A Real-World Multicentre Retrospective Study of Low-Dose Apatinib for Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. Treatment
2.3. Efficacy Assessments
2.4. Statistical Analyses
3. Results
3.1. Baseline Patient Characteristics
3.2. Efficacy Outcome
3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, L.; Strasser-Weippl, K.; Li, J.J.; St Louis, J.; Finkelstein, D.M.; Yu, K.D.; Chen, W.Q.; Shao, Z.M.; Goss, P.E. Breast cancer in China. Lancet Oncol. 2014, 15, e279–e289. [Google Scholar] [CrossRef]
- Burstein, H.J.; Elias, A.D.; Rugo, H.S.; Cobleigh, M.A.; Wolff, A.C.; Eisenberg, P.D.; Lehman, M.; Adams, B.J.; Bello, C.L.; DePrimo, S.E.; et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2008, 26, 1810–1816. [Google Scholar] [CrossRef]
- Cortes, J.; O’Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Dieras, V.; Delozier, T.; et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef]
- Polyzos, A.; Kalbakis, K.; Kentepozidis, N.; Giassas, S.; Kalykaki, A.; Vardakis, N.; Bozionelou, V.; Saloustros, E.; Kontopodis, E.; Georgoulias, V.; et al. Salvage treatment in metastatic breast cancer with weekly paclitaxel and bevacizumab. Cancer Chemother. Pharmacol. 2011, 68, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, B.; Wang, Z.; Ragaz, J.; Zhang, J.; Sun, S.; Cao, J.; Lv, F.; Wang, L.; Zhang, S.; et al. Bevacizumab in Combination with Modified FOLFOX6 in Heavily Pretreated Patients with HER2/Neu-Negative Metastatic Breast Cancer: A Phase II Clinical Trial. PLoS ONE 2015, 10, e0133133. [Google Scholar] [CrossRef]
- Hu, X.; Cao, J.; Hu, W.; Wu, C.; Pan, Y.; Cai, L.; Tong, Z.; Wang, S.; Li, J.; Wang, Z.; et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014, 14, 820. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Xu, B.; Jiang, Z.; Ragaz, J.; Tong, Z.; Zhang, Q.; Wang, X.; Feng, J.; Pang, D.; et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int. J. Cancer 2014, 135, 1961–1969. [Google Scholar] [CrossRef]
- Feng, J.; Qin, S. The synergistic effects of Apatinib combined with cytotoxic chemotherapeutic agents on gastric cancer cells and in a fluorescence imaging gastric cancer xenograft model. Onco Targets Ther. 2018, 11, 3047–3057. [Google Scholar] [CrossRef]
- Harlozinska, A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005, 25, 3327–3333. [Google Scholar]
- Zhang, H. Apatinib for molecular targeted therapy in tumor. Drug Des. Devel. Ther. 2015, 9, 6075–6081. [Google Scholar] [CrossRef]
- Tian, S.; Quan, H.; Xie, C.; Guo, H.; Lu, F.; Xu, Y.; Li, J.; Lou, L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011, 102, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, C.; Huang, J.; Guan, Y.; Guo, Q. Clinical investigation of the efficacy and toxicity of apatinib (YN968D1) in stage III/IV non-small cell lung cancer after second-line chemotherapy treatment: A retrospective study. Thorac. Cancer 2018, 9, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.J.; Liang, Y.J.; Huang, H.B.; Zhao, H.Y.; Wu, C.P.; Wang, F.; Tao, L.Y.; Zhang, C.Z.; Dai, C.L.; Tiwari, A.K.; et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010, 70, 7981–7991. [Google Scholar] [CrossRef]
- Tong, X.Z.; Wang, F.; Liang, S.; Zhang, X.; He, J.H.; Chen, X.G.; Liang, Y.J.; Mi, Y.J.; To, K.K.; Fu, L.W. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem. Pharmacol. 2012, 83, 586–597. [Google Scholar] [CrossRef]
- Zhu, A.; Yuan, P.; Wang, J.; Fan, Y.; Luo, Y.; Cai, R.; Zhang, P.; Li, Q.; Ma, F.; Xu, B. Apatinib combined with chemotherapy in patients with previously treated advanced breast cancer: An observational study. Oncol. Lett. 2019, 17, 4768–4778. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Geng, C.; Zhao, H.; Jiang, H.; Song, G.; Zhang, J.; Liu, Y.; Gui, X.; Wang, J.; Li, K.; et al. Multicenter phase II study of apatinib single or combination therapy in HER2-negative breast cancer involving chest wall metastasis. Chin. J. Cancer Res. 2021, 33, 243–255. [Google Scholar] [CrossRef]
- Hu, N.; Zhu, A.; Si, Y.; Yue, J.; Wang, X.; Wang, J.; Ma, F.; Xu, B.; Yuan, P. A Phase II, Single-Arm Study of Apatinib and Oral Etoposide in Heavily Pre-Treated Metastatic Breast Cancer. Front. Oncol. 2020, 10, 565384. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Li, Y.; Li, Q.; Su, F.; Yao, H.; Su, S.; Wang, Q.; Jin, L.; Wang, Y.; et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: An open-label phase II trial. J. Immunother. Cancer 2020, 8, e000696. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Tian, Z.; Lin, Y.; Li, H.; Zhu, Z.; Liu, Q.; Su, S.; Zeng, Y.; Jia, W.; et al. Multicenter phase II trial of Camrelizumab combined with Apatinib and Eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat. Commun. 2022, 13, 3011. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, J.; Yu, Q.; Wang, X.; Song, X.; Wang, F.; Li, C.; Yu, Z.; Yu, J. Real-World Data on Apatinib Efficacy—Results of a Retrospective Study in Metastatic Breast Cancer Patients Pretreated With Multiline Treatment. Front. Oncol. 2021, 11, 643654. [Google Scholar] [CrossRef]
- Zhu, A.; Yuan, P.; Hu, N.; Li, M.; Wang, W.; Wang, X.; Yue, J.; Wang, J.; Luo, Y.; Ma, F.; et al. Phase II study of apatinib in combination with oral vinorelbine in heavily pretreated HER2-negative metastatic breast cancer and clinical implications of monitoring ctDNA. Cancer Biol. Med. 2021, 18, 875. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Okada, M.; Kaji, Y.; Satouchi, M.; Sato, Y.; Yamabe, Y.; Onaya, H.; Endo, M.; Sone, M.; Arai, Y. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan Kagaku Ryoho 2009, 36, 2495–2501. [Google Scholar]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Ha, L.; Cheng, G.; Wan, Y.; Xia, Y.; Sosnoski, D.M.; Mastro, A.M.; Zheng, S.Y. A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 2018, 14, e1702787. [Google Scholar] [CrossRef]
- Cui, H.; Esworthy, T.; Zhou, X.; Hann, S.Y.; Glazer, R.I.; Li, R.; Zhang, L.G. Engineering a Novel 3D Printed Vascularized Tissue Model for Investigating Breast Cancer Metastasis to Bone. Adv. Healthc. Mater. 2020, 9, e1900924. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, J.; Xiong, J.; Wu, C.; Bai, Y.; Liu, W.; Tong, J.; Liu, Y.; Xu, R.; et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J. Clin. Oncol. 2016, 34, 1448–1454. [Google Scholar] [CrossRef]
- Gao, Z.; Shi, M.; Wang, Y.; Chen, J.; Ou, Y. Apatinib enhanced anti-tumor activity of cisplatin on triple-negative breast cancer through inhibition of VEGFR-2. Pathol. Res. Pract. 2019, 215, 152422. [Google Scholar] [CrossRef]
- Chen, J.; Deng, S.; Zhang, Y.; Wang, C.; Hu, X.; Kong, D.; Liang, G.; Yuan, X.; Li, Y.; Wang, X. Apatinib enhances the anti-tumor effect of paclitaxel via the PI3K/p65/Bcl-xl pathway in triple-negative breast cancer. Ann. Transl. Med. 2021, 9, 1001. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.K.; Kathawala, R.J.; Chen, Z.S. Repositioning of Tyrosine Kinase Inhibitors as Antagonists of ATP-Binding Cassette Transporters in Anticancer Drug Resistance. Cancers 2014, 6, 1925–1952. [Google Scholar] [CrossRef]
- Fontanella, C.; Ongaro, E.; Bolzonello, S.; Guardascione, M.; Fasola, G.; Aprile, G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann. Transl. Med. 2014, 2, 123. [Google Scholar]
- Blum, J.L.; Jones, S.E.; Buzdar, A.U.; LoRusso, P.M.; Kuter, I.; Vogel, C.; Osterwalder, B.; Burger, H.U.; Brown, C.S.; Griffin, T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J. Clin. Oncol. 1999, 17, 485–493. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.; Li, Q.; Xiong, J.; Ma, Y.; Wu, G.; Zhao, Y. Apatinib + CPT-11 + S-1 for treatment of refractory brain metastases in patient with triple-negative breast cancer: Case report and literature review. Medicine 2018, 97, e0349. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhou, Y.; Wang, Y.W.; Tong, L.; Jiang, R.X.; Xiao, L.; Zhang, G.J.; Xing, S.S.; Qian, F.; Feng, J.Q.; et al. Comparison of apatinib and capecitabine (Xeloda) with capecitabine (Xeloda) in advanced triple-negative breast cancer as third-line therapy: A retrospective study. Medicine 2018, 97, e12222. [Google Scholar] [CrossRef] [PubMed]
- Jehn, C.F.; Hemmati, P.; Lehenbauer-Dehm, S.; Kummel, S.; Flath, B.; Schmid, P. Biweekly Pegylated Liposomal Doxorubicin (Caelyx) in Heavily Pretreated Metastatic Breast Cancer: A Phase 2 Study. Clin. Breast Cancer 2016, 16, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Koutras, A.; Zagouri, F.; Koliou, G.A.; Psoma, E.; Chryssogonidis, I.; Lazaridis, G.; Tryfonopoulos, D.; Kotsakis, A.; Res, E.; Kentepozidis, N.K.; et al. Phase 2 study of cabazitaxel as second-line treatment in patients with HER2-negative metastatic breast cancer previously treated with taxanes-a Hellenic Cooperative Oncology Group (HeCOG) Trial. Br. J. Cancer 2020, 123, 355–361. [Google Scholar] [CrossRef]
- Shah, A.N.; Flaum, L.; Helenowski, I.; Santa-Maria, C.A.; Jain, S.; Rademaker, A.; Nelson, V.; Tsarwhas, D.; Cristofanilli, M.; Gradishar, W. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J. Immunother. Cancer 2020, 8, e000173. [Google Scholar] [CrossRef]
- Kaufman, P.A.; Awada, A.; Twelves, C.; Yelle, L.; Perez, E.A.; Velikova, G.; Olivo, M.S.; He, Y.; Dutcus, C.E.; Cortes, J. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2015, 33, 594–601. [Google Scholar] [CrossRef]
- Perez, E.A.; Awada, A.; O’Shaughnessy, J.; Rugo, H.S.; Twelves, C.; Im, S.A.; Gomez-Pardo, P.; Schwartzberg, L.S.; Dieras, V.; Yardley, D.A.; et al. Etirinotecan pegol (NKTR-102) versus treatment of physician’s choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 1556–1568. [Google Scholar] [CrossRef]
- Yuan, P.; Hu, X.; Sun, T.; Li, W.; Zhang, Q.; Cui, S.; Cheng, Y.; Ouyang, Q.; Wang, X.; Chen, Z.; et al. Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: A randomised clinical trial. Eur. J. Cancer 2019, 112, 57–65. [Google Scholar] [CrossRef]
- Jacot, W.; Heudel, P.E.; Fraisse, J.; Gourgou, S.; Guiu, S.; Dalenc, F.; Pistilli, B.; Campone, M.; Levy, C.; Debled, M.; et al. Real-life activity of eribulin mesylate among metastatic breast cancer patients in the multicenter national observational ESME program. Int. J. Cancer 2019, 145, 3359–3369. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, J.; Guo, W.; Xiong, J.; Bai, Y.; Sun, G.; Yang, Y.; Wang, L.; Xu, N.; et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: Results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol. 2013, 31, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Brower, V. Apatinib in treatment of refractory gastric cancer. Lancet Oncol. 2016, 17, e137. [Google Scholar] [CrossRef]
- Lim, J.J.; Yang, K.; Taylor-Harding, B.; Wiedemeyer, W.R.; Buckanovich, R.J. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2. Neoplasia 2014, 16, 343–353.e2. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.J.; Wei, X.; Peng, Y.; Zha, L.; Zhou, R.B.; Shi, H.; Zhou, Q.; Liang, H.J. Neuropilin-2 mediates lymphangiogenesis of colorectal carcinoma via a VEGFC/VEGFR3 independent signaling. Cancer Lett. 2015, 358, 200–209. [Google Scholar] [CrossRef]

| Characteristics | No. | % |
|---|---|---|
| ECOG performance status | ||
| 0–1 | 62 | 48.4 |
| 2 | 66 | 51.6 |
| ER | ||
| positive | 65 | 50.8 |
| negative | 63 | 49.2 |
| PR | ||
| positive | 52 | 40.6 |
| negative | 76 | 59.4 |
| Number of prior systemic therapy lines | ||
| 1 | 14 | 10.9 |
| 2 | 42 | 32.8 |
| 3 | 32 | 25.0 |
| >3 | 40 | 31.3 |
| Metastatic sites | ||
| bone | 59 | 46.1 |
| liver | 56 | 43.8 |
| lung | 69 | 53.9 |
| chest wall | 24 | 18.8 |
| brain | 11 | 8.6 |
| Ki67 | ||
| ≤15 | 16 | 12.5 |
| >15 | 94 | 73.4 |
| Unknown | 18 | 14.1 |
| Axillary lymph node | ||
| positive | 79 | 61.7 |
| negative | 49 | 38.3 |
| Radiotherapy | ||
| yes | 72 | 56.3 |
| no | 56 | 43.7 |
| Age | ||
| ≤50 | 61 | 47.7 |
| >50 | 67 | 52.3 |
| Type of metastatic sites | ||
| Visceral | 115 | 89.8 |
| Only non-visceral | 13 | 10.2 |
| Characteristic | Progression-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| Median PFS, Months (95% CI) | p-Value | Median OS, Months (95% CI) | p-Value | |
| Age, years | 0.442 | 0.328 | ||
| ≤50 | 4.5 (3.0–6.0) | 14.2 (11.4–17.0) | ||
| >50 | 4.9 (4.2–5.6) | 16.0 (14.4–17.6) | ||
| Molecular type | 0.109 | 0.062 | ||
| HR+ | 4.0 (2.6–5.4) | 17.2 (15.0–19.4) | ||
| TNBC | 5.1 (4.6–5.6) | 10.7 (8.5–12.9) | ||
| Ki67 | 0.425 | 0.133 | ||
| ≤15% | 5.0 (4.1–5.9) | 15.7 (13.7–17.7) | ||
| >15% | 4.5 (3.1–5.9) | 14.2 (11.7–16.7) | ||
| Micropapillary | 0.390 | 0.514 | ||
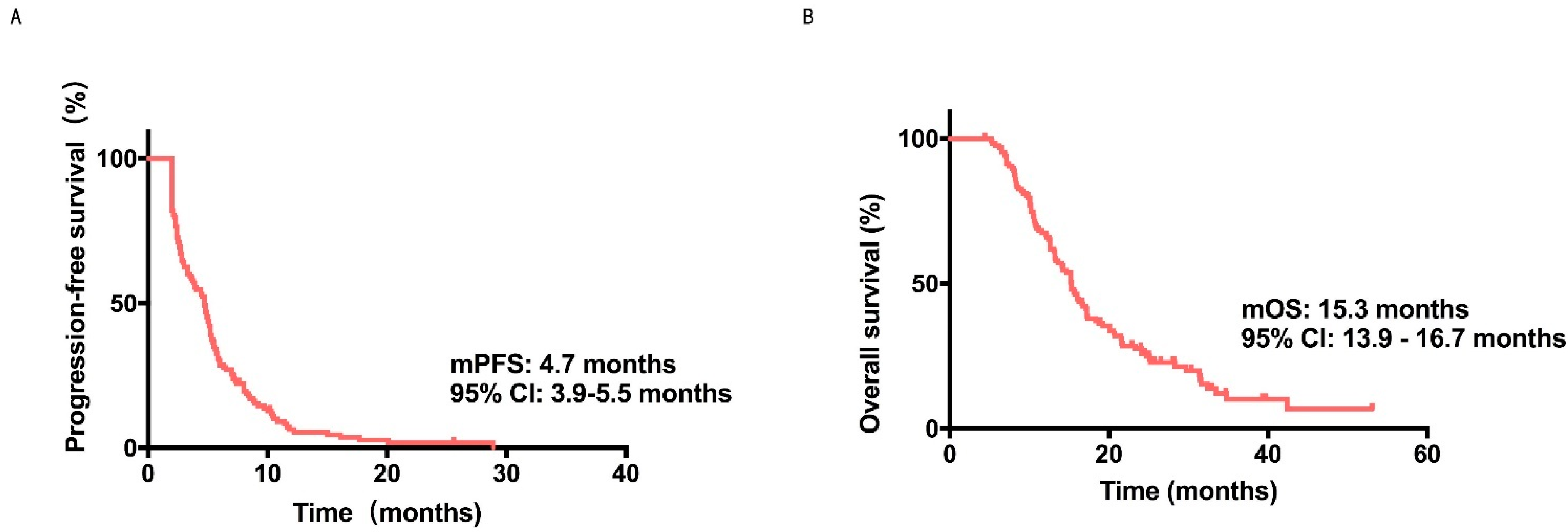
| No | 4.8 (4.0–5.6) | 15.3 (13.9–16.7) | ||
| Yes | 3.9 (2.4–5.4) | NR | ||
| Vascular cancer embolus | 0.890 | 0.173 | ||
| No | 4.7 (3.8–5.6) | 15.2 (13.8–16.6) | ||
| Yes | 4.7 (2.8–6.6) | 17.2 (9.7–24.7) | ||
| Axillary lymph nodes | 0.697 | 0.024 | ||
| No | 3.9 (2.3–5.5) | 15.2 (12.3–18.1) | ||
| Yes | 4.8 (4.2–5.4) | 15.3 (13.3–17.3) | ||
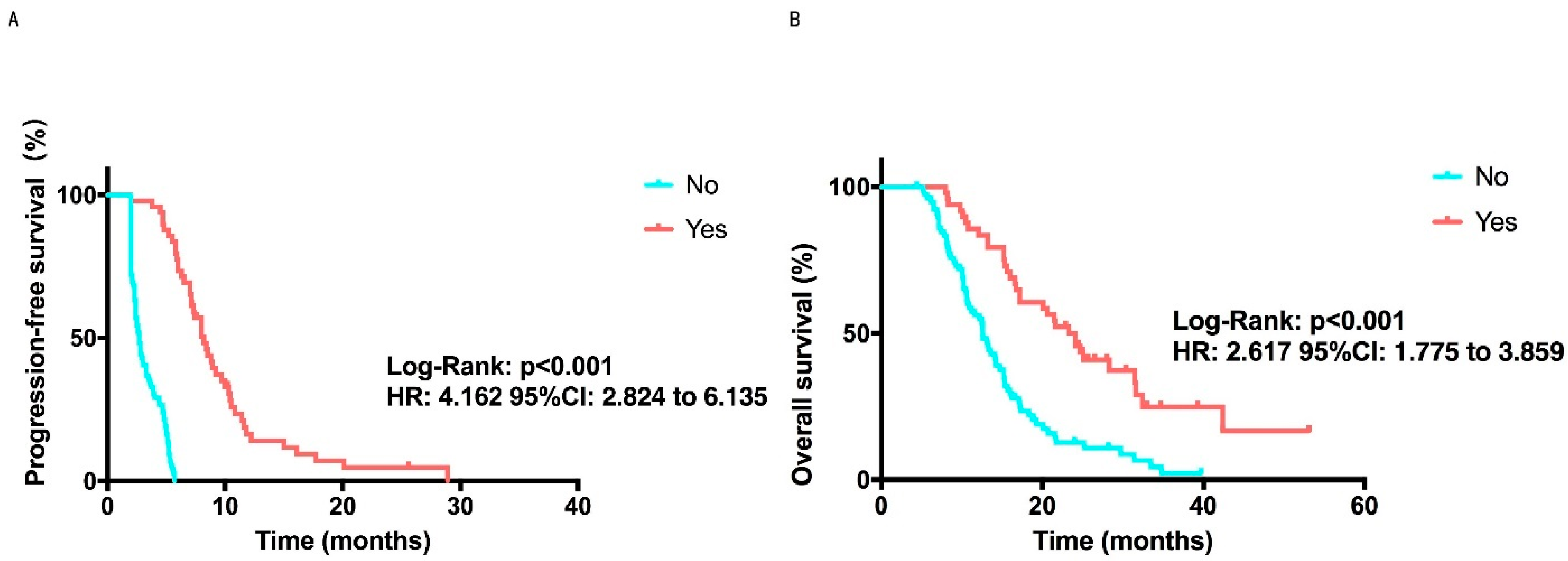
| Radiotherapy | 0.003 | 0.521 | ||
| No | 5.2 (4.1–6.3) | 16.6 (13.0–20.2) | ||
| Yes | 3.9 (2.3–5.5) | 15.2 (13.7–16.7) | ||
| BRCA | 0.018 | 0.021 | ||
| Mutated | 8.9 (3.5–14.3) | NR | ||
| Wild type | 4.7 (3.7–5.7) | 14.1 (12.1–16.1) | ||
| Lines of treatment | 0.034 | 0.213 | ||
| <3 | 5.1 (4.8–5.4) | 17.1 (14.6–19.6) | ||
| ≥3 | 3.5 (2.6–4.4) | 14.7 (12.7–16.7) | ||
| Liver metastasis | 0.125 | 0.869 | ||
| No | 5.1 (4.4–5.8) | 14.1 (12.5–15.7) | ||
| Yes | 3.9 (2.4–5.4) | 17.2 (14.9–19.5) | ||
| Lung metastasis | 0.456 | 0.156 | ||
| No | 3.5 (2.0–5.0) | 14.1 (11.9–16.3) | ||
| Yes | 5.0 (4.5–5.5) | 16.6 (14.1–19.1) | ||
| Brain metastasis | 0.834 | 0.673 | ||
| No | 4.7 (3.9–5.5) | 15.3 (13.7–16.9) | ||
| Yes | 5.3 (2.3–8.3) | 15.2 (10.8–19.6) | ||
| Chest wall metastasis | 0.769 | 0.920 | ||
| No | 4.5 (3.6–5.4) | 15.2 (13.5–16.9) | ||
| Yes | 5.1 (3.9–6.3) | 15.7 (12.5–18.9) | ||
| Bone metastasis | 0.014 | 0.107 | ||
| No | 5.2 (4.8–5.6) | 17.1 (14.8–19.4) | ||
| Yes | 3.8 (2.3–5.3) | 14.7 (12.7–16.7) | ||
| Hypertension | 0.012 | 0.232 | ||
| No | 3.8 (1.8–5.8) | 14.2 (12.1–16.3) | ||
| Yes | 4.8 (4.1–5.6) | 16.2 (14.1–18.3) | ||
| Hand-foot syndrome | 0.023 | 0.226 | ||
| No | 3.9 (2.6–5.2) | 14.7 (12.3–17.1) | ||
| Yes | 5.3 (4.4–6.2) | 16.2 (14.4–18.0) | ||
| Proteinuria | 0.500 | 0.592 | ||
| No | 4.7 (3.9–5.5) | 15.3 (14.0–16.6) | ||
| Yes | 4.0 (1.4–6.6) | 13.7 (9.7–16.9) | ||
| Apatinb order | 0.017 | 0.210 | ||
| First | 4.7 (3.9–5.5) | 15.2 (13.5–17.0) | ||
| Second | 8.0 (2.0–14.0) | 16.6 (6.2–27.0) | ||
| Chemotherapy regimen | <0.001 | 0.001 | ||
| Immunotherapy | 11.8 (2.0–21.6) | NR | ||
| Paclitaxel and platinum | 7.1 (5.4–8.8) | 25.1 (8.5–41.7) | ||
| Paclitaxel | 5.7 (4.3–7.1) | 20.6 (17.3–23.9) | ||
| Pemetrexed | 3.3 (1.2–5.4) | 8.1 (2.0–14.6) | ||
| Epirubicin | 4.0 (1.8–6.2) | 16.6 (8.6–24.6) | ||
| Vinorelbine | 3.0 (2.2–3.8) | 12.6 (6.3–18.9) | ||
| Gemcitabine | 2.7 (1.4–4.0) | 12.2 (10.2–14.2) | ||
| Capecitabine | 2.5 (2.0–3.0) | 13.2 (9.8–16.6) | ||
| Clinical benefit | <0.001 | <0.001 | ||
| No | 2.7 (2.4–3.0) | 12.6 (11.0–14.2) | ||
| Yes | 8.2 (7.0–9.4) | 24.1 (19.6–28.6) | ||
| AE | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hypertension | 42 (32.8) | 23 (18.0) | 13 (10.2) | 0 |
| Anemia | 19 (14.8) | 11 (8.6) | 3 (2.3) | 0 |
| Proteinuria | 16(12.5) | 6 (4.7) | 1 (0.8) | 0 |
| Hand-foot syndrome | 18 (14.1) | 30 (23.4) | 7 (5.5) | 0 |
| Thrombocytopenia | 18 (14.1) | 9 (7.0) | 3 (2.3) | 0 |
| Leukopenia | 27 (21.1) | 13 (10.2) | 5 (3.9) | 1 (0.8) |
| Hepatotoxicity | 17 (13.3) | 11 (8.6) | 1 (0.8) | 0 |
| Fatigue | 18 (14.1) | 27 (21.1) | 3 (2.3) | 0 |
| Anorexia | 28 (21.) | 31 (24.2) | 10 (7.8) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, T.; Sun, C.; Liang, Y.; Yang, F.; Yan, X.; Bao, S.; Zhang, Y.; Huang, X.; Fu, Z.; Li, W.; et al. A Real-World Multicentre Retrospective Study of Low-Dose Apatinib for Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. Cancers 2022, 14, 4084. https://doi.org/10.3390/cancers14174084
Zeng T, Sun C, Liang Y, Yang F, Yan X, Bao S, Zhang Y, Huang X, Fu Z, Li W, et al. A Real-World Multicentre Retrospective Study of Low-Dose Apatinib for Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. Cancers. 2022; 14(17):4084. https://doi.org/10.3390/cancers14174084
Chicago/Turabian StyleZeng, Tianyu, Chunxiao Sun, Yan Liang, Fan Yang, Xueqi Yan, Shengnan Bao, Yucheng Zhang, Xiang Huang, Ziyi Fu, Wei Li, and et al. 2022. "A Real-World Multicentre Retrospective Study of Low-Dose Apatinib for Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer" Cancers 14, no. 17: 4084. https://doi.org/10.3390/cancers14174084
APA StyleZeng, T., Sun, C., Liang, Y., Yang, F., Yan, X., Bao, S., Zhang, Y., Huang, X., Fu, Z., Li, W., & Yin, Y. (2022). A Real-World Multicentre Retrospective Study of Low-Dose Apatinib for Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. Cancers, 14(17), 4084. https://doi.org/10.3390/cancers14174084






