.97 (0.54–1.75) | ||
| CEA, ng/mL | 0.82 | 0.11 | ||
| ≤5 | 1 | 1 | ||
| >5 | 1.04 (0.73–1.48) | 1.53 (0.91–2.58) | ||
| Surgery | 0.01 | 0.36 | ||
| LAR | 1 | 1 | ||
| APR | 1.77 (1.16–2.72) | 1.39 (0.68–2.84) | ||
| Pathologic T | 0.03 | 0.28 | ||
| ypT0-2 | 1 | 1 | ||
| ypT3-4 | 2.12 (1.08–4.18) | 1.66 (0.66–4.17) | ||
| Pathologic N | <0.01 | <0.01 | 0.04 | 0.08 |
| ypN0 | 1 | 1 | 1 | 1 |
| ypN+ | 2.57 (1.76–3.75) | 2.33 (1.57–3.46) | 1.76 (1.03–3.00) | 1.62 (0.95–2.76) |
| Histologic grade | 0.04 | 0.17 | ||
| Low | 1 | 1 | ||
| High | 2.05 (1.24–3.38) | 1.75 (0.79–3.86) | ||
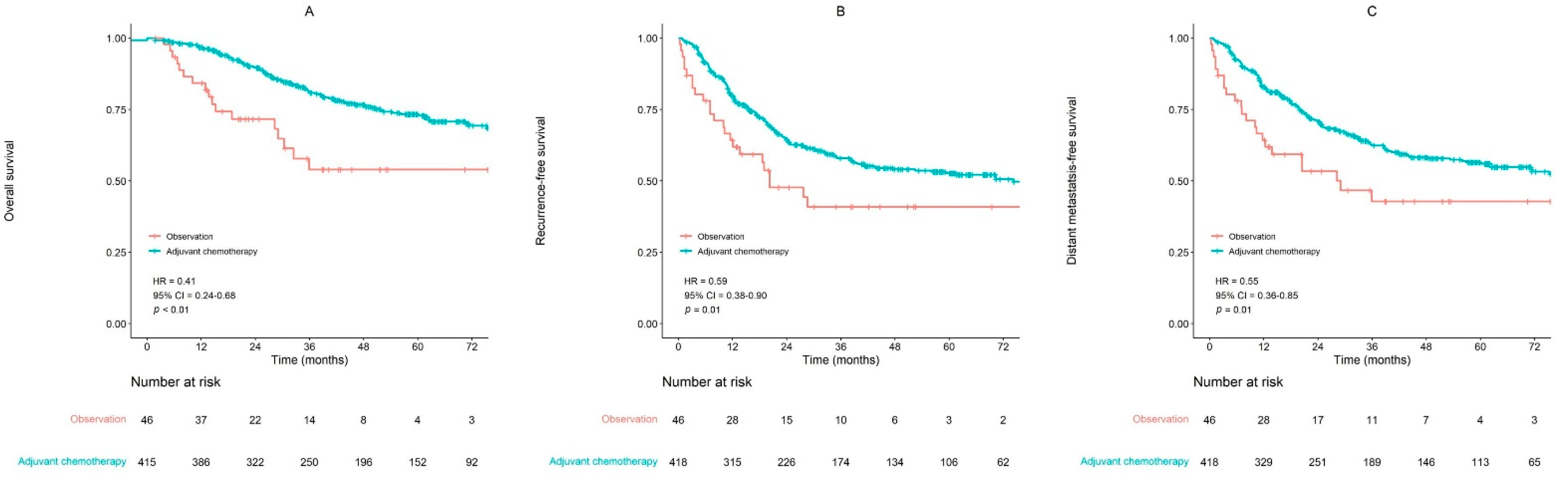
| Adjuvant Chemotherapy | <0.01 | <0.01 | 0.35 | |
| No | 1 | 1 | 1 | |
| Yes | 0.41 (0.24–0.68) | 0.39 (0.23–0.66) | 0.66 (0.28–1.57) | |
| Variable | High-Risk Group | Low-Risk Group | ||
|---|---|---|---|---|
| Univariate (p) Hazard Ratio (95% CI) | Multivariate (p) Hazard Ratio (95% CI) | Univariate (p) Hazard Ratio (95% CI) | Multivariate (p) Hazard Ratio (95% CI) | |
| Age, year | 0.07 | 0.04 | 0.51 | |
| ≤65 | 1 | 1 | 1 | |
| >65 | 1.30 (0.98–1.72) | 1.34 (1.01–1.77) | 1.13 (0.78–1.64) | |
| Gender | 0.14 | 0.57 | ||
| Male | 1 | 1 | ||
| Female | 1.25 (0.93–1.68) | 1.12 (0.76–1.66) | ||
| CEA, ng/mL | 0.55 | 0.06 | ||
| ≤5 | 1 | 1 | ||
| >5 | 1.09 (0.83–1.43) | 1.41 (0.99–2.00) | ||
| Surgery | <0.01 | <0.01 | 0.10 | |
| LAR | 1 | 1 | 1 | |
| APR | 1.94 (1.38–2.73) | 1.74 (1.21–2.51) | 1.50 (0.92–2.45) | |
| Pathologic T | <0.01 | 0.01 | ||
| ypT0-2 | 1 | 1 | ||
| ypT3-4 | 2.11 (1.27–3.52) | 2.83 (1.32–6.07) | ||
| Pathologic N | <0.01 | <0.01 | <0.01 | 0.02 |
| ypN0 | 1 | 1 | 1 | 1 |
| ypN1-2 | 2.04 (1.53–2.70) | 1.81 (1.35–2.44) | 2.08 (1.44–3.00) | 1.53 (1.08–2.16) |
| Histologic grade | 0.04 | 0.02 | ||
| Low | 1 | 1 | ||
| High | 1.55 (1.01–2.37) | 1.87 (1.09–3.21) | ||
| Adjuvant Chemotherapy | 0.01 | 0.03 | 0.31 | |
| No | 1 | 1 | 1 | |
| Yes | 0.59 (0.38–0.90) | 0.61 (0.39–0.94) | 1.40 (0.73–2.69) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.-Y.; Kim, S.H.; Jang, H.S.; Song, J.H.; Jeong, S.; Jung, J.-H.; Lee, J.H. Pathologic Implications of Radial Resection Margin and Perineural Invasion to Adjuvant Chemotherapy after Preoperative Chemoradiotherapy and Surgery for Rectal Cancer: A Multi-Institutional and Case-Matched Control Study. Cancers 2022, 14, 4112. https://doi.org/10.3390/cancers14174112
Sung S-Y, Kim SH, Jang HS, Song JH, Jeong S, Jung J-H, Lee JH. Pathologic Implications of Radial Resection Margin and Perineural Invasion to Adjuvant Chemotherapy after Preoperative Chemoradiotherapy and Surgery for Rectal Cancer: A Multi-Institutional and Case-Matched Control Study. Cancers. 2022; 14(17):4112. https://doi.org/10.3390/cancers14174112
Chicago/Turabian StyleSung, Soo-Yoon, Sung Hwan Kim, Hong Seok Jang, Jin Ho Song, Songmi Jeong, Ji-Han Jung, and Jong Hoon Lee. 2022. "Pathologic Implications of Radial Resection Margin and Perineural Invasion to Adjuvant Chemotherapy after Preoperative Chemoradiotherapy and Surgery for Rectal Cancer: A Multi-Institutional and Case-Matched Control Study" Cancers 14, no. 17: 4112. https://doi.org/10.3390/cancers14174112
APA StyleSung, S.-Y., Kim, S. H., Jang, H. S., Song, J. H., Jeong, S., Jung, J.-H., & Lee, J. H. (2022). Pathologic Implications of Radial Resection Margin and Perineural Invasion to Adjuvant Chemotherapy after Preoperative Chemoradiotherapy and Surgery for Rectal Cancer: A Multi-Institutional and Case-Matched Control Study. Cancers, 14(17), 4112. https://doi.org/10.3390/cancers14174112






