The Prognostic Value of Liquid Biopsies for Benefit of Salvage Radiotherapy in Relapsed Oligometastatic Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients Selection Criteria
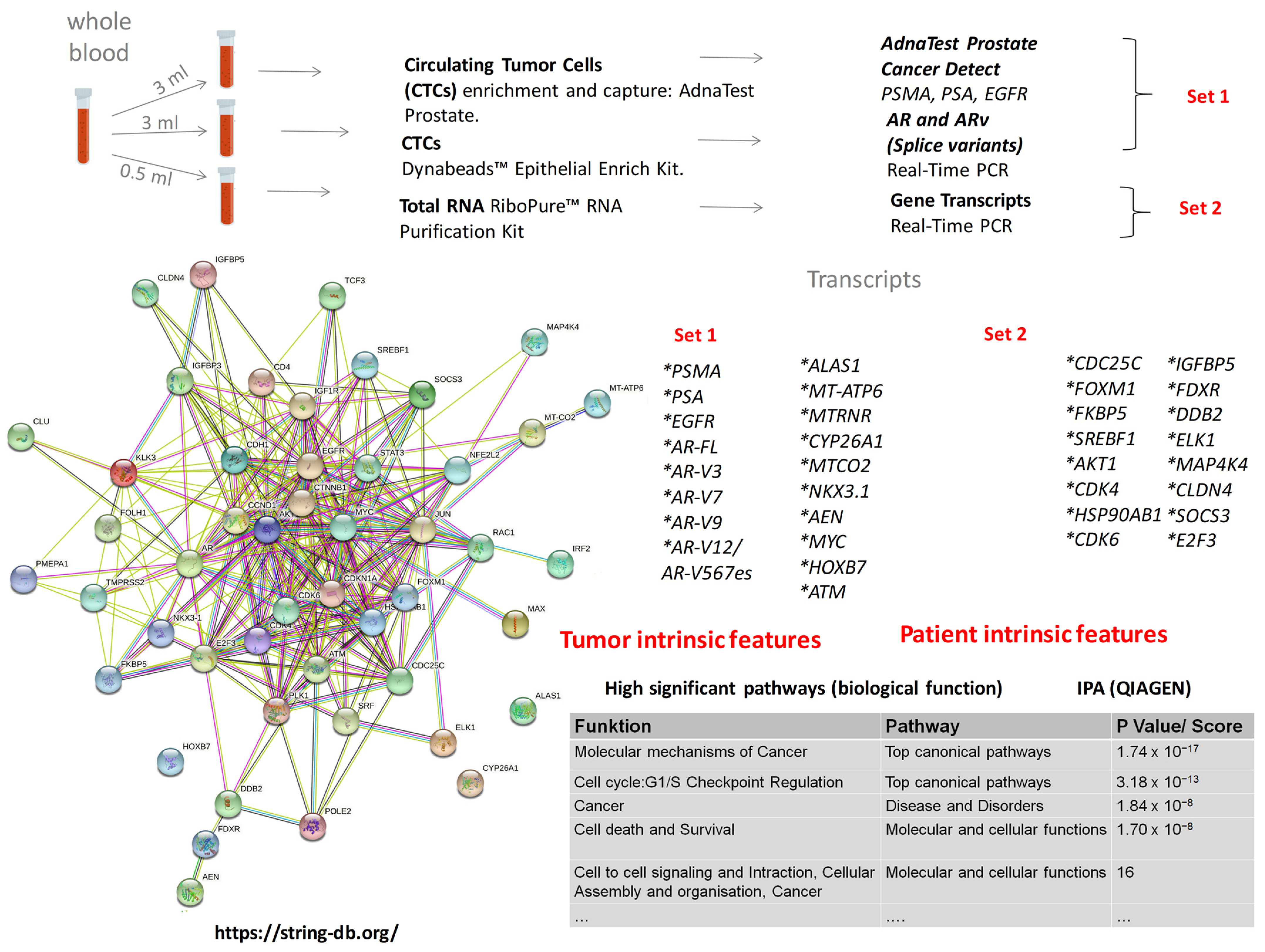
2.2. Gene Panel Development
2.3. Samples Collection and Processing
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Data Processing and Statistical Evaluations
3. Results
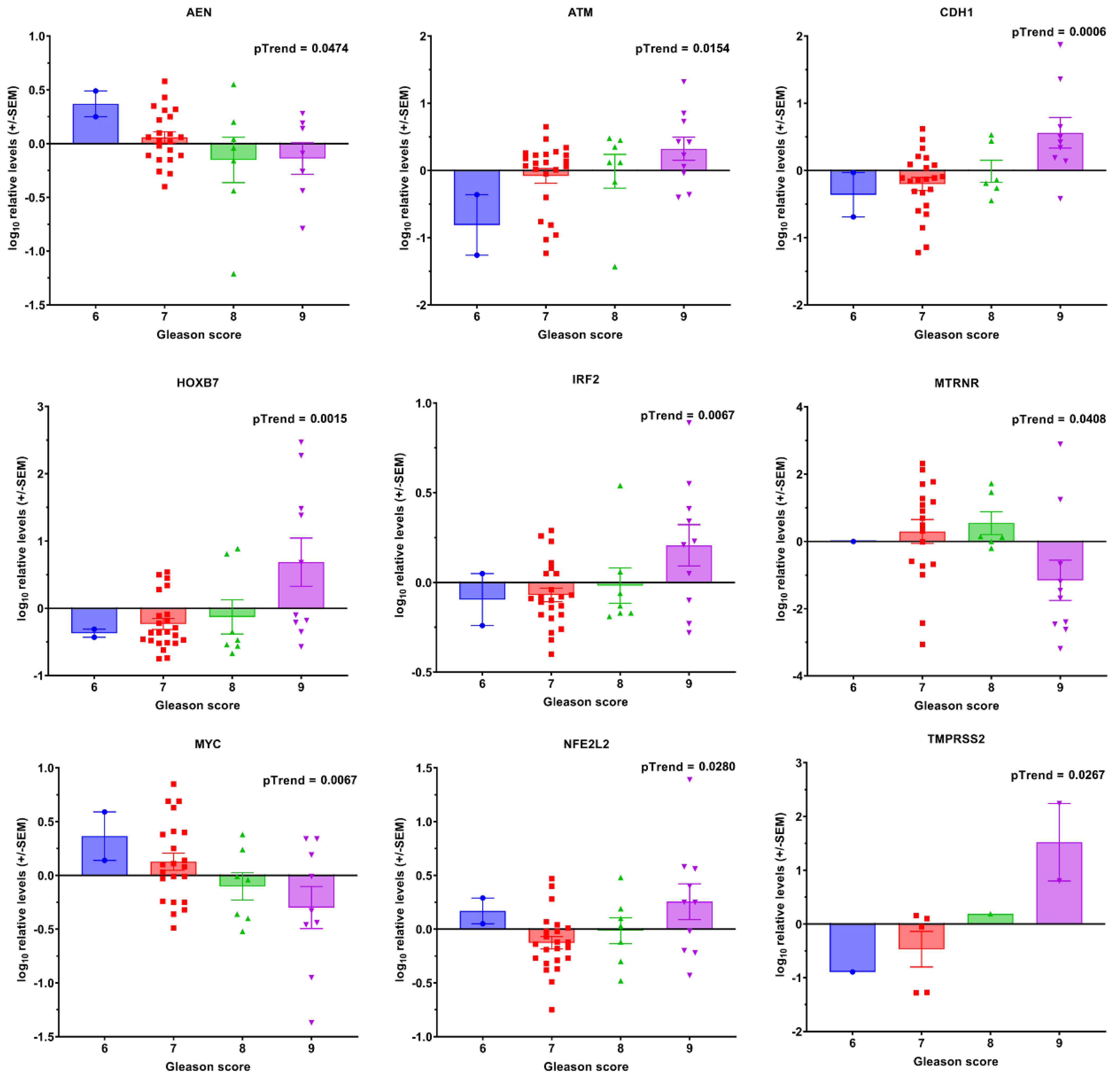
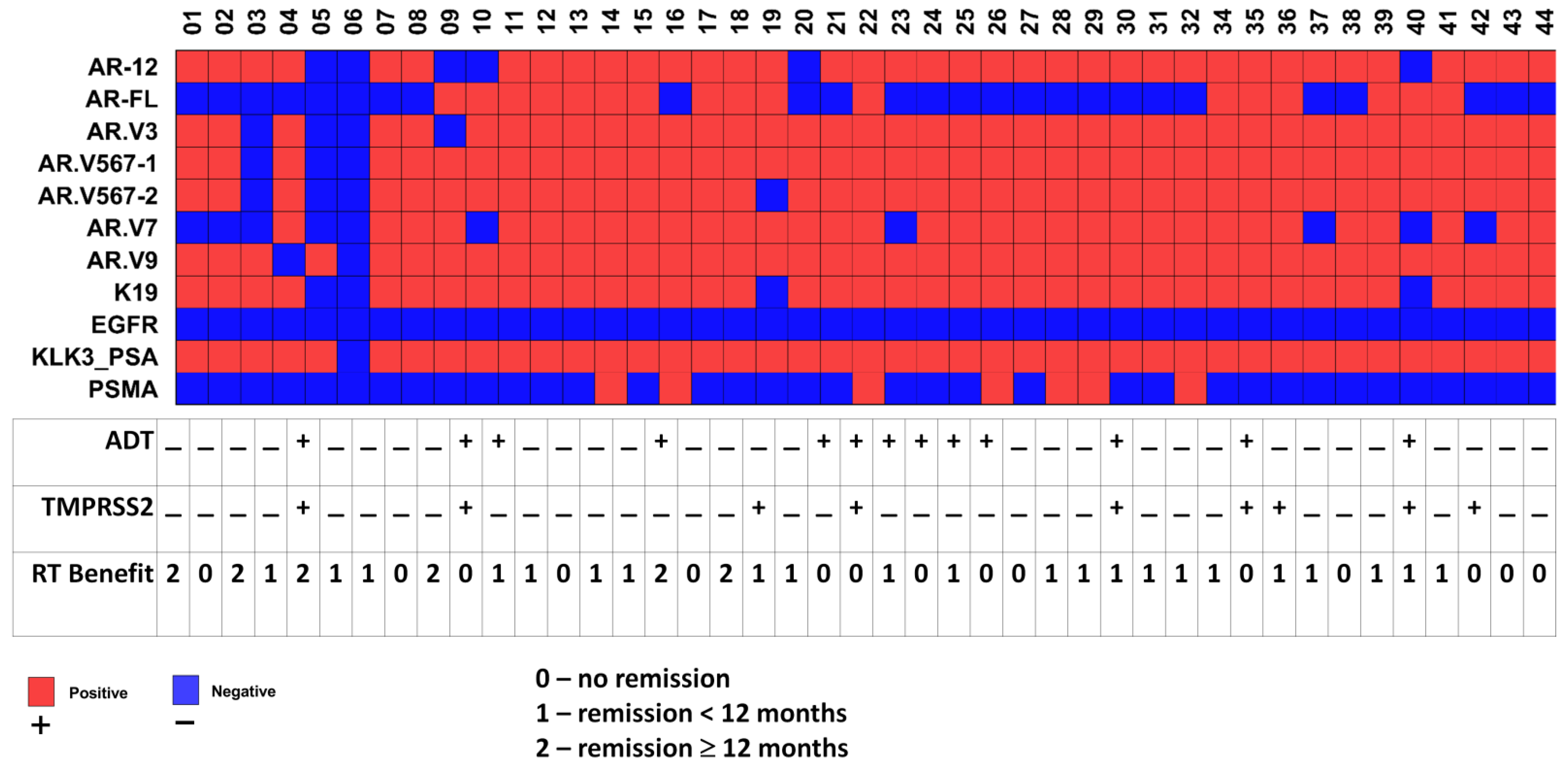
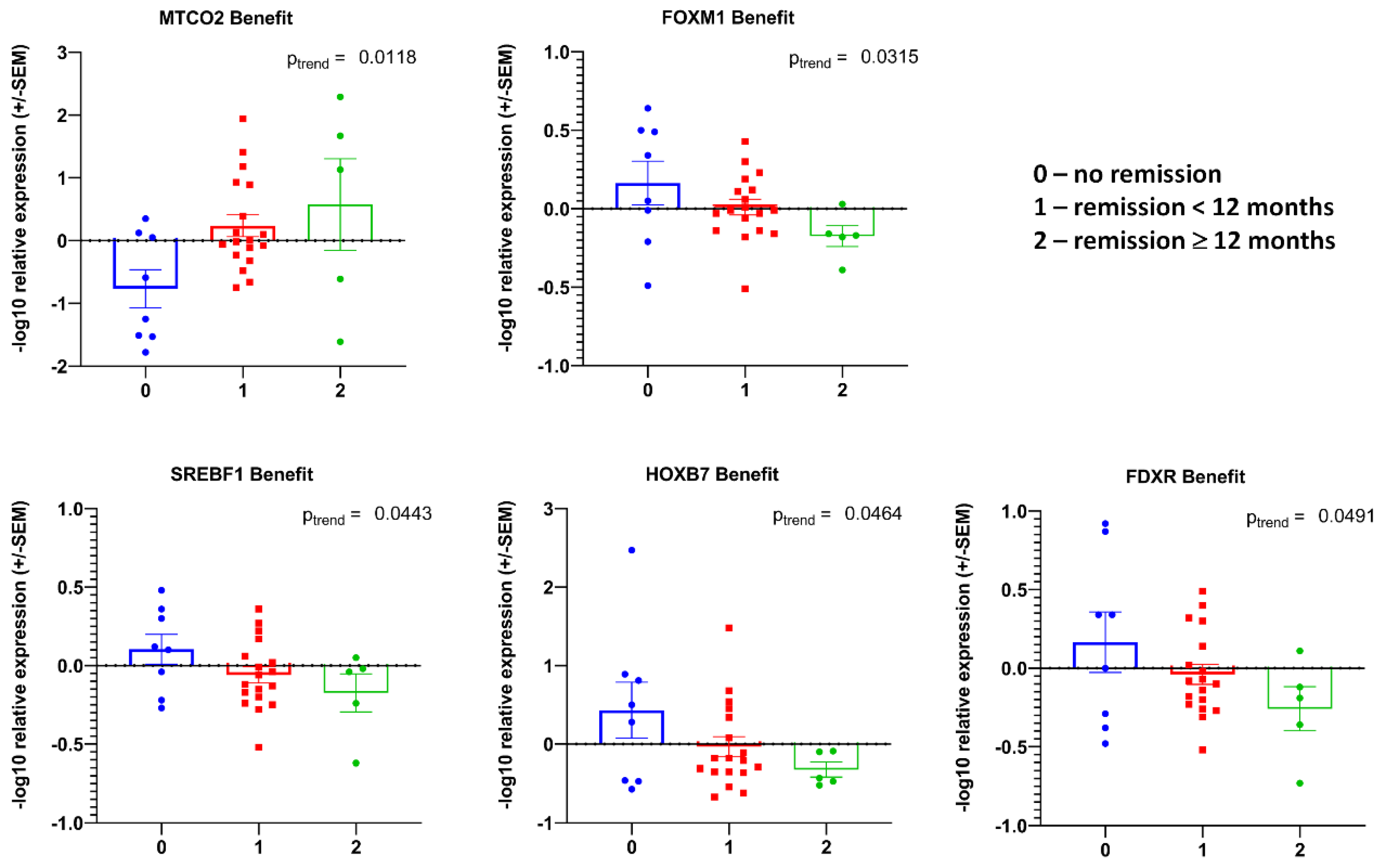
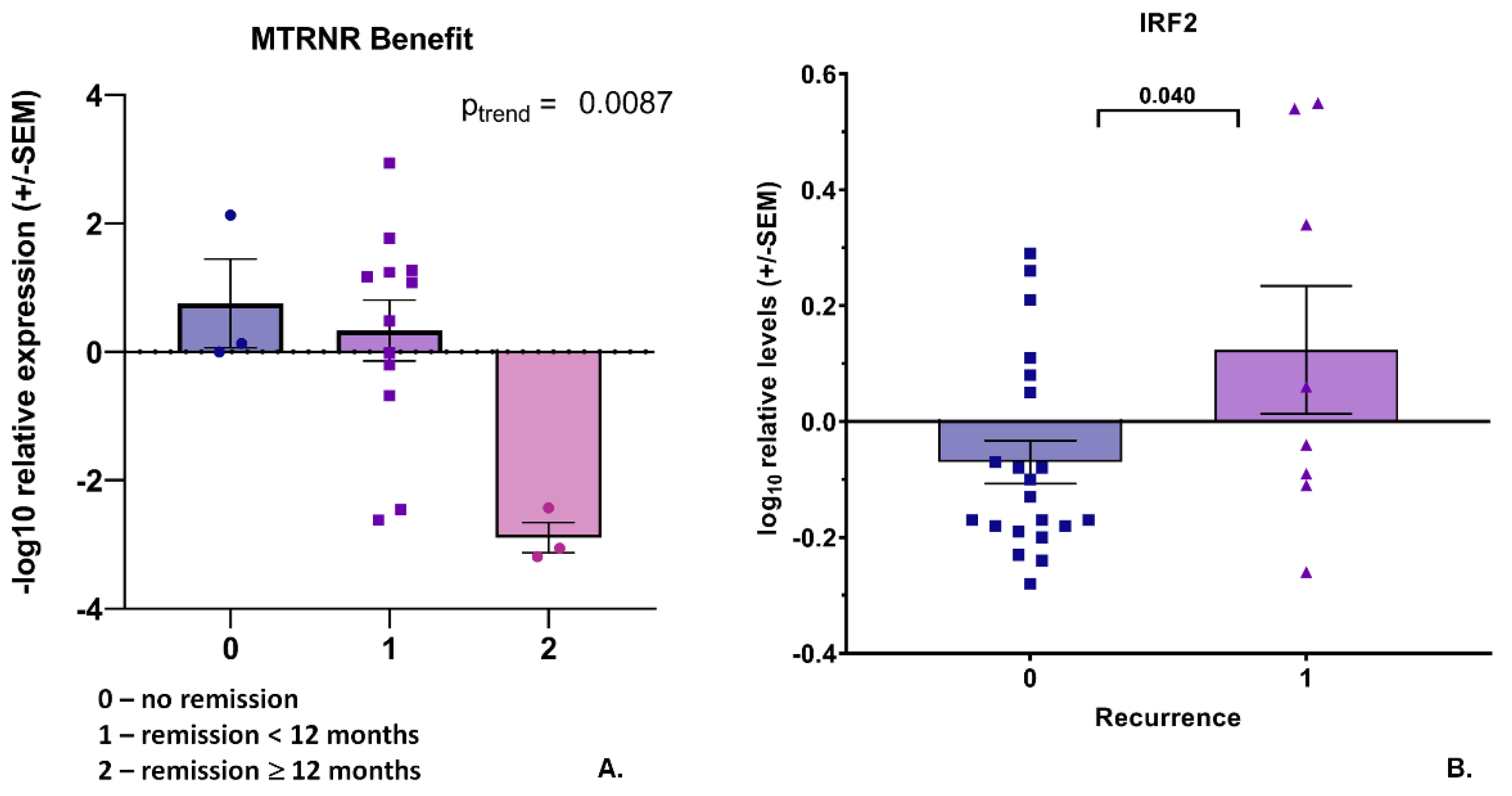
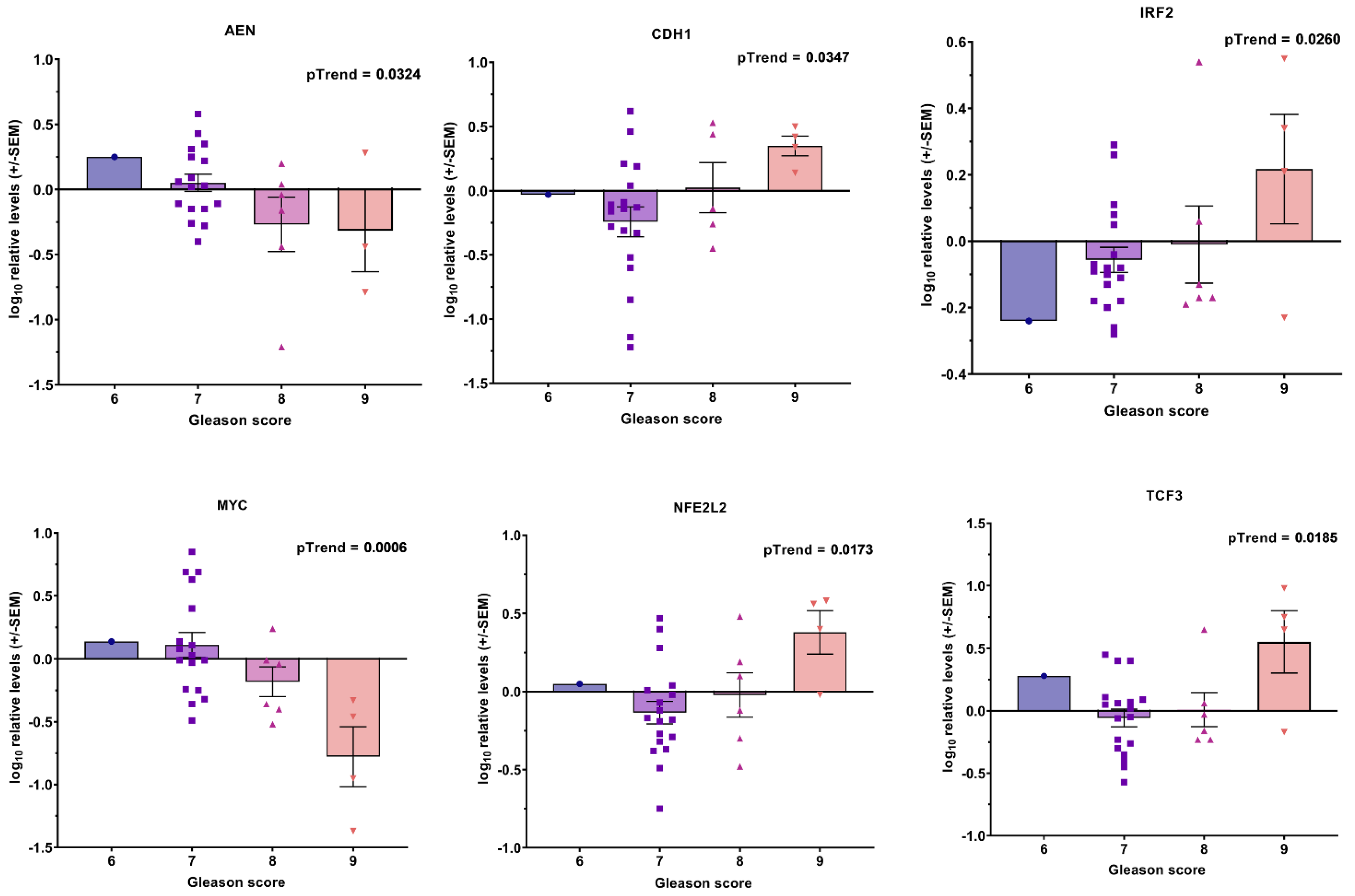
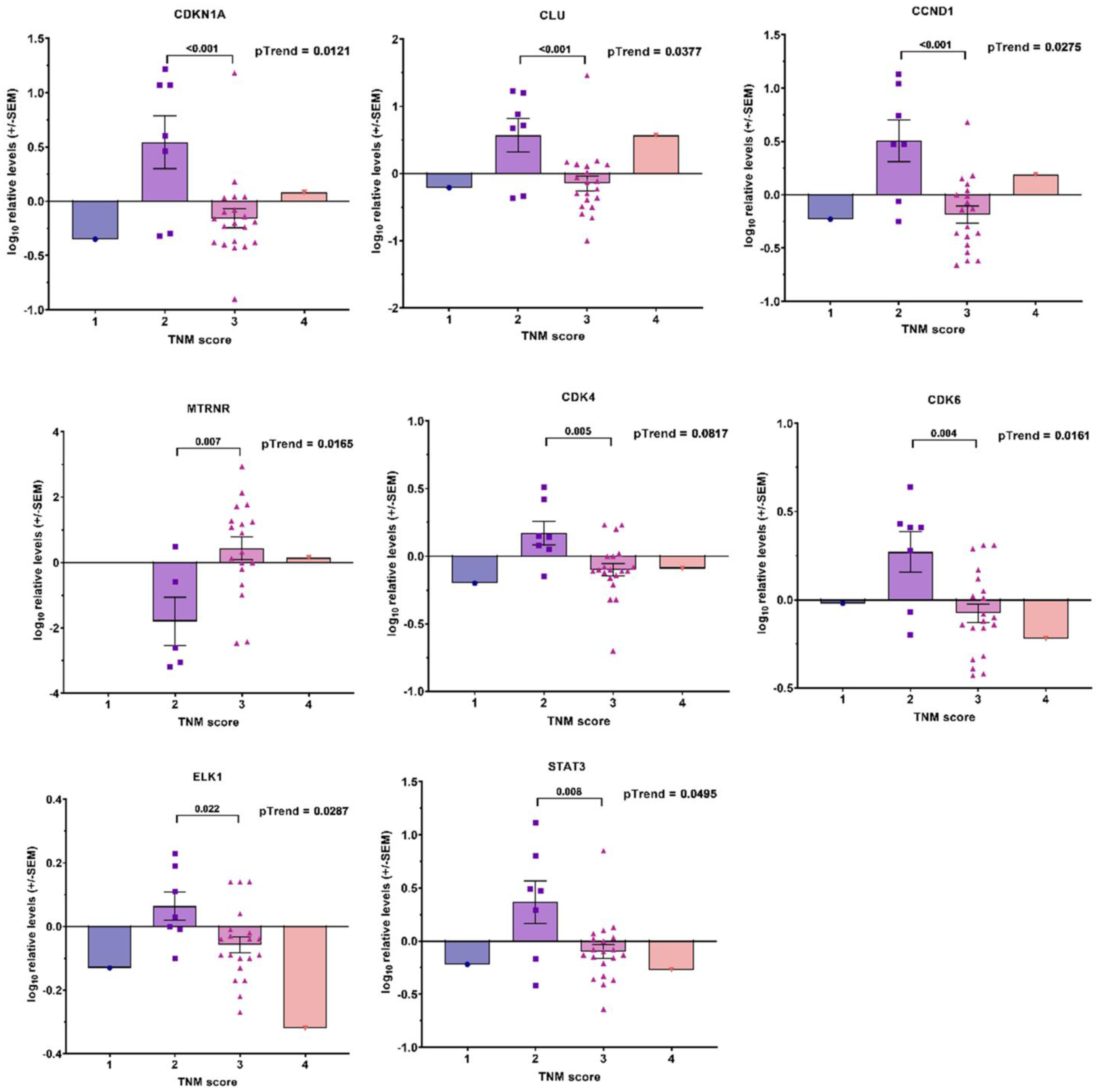
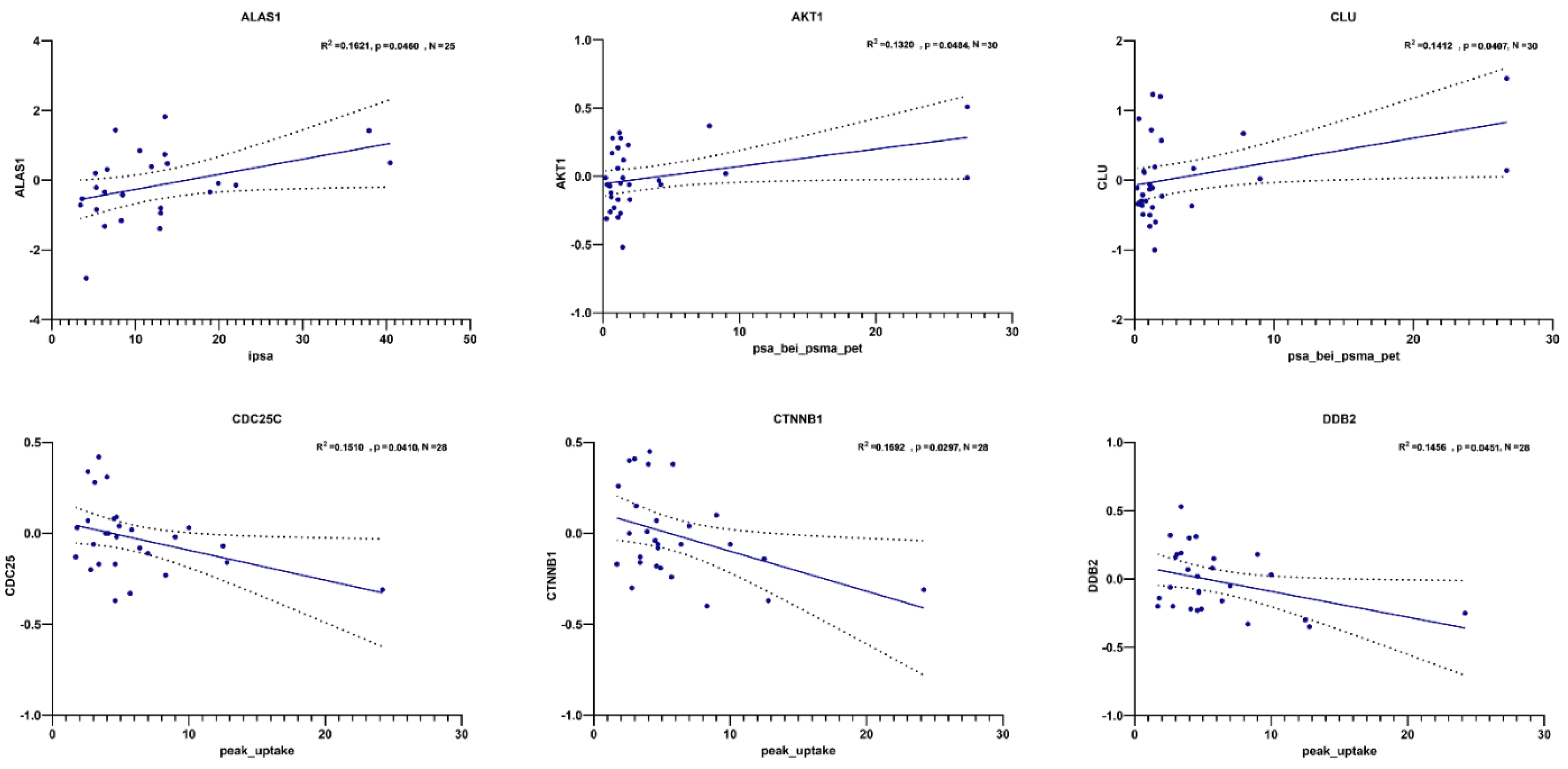
3.1. Transcript Expression of Patient-Derived CTCs—Associated mRNA
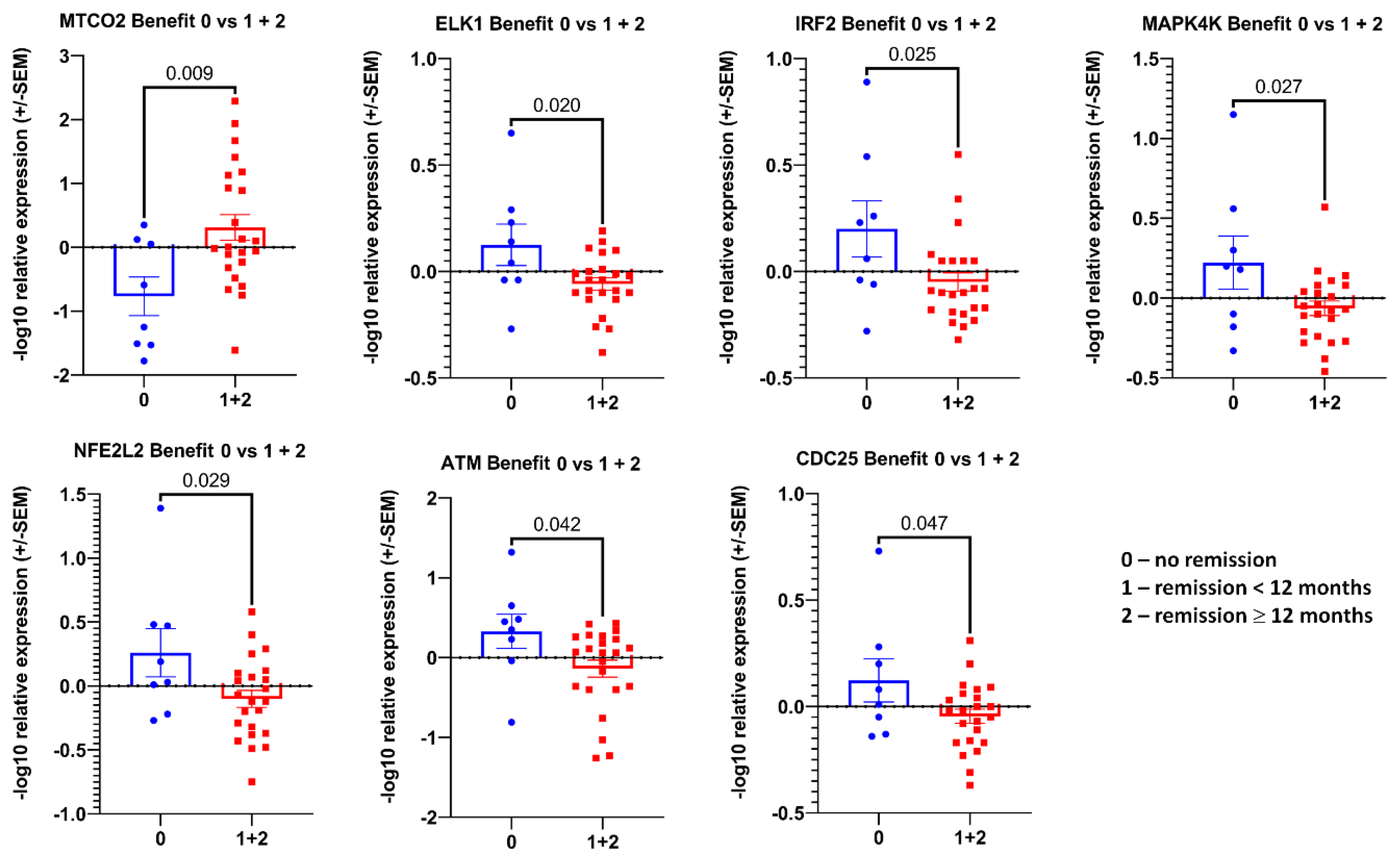
3.2. Transcript Expression in Blood-Derived mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Son: Hoboken, NJ, USA, 2017. [Google Scholar]
- Den, R.B.; Yousefi, K.; Trabulsi, E.J.; Abdollah, F.; Choeurng, V.; Feng, F.Y.; Dicker, A.P.; Lallas, C.D.; Gomella, L.G.; Davicioni, E.; et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J. Clin. Oncol. 2015, 33, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Johnson, M.H.; Yousefi, K.; Davicioni, E.; Netto, G.J.; Marchionni, L.; Fedor, H.L.; Glavaris, S.; Choeurng, V.; Buerki, C.; et al. Tissue-based Genomics Augments Post-prostatectomy Risk Stratification in a Natural History Cohort of Intermediate- and High-Risk Men. Eur. Urol. 2016, 69, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Swanton, C. Metastasis as an evolutionary process. Science 2016, 352, 169–175. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schäfer, M.; et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1258–1268. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Beer, A.J.; Graner, F.P.; Haller, B.; Weirich, G.; Doherty, A.; Gschwend, J.E.; Schwaiger, M.; Eiber, M. Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J. Nucl. Med. 2016, 57, 1713–1719. [Google Scholar] [CrossRef]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Siddiqui, J.; Wu, Y.M.; Robinson, D.; Lonigro, R.J.; et al. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results From NCI 9012. J. Clin. Oncol. 2018, 36, 991–999. [Google Scholar] [CrossRef]
- Duchesne, G.M.; Woo, H.H.; Bassett, J.K.; Bowe, S.J.; D’Este, C.; Frydenberg, M.; King, M.; Ledwich, L.; Loblaw, A.; Malone, S.; et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016, 17, 727–737. [Google Scholar] [CrossRef]
- Magnan, S.; Zarychanski, R.; Pilote, L.; Bernier, L.; Shemilt, M.; Vigneault, E.; Fradet, V.; Turgeon, A.F. Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2015, 1, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L.; Burdett, S.; Rydzewska, L.H.M.; Albiges, L.; Clarke, N.W.; Fisher, D.; Fizazi, K.; Gravis, G.; James, N.D.; Mason, M.D.; et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: A systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016, 17, 243–256. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, A.; von Klot, C.A.J.; Walacides, D.; Derlin, T.; Bengel, F.M.; Ross, T.L.; Wester, H.J.; Derlin, K.; Kuczyk, M.A.; Christiansen, H.; et al. Patterns of Progression After 68Ga-PSMA-Ligand PET/CT-Guided Radiation Therapy for Recurrent Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Mader, S.; Pantel, K. Liquid Biopsy: Current Status and Future Perspectives. Oncol. Res. Treat. 2017, 40, 404–408. [Google Scholar] [CrossRef]
- Ward Gahlawat, A.; Lenhardt, J.; Witte, T.; Keitel, D.; Kaufhold, A.; Maass, K.K.; Pajtler, K.W.; Sohn, C.; Schott, S. Evaluation of Storage Tubes for Combined Analysis of Circulating Nucleic Acids in Liquid Biopsies. Int. J. Mol. Sci. 2019, 20, 704. [Google Scholar] [CrossRef]
- Steinestel, J.; Luedeke, M.; Arndt, A.; Schnoeller, T.J.; Lennerz, J.K.; Wurm, C.; Maier, C.; Cronauer, M.V.; Steinestel, K.; Schrader, A.J. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget 2015, 10, 4213–4223. [Google Scholar] [CrossRef]
- Mulcahy, D.G.; Macdonald, K.S., III; Brady, S.G.; Meyer, C.; Barker, K.B.; Coddington, J. Greater than X kb: A quantitative assessment of preservation conditions on genomic DNA quality, and a proposed standard for genome-quality DNA. PeerJ 2016, 4, e2528. [Google Scholar] [CrossRef]
- Bernemann, C.; Humberg, V.; Thielen, B.; Steinestel, J.; Chen, X.; Duensing, S.; Schrader, A.J.; Boegemann, M. Comparative Analysis of AR Variant AR-V567es mRNA Detection Systems Reveals Eminent Variability and Questions the Role as a Clinical Biomarker in Prostate Cancer. Clin. Cancer Res. 2019, 25, 3856–3864. [Google Scholar] [CrossRef]
- Nagaya, N.; Nagata, M.; Lu, Y.; Kanayama, M.; Hou, Q.; Hotta, Z.U.; China, T.; Kitamura, K.; Matsushita, K.; Isotani, S.; et al. Prostate-specific membrane antigen in circulating tumor cells is a new poor prognostic marker for castration-resistant prostate cancer. PLoS ONE 2020, 15, e0226219. [Google Scholar] [CrossRef] [Green Version]
- Moll, R.; Franke, W.W.; Schiller, D.L. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Wadosky, K.M.; Koochekpour, S. Androgen receptor splice variants and prostate cancer: From bench to bedside. Oncotarget 2017, 8, 18550–18576. [Google Scholar] [CrossRef] [PubMed]
- Den, R.B.; Feng, F.Y.; Showalter, T.N.; Mishra, M.V.; Trabulsi, E.J.; Lallas, C.D.; Gomella, L.G.; Kelly, W.K.; Birbe, R.C.; McCue, P.A.; et al. Genomic prostate cancer classifier predicts biochemical failure and metastases in patients after postoperative radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1038–1046. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Davicioni, E.; Crisan, A.; Jenkins, R.B.; Ghadessi, M.; Karnes, R.J. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur. Urol. 2015, 67, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Yousefi, K.; Haddad, Z.; Choeurng, V.; Buerki, C.; Stephenson, A.J.; Li, J.; Kattan, M.W.; Magi-Galluzzi, C.; Davicioni, E. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur. Urol. 2015, 67, 778–786. [Google Scholar] [CrossRef]
- Freedland, S.J.; Choeurng, V.; Howard, L.; De Hoedt, A.; du Plessis, M.; Yousefi, K.; Lam, L.L.; Buerki, C.; Ra, S.; Robbins, B.; et al. Utilization of a Genomic Classifier for Prediction of Metastasis Following Salvage Radiation Therapy after Radical Prostatectomy. Eur. Urol. 2016, 70, 588–596. [Google Scholar] [CrossRef]
- Karnes, R.J.; Choeurng, V.; Ross, A.E.; Schaeffer, E.M.; Klein, E.A.; Freedland, S.J.; Erho, N.; Yousefi, K.; Takhar, M.; Davicioni, E.; et al. Validation of a Genomic Risk Classifier to Predict Prostate Cancer-specific Mortality in Men with Adverse Pathologic Features. Eur. Urol. 2018, 73, 168–175. [Google Scholar] [CrossRef]
- Spratt, D.E.; Dai, D.L.Y.; Den, R.B.; Troncoso, P.; Yousefi, K.; Ross, A.E.; Schaeffer, E.M.; Haddad, Z.; Davicioni, E.; Mehra, R.; et al. Performance of a Prostate Cancer Genomic Classifier in Predicting Metastasis in Men with Prostate-specific Antigen Persistence Postprostatectomy. Eur. Urol. 2018, 74, 107–114. [Google Scholar] [CrossRef]
- Jairath, N.K.; Dal Pra, A.; Vince, R., Jr.; Dess, R.T.; Jackson, W.C.; Tosoian, J.J.; McBride, S.M.; Zhao, S.G.; Berlin, A.; Mahal, B.A.; et al. A Systematic Review of the Evidence for the Decipher Genomic Classifier in Prostate Cancer. Eur. Urol. 2021, 79, 374–383. [Google Scholar] [CrossRef]
- Pra, A.D.; Ghadjar, P.; Hayoz, S.; Liu, V.Y.T.; Spratt, D.E.; Thompson, D.J.S.; Davicioni, E.; Huang, H.-C.; Zhao, X.; Liu, Y.; et al. Validation of the Decipher genomic classifier in patients receiving salvage radiotherapy without hormone therapy after radical prostatectomy—An ancillary study of the SAKK 09/10 randomized clinical trial. Ann. Oncol. 2022. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- van der Toom, E.E.; Verdone, J.E.; Gorin, M.A.; Pienta, K.J. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget 2016, 7, 62754–62766. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ledet, E.; Li, D.; Dotiwala, A.; Steinberger, A.; Feibus, A.; Li, J.; Qi, Y.; Silberstein, J.; Lee, B.; et al. A Whole Blood Assay for AR-V7 and ARv567es in Prostate Cancer Patients. J. Urol. 2016, 196, 1758. [Google Scholar] [CrossRef]
- Todenhofer, T.; Azad, A.; Stewart, C.; Gao, J.; Eigl, B.J.; Gleave, M.E.; Joshua, A.M.; Black, P.C.; Chi, K.N. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to Abiraterone acetate. J. Urol. 2017, 197, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Xie, W.; Nakabayashi, M.; Zhang, H.; Jeong, S.H.; Wang, X.; Komura, K.; Sweeney, C.J.; Sartor, O.; Lee, G.M.; et al. Association of AR-V7 and prostate specific antigen RNA levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin. Cancer Res. 2016, 23, 726–734. [Google Scholar] [CrossRef]
- Sperger, J.M.; Emamekhoo, H.; McKay, R.R.; Stahlfeld, C.N.; Singh, A.; Chen, X.E.; Kwak, L.; Gilsdorf, C.S.; Wolfe, S.K.; Wei, X.X.; et al. Prospective Evaluation of Clinical Outcomes Using a Multiplex Liquid Biopsy Targeting Diverse Resistance Mechanisms in Metastatic Prostate Cancer. J. Clin. Oncol. 2021, 39, 2926–2937. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, M.; Wieser, M.; Eigentler, A.; Seewald, M.; Dobler, G.; Neuwirt, H.; Kafka, M.; Heidegger, I.; Horninger, W.; Bektic, J.; et al. Validation of Cell-Free RNA and Circulating Tumor Cells for Molecular Marker Analysis in Metastatic Prostate Cancer. Biomedicines 2021, 9, 1004. [Google Scholar] [CrossRef]
- Henshall, S.M.; Afar, D.E.; Hiller, J.; Horvath, L.G.; Quinn, D.I.; Rasiah, K.K.; Gish, K.; Willhite, D.; Kench, J.G.; Gardiner-Garden, M.; et al. Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 2003, 63, 4196–4203. [Google Scholar]
- Glinsky, G.V.; Glinskii, A.B.; Stephenson, A.J.; Hoffman, R.M.; Gerald, W.L. Gene expression profiling predicts clinical outcome of prostate cancer. J. Clin. Investig. 2004, 113, 913–923. [Google Scholar] [CrossRef]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; Brothman, A.R.; Berney, D.M.; Reid, J.E.; Mesher, D.; Speights, V.O.; Stankiewicz, E.; Foster, C.S.; et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef]
- Rubicz, R.; Zhao, S.; Wright, J.L.; Coleman, I.; Grasso, C.; Geybels, M.S.; Leonardson, A.; Kolb, S.; April, C.; Bibikova, M.; et al. Gene expression panel predicts metastatic-lethal prostate cancer outcomes in men diagnosed with clinically localized prostate cancer. Mol. Oncol. 2017, 11, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamy, P.J.; Allory, Y.; Gauchez, A.S.; Asselain, B.; Beuzeboc, P.; de Cremoux, P.; Fontugne, J.; Georges, A.; Hennequin, C.; Lehmann-Che, J.; et al. Prognostic Biomarkers Used for Localised Prostate Cancer Management: A Systematic Review. Eur. Urol. Focus 2018, 4, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.L.; Catton, C.N.; Swallow, C.J. Unbiased data mining identifies cell cycle transcripts that predict non-indolent Gleason score 7 prostate cancer. BMC Urol. 2019, 19, 4. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.A. Gleason score-related outcome model for human prostate cancer: A comprehensive study based on weighted gene co-expression network analysis. Cancer Cell Int. 2020, 20, 159. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Zhao, Y.; Liang, K.; Huang, Y. Dentification of Potential Key Genes for Pathogenesis and Prognosis in Prostate Cancer by Integrated Analysis of Gene Expression Profiles and the Cancer Genome Atlas. Front. Oncol. 2020, 10, 809. [Google Scholar] [CrossRef]
- Meng, J.; Guan, Y.; Wang, B.; Chen, L.; Chen, J.; Zhang, M.; Liang, C.H. Risk subtyping and prognostic assessment of prostate cancer based on consensus genes. Commun. Biol. 2022, 5, 233. [Google Scholar] [CrossRef]
- Agemy, L.; Kela, I.; Waks, T.; Pfeffer, R.; Bar-Shira, A.; Orr-Urtreger, A.; Domany, E.; Eshhar, Z. Gene Expression Profiles Predict Sensitivity of Prostate Cancer to Radiotherapy. J. Cancer Ther. 2013, 4, 11–26. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd; John-Aryankalayil, M.; Palayoor, S.T.; Makinde, A.Y.; Cerna, D.; Falduto, M.T.; Magnuson, S.R.; Coleman, C.N. mRNA Expression Profiles for Prostate Cancer following Fractionated Irradiation Are Influenced by p53 Status. Transl. Oncol. 2013, 6, 573–585. [Google Scholar] [CrossRef]
- Tseng, C.S.; Wang, Y.J.; Chen, C.H.; Wang, S.M.; Huang, K.H.; Chow, P.M.; Pu, Y.S.; Huang, C.Y.; Cheng, J.C. Outcomes and Prediction Models for Exclusive Prostate Bed Salvage Radiotherapy among Patients with Biochemical Recurrence after Radical Prostatectomy. Cancers 2021, 13, 2672. [Google Scholar] [CrossRef]
- Bibikova, M.; Chudin, E.; Arsanjani, A.; Zhou, L.; Garcia, E.W.; Modder, J.; Kostelec, M.; Barker, D.; Downs, T.; Fan, J.B.; et al. Expression signatures that correlated with Gleason score and relapse in prostate cancer. Genomics 2007, 89, 666–672. [Google Scholar] [CrossRef]
- Penney, K.L.; Sinnott, J.A.; Fall, K.; Pawitan, Y.; Hoshida, Y.; Kraft, P.; Stark, J.R.; Fiorentino, M.; Perner, S.; Finn, S.; et al. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J. Clin. Oncol. 2011, 29, 2391–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinnott, J.A.; Peisch, S.; Tyekucheva, S.; Gerke, T.A.; Lis, R.T.; Rider, J.R.; Fiorentino, M.; Stampfer, M.J.; Mucci, L.A.; Loda, M.; et al. Prognostic utility of a new mRNA expression signature of Gleason score. Clin. Cancer Res. 2016, 23, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jhun, M.A.; Geybels, M.S.; Wright, J.L.; Kolb, S.; April, C.; Bibikova, M.; Ostrander, E.A.; Fan, J.B.; Feng, Z.; Stanford, J.L. Gene expression signature of Gleason score is associated with prostate cancer outcomes in a radical prostatectomy cohort. Oncotarget 2017, 8, 43035–43047. [Google Scholar] [CrossRef]
- Abril, J.; De Heredia, M.L.; González, L.; Cléries, R.; Nadal, M.; Condom, E.; Aguiló, F.; Gómez-Zaera, M.; Nunes, V. Altered expression of 12S/MT-RNR1, MT-CO2/COX2, and MT-ATP6 mitochondrial genes in prostate cancer. Prostate 2008, 68, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.; Mehrabi, S.; Bacanamwo, M.; Yao, X.; Aikhionbare, F.O. Expression of mitochondrial genes MT-ND1, MT-ND6, MT-CYB, MT-COI, MT-ATP6, and 12S/MT-RNR1 in colorectal adenopolyps. Tumor. Biol. 2016, 37, 12465–12475. [Google Scholar] [CrossRef] [PubMed]
- Han, C.B.; Ma, J.M.; Xin, Y.; Mao, X.Y.; Zhao, Y.J.; Wu, D.Y.; Zhang, S.M.; Zhang, Y.K. Mutations of mitochondrial 12S rRNA in gastric carcinoma and their significance. World J. Gastroenterol. 2005, 11, 31–35. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chu, Y.D.; Lim, S.N.; Chen, C.W.; Yeh, C.T.; Lin, W.R. Impact of an MT-RNR1 gene polymorphism on hepatocellular carcinoma progression and clinical characteristics. Int. J. Mol. Sci. 2021, 22, 1119. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lim, S.N.; Chen, C.Y.; Chi, H.C.; Yeh, C.T.; Lin, W.R. Functional Role of Mitochondrial DNA in Cancer Progression. Int. J. Mol. Sci. 2022, 23, 1659. [Google Scholar] [CrossRef]

| Total Number of Patients/Blood Samples Available | 44/43 |
|---|---|
| Age [years, Mean (Range)] | 63.6 (47–80) |
| TNM at primary diagnosis [number of patients] | |
| T1 | 6 |
| T2 | 9 |
| T3 | 27 |
| T4 | 1 |
| N 0 | 38 |
| N 1 | 5 |
| M 0 | 43 |
| M 1 | 0 |
| Gleason—Score at primary diagnosis [number of patients] | |
| Gleason sum 6 | 1 |
| Gleason sum 7a | 22 |
| Gleason sum 7b | 3 |
| Gleason sum 8 | 7 |
| Gleason sum 9 | 10 |
| Gleason sum 10 | 0 |
| Therapy after primary diagnosis | |
| Prostatectomy | 39 |
| Radiotherapy | 4 |
| PSA Level in Blood sample [ng/mL, mean (range)] | |
| At primary diagnosis | 12.15 (2.6–40.4) |
| At relapse and PSMA-PET | 5.15 (0.19–64) |
| Doubling time at PSMA-detected relapse [month] | 22 (1–46) |
| Site of relapse [number of patients] | |
| Former prostate region only | 3 |
| Pelvic lymphatic nodes only | 22 |
| Extra pelvic lymphatic nodes only | 0 |
| Bone metastases only | 7 |
| Metastases at lung/liver only | 1 |
| Both pelvic and extra pelvic lymphatic nodes | 2 |
| Both lymphatic nodes and bone metastases | 4 |
| Both former prostate region and bone metastases | 2 |
| Both former prostate region and pelvic lymphatic nodes | 2 |
| Peak uptake [mean (range)] | 7.7 (1.8–49.8) |
| Number of patients not receiving ADT during follow up (ADT−) | 30 |
| Number of patients receiving ADT during follow up (ADT+) | 13 |
| Treatment outcome after Radiotherapy of relapse | |
| Time to progression [month, mean (range)] | 7.1 (2–19) |
| ADT− patients | |
| Remission < 12 months [number of patients] | 20 |
| Remission ≥ 12 months [number of patients] | 3 |
| No remission | 7 |
| ADT+ patients | |
| Remission < 12 months [number of patients] | 5 |
| Remission ≥ 12 months [number of patients] | 2 |
| No remission | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanova, N.V.; Radmanesh, H.; Ramachandran, D.; Knoechelmann, A.C.; Christiansen, H.; Derlin, T.; von Klot, C.A.J.; Merten, R.; Henkenberens, C. The Prognostic Value of Liquid Biopsies for Benefit of Salvage Radiotherapy in Relapsed Oligometastatic Prostate Cancer. Cancers 2022, 14, 4095. https://doi.org/10.3390/cancers14174095
Bogdanova NV, Radmanesh H, Ramachandran D, Knoechelmann AC, Christiansen H, Derlin T, von Klot CAJ, Merten R, Henkenberens C. The Prognostic Value of Liquid Biopsies for Benefit of Salvage Radiotherapy in Relapsed Oligometastatic Prostate Cancer. Cancers. 2022; 14(17):4095. https://doi.org/10.3390/cancers14174095
Chicago/Turabian StyleBogdanova, Natalia V., Hoda Radmanesh, Dhanya Ramachandran, Anne Caroline Knoechelmann, Hans Christiansen, Thorsten Derlin, Christoph Alexander Joachim von Klot, Roland Merten, and Christoph Henkenberens. 2022. "The Prognostic Value of Liquid Biopsies for Benefit of Salvage Radiotherapy in Relapsed Oligometastatic Prostate Cancer" Cancers 14, no. 17: 4095. https://doi.org/10.3390/cancers14174095
APA StyleBogdanova, N. V., Radmanesh, H., Ramachandran, D., Knoechelmann, A. C., Christiansen, H., Derlin, T., von Klot, C. A. J., Merten, R., & Henkenberens, C. (2022). The Prognostic Value of Liquid Biopsies for Benefit of Salvage Radiotherapy in Relapsed Oligometastatic Prostate Cancer. Cancers, 14(17), 4095. https://doi.org/10.3390/cancers14174095







