BMS-986158, a Small Molecule Inhibitor of the Bromodomain and Extraterminal Domain Proteins, in Patients with Selected Advanced Solid Tumors: Results from a Phase 1/2a Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Study Endpoints and Assessments
2.4. Statistical Analyses
3. Results
3.1. Patients
3.2. Safety
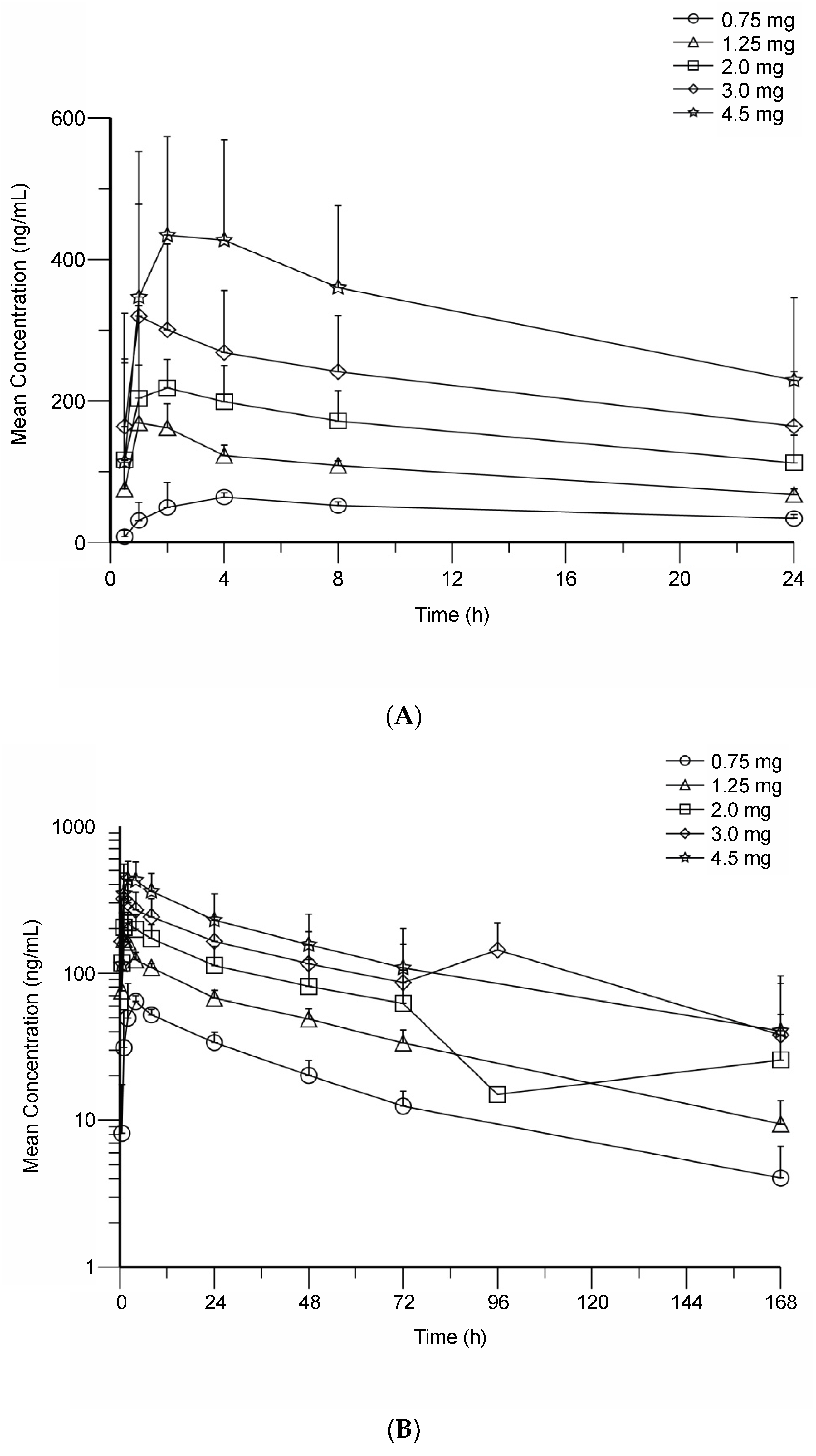
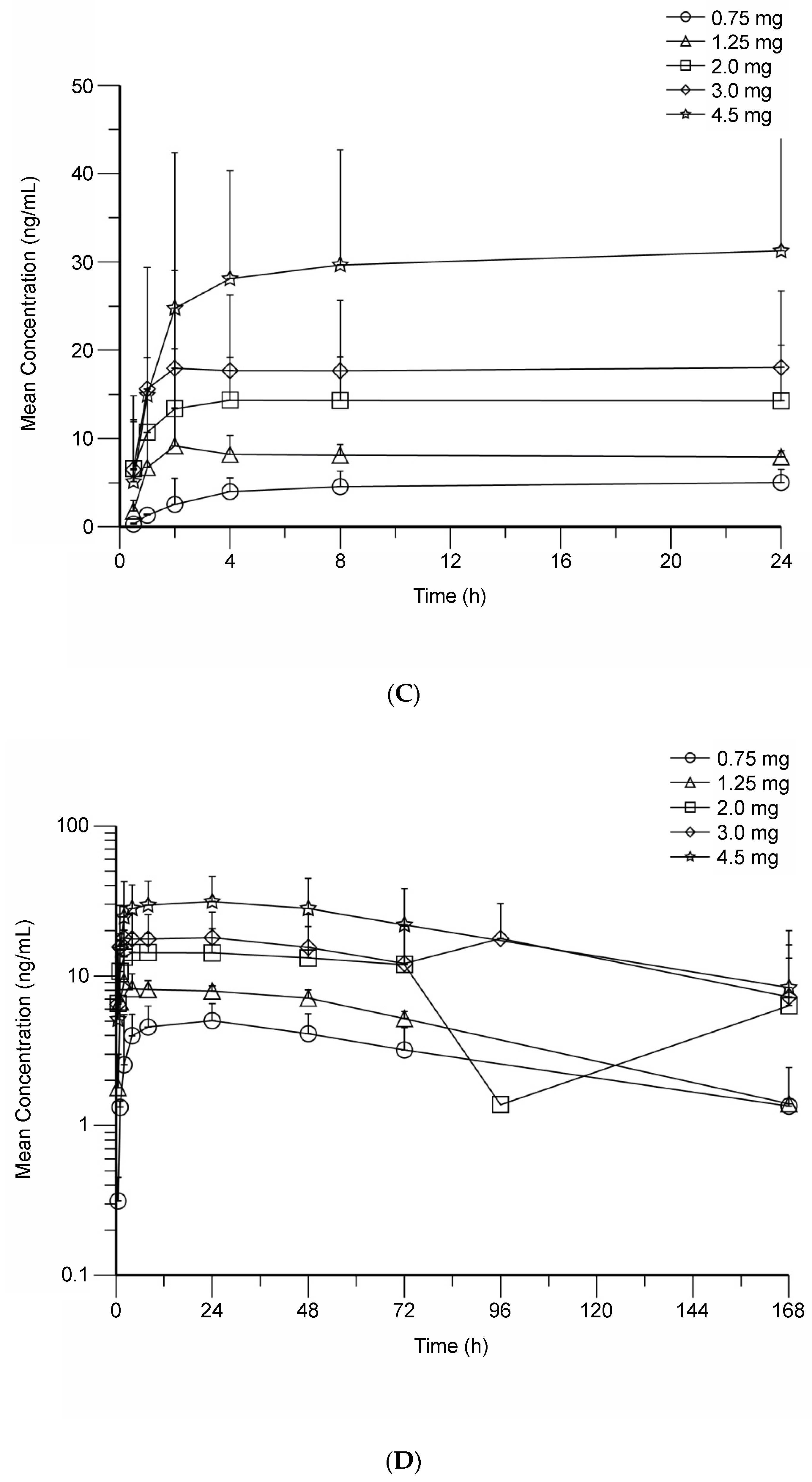
3.3. BMS-986158 Pharmacokinetics
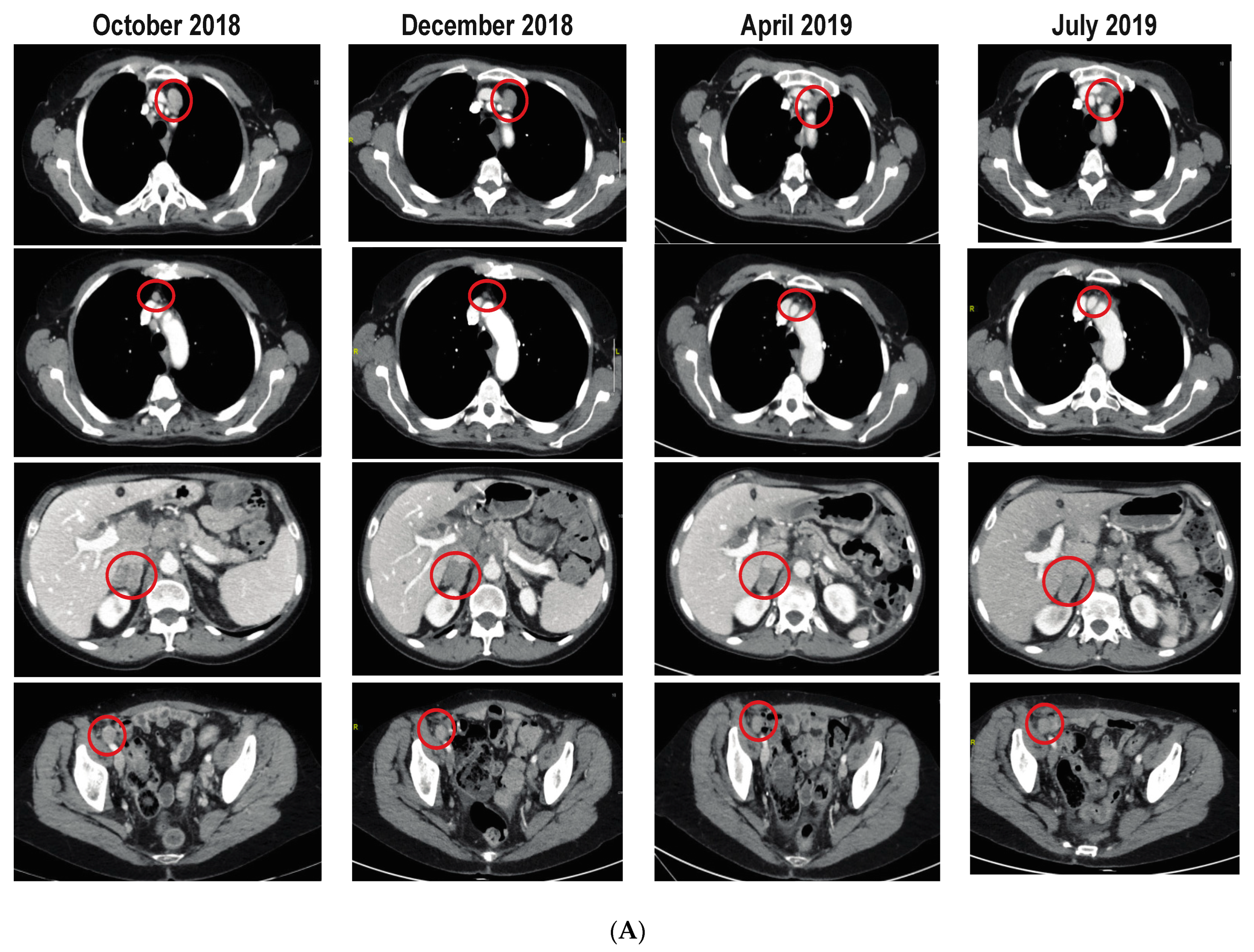
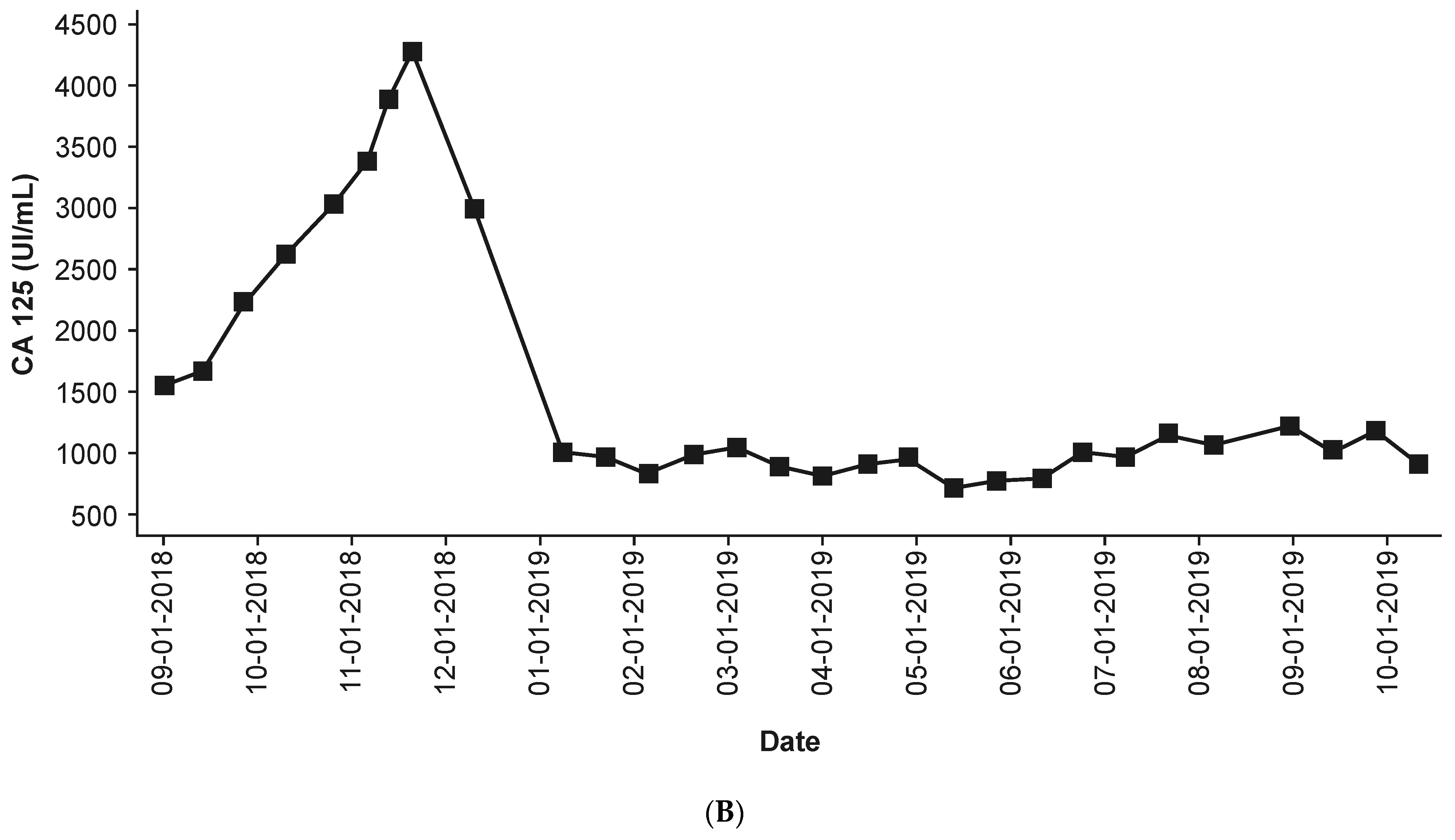
3.4. Efficacy
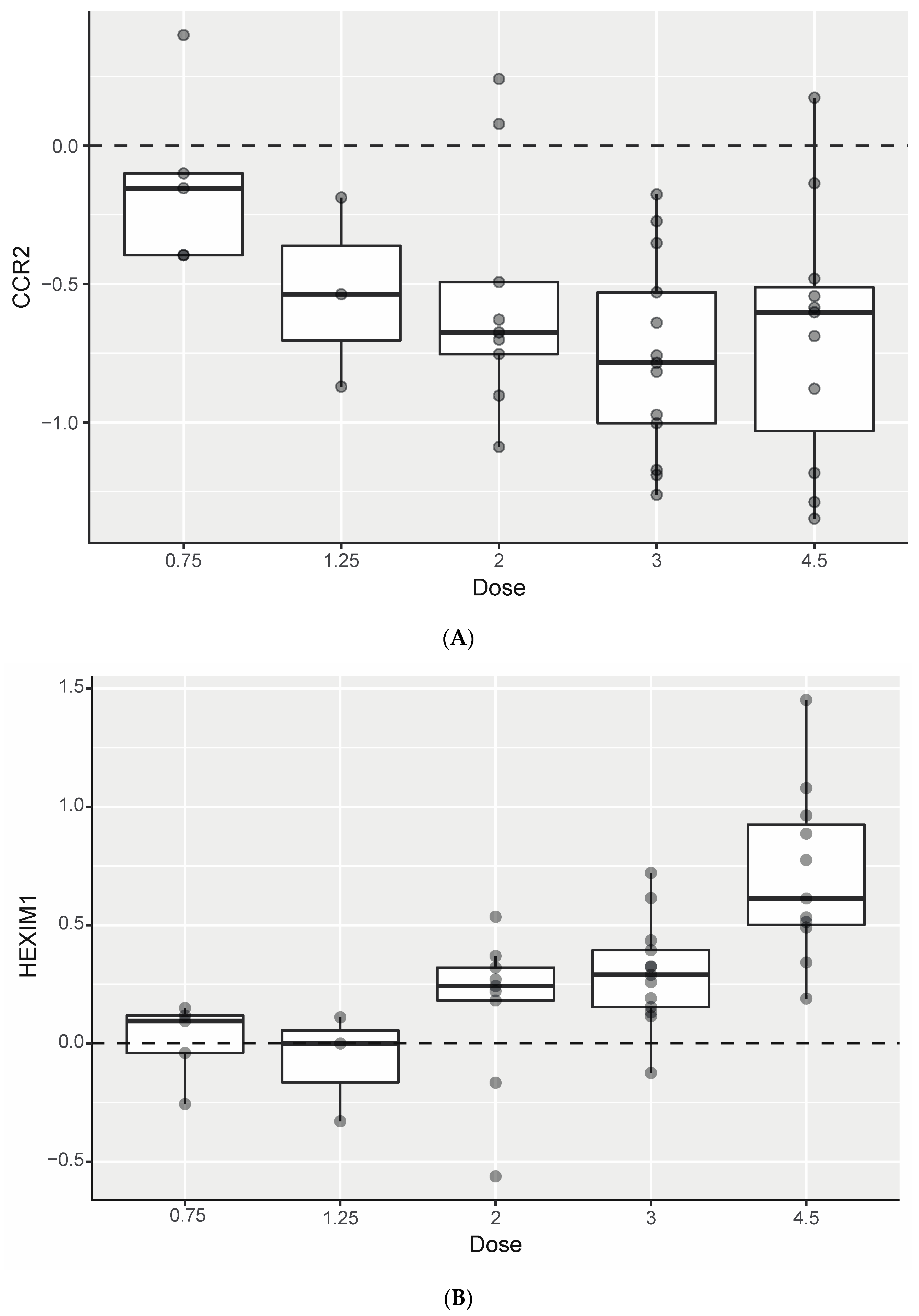
3.5. Biomarker Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, R.L.; Licht, J.D. Targeting epigenetics in cancer. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 187–207. [Google Scholar] [CrossRef]
- Kumar, V.E.; Nambiar, R.; De Souza, C.; Nguyen, A.; Chien, J.; Lam, K.S. Targeting epigenetic modifiers of tumor plasticity and cancer stem cell behavior. Cells 2022, 11, 1403. [Google Scholar] [CrossRef]
- Kulikowski, E.; Rakai, B.D.; Wong, N.C.W. Inhibitors of bromodomain and extra-terminal proteins for treating multiple human diseases. Med. Res. Rev. 2021, 41, 223–245. [Google Scholar] [CrossRef]
- Loven, J.; Hoke, H.A.; Lin, C.Y.; Lau, A.; Orlando, D.A.; Vakoc, C.R.; Bradner, J.E.; Lee, T.I.; Young, R.A. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013, 153, 320–334. [Google Scholar] [CrossRef]
- Rahman, S.; Sowa, M.E.; Ottinger, M.; Smith, J.A.; Shi, Y.; Harper, J.W.; Howley, P.M. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol. Cell. Biol. 2011, 31, 2641–2652. [Google Scholar] [CrossRef]
- Taniguchi, Y. The bromodomain and extra-terminal domain (BET) family: Functional anatomy of BET paralogous proteins. Int. J. Mol. Sci. 2016, 17, 1849. [Google Scholar] [CrossRef]
- Donati, B.; Lorenzini, E.; Ciarrocchi, A. BRD4 and cancer: Going beyond transcriptional regulation. Mol. Cancer 2018, 17, 164. [Google Scholar] [CrossRef]
- French, C.A.; Miyoshi, I.; Kubonishi, I.; Grier, H.E.; Perez-Atayde, A.R.; Fletcher, J.A. BRD4-NUT fusion oncogene: A novel mechanism in aggressive carcinoma. Cancer Res. 2003, 63, 304–307. [Google Scholar]
- French, C.A.; Ramirez, C.L.; Kolmakova, J.; Hickman, T.T.; Cameron, M.J.; Thyne, M.E.; Kutok, J.L.; Toretsky, J.A.; Tadavarthy, A.K.; Kees, U.R.; et al. BRD-NUT oncoproteins: A family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 2008, 27, 2237–2242. [Google Scholar] [CrossRef]
- Liao, Y.F.; Wu, Y.B.; Long, X.; Zhu, S.Q.; Jin, C.; Xu, J.J.; Ding, Y.J. High level of BRD4 promotes non-small cell lung cancer progression. Oncotarget 2016, 7, 9491–9500. [Google Scholar] [CrossRef]
- Crawford, N.P.S.; Alsarraj, J.; Lukes, L.; Walker, R.C.; Officewala, J.S.; Yang, H.H.; Lee, M.P.; Ozato, K.; Hunter, K.W. Bromodomain 4 activation predicts breast cancer survival. Proc. Natl. Acad. Sci. USA 2008, 105, 6380–6385. [Google Scholar] [CrossRef]
- Urbanucci, A.; Barfeld, S.J.; Kytola, V.; Itkonen, H.M.; Coleman, I.M.; Vodak, D.; Sjoblom, L.; Sheng, X.; Tolonen, T.; Minner, S.; et al. Androgen receptor deregulation drives bromodomain-mediated chromatin alterations in prostate cancer. Cell Rep. 2017, 19, 2045–2059. [Google Scholar] [CrossRef]
- Baratta, M.G.; Schinzel, A.C.; Zwang, Y.; Bandopadhayay, P.; Bowman-Colin, C.; Kutt, J.; Curtis, J.; Piao, H.; Wong, L.C.; Kung, A.L.; et al. An in-tumor genetic screen reveals that the BET bromodomain protein, BRD4, is a potential therapeutic target in ovarian carcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, 232–237. [Google Scholar] [CrossRef]
- Ambrosini, G.; Sawle, A.D.; Musi, E.; Schwartz, G.K. BRD4-targeted therapy induces Myc-independent cytotoxicity in Gnaq/11-mutatant uveal melanoma cells. Oncotarget 2015, 6, 33397–33409. [Google Scholar] [CrossRef]
- Lockwood, W.W.; Zejnullahu, K.; Bradner, J.E.; Varmus, H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 19408–19413. [Google Scholar] [CrossRef]
- Shorstova, T.; Marques, M.; Su, J.; Johnston, J.; Kleinman, C.L.; Hamel, N.; Huang, S.; Alaoui-Jamali, M.A.; Foulkes, W.D.; Witcher, M. SWI/SNF-compromised cancers are susceptible to bromodomain inhibitors. Cancer Res. 2019, 79, 2761–2774. [Google Scholar] [CrossRef]
- Loganathan, S.N.; Tang, N.; Fleming, J.T.; Ma, Y.; Guo, Y.; Borinstein, S.C.; Chiang, C.; Wang, J. BET bromodomain inhibitors suppress EWS-FLI1-dependent transcription and the IGF1 autocrine mechanism in Ewing sarcoma. Oncotarget 2016, 7, 43504–43517. [Google Scholar] [CrossRef]
- Stathis, A.; Zucca, E.; Bekradda, M.; Gomez-Roca, C.; Delord, J.-P.; de la Motte Rouge, T.; Uro-Coste, E.; de Braud, F.; Pelosi, G.; French, C.A. Clinical response of carcinomas harboring the BRD4–NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016, 6, 492–500. [Google Scholar] [CrossRef]
- Gavai, A.V.; Norris, D.; Delucca, G.; Tortolani, D.; Tokarski, J.S.; Dodd, D.; O’Malley, D.; Zhao, Y.; Quesnelle, C.; Gill, P.; et al. Discovery and preclinical pharmacology of an oral bromodomain and extra-terminal (BET) inhibitor using scaffold-hopping and structure-guided drug design. J. Med. Chem. 2021, 64, 14247–14265. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, P.; Li, Y.; Bekele, B.N. A modified toxicity probability interval method for dose-finding trials. Clin. Trials 2010, 7, 653–663. [Google Scholar] [CrossRef]
- Hummel, J.; McKendrick, S.; Brindley, C.; French, R. Exploratory assessment of dose proportionality: Review of current approaches and proposal for a practical criterion. Pharm. Stat. 2009, 8, 38–49. [Google Scholar] [CrossRef]
- Bartholomeeusen, K.; Xiang, Y.; Fujinaga, K.; Peterlin, B.M. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J. Biol. Chem. 2012, 287, 36609–36616. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Baldini, C.; Postel-Vinay, S. How much can we bet on activity of BET inhibitors beyond NUT-midline carcinoma? JNCI Cancer Spectr. 2020, 4, pkz092. [Google Scholar] [CrossRef]
- Sun, Y.; Han, J.; Wang, Z.; Li, X.; Sun, Y.; Hu, Z. Safety and efficacy of bromodomain and extra-terminal inhibitors for the treatment of hematological malignancies and solid tumors: A systematic study of clinical trials. Front. Pharmacol. 2021, 11, 621093. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Eder, J.P.; LoRusso, P.M. BET inhibitors: A novel epigenetic approach. Ann. Oncol. 2017, 28, 1776–1787. [Google Scholar] [CrossRef]
- Devaraj, S.G.; Fiskus, W.; Shah, B.; Qi, J.; Sun, B.; Iyer, S.P.; Sharma, S.; Bradner, J.E.; Bhalla, K.N. HEXIM1 induction is mechanistically involved in mediating anti-AML activity of BET protein bromodomain antagonist. Leukemia 2016, 30, 504–508. [Google Scholar] [CrossRef][Green Version]
- Coude, M.M.; Braun, T.; Berrou, J.; Dupont, M.; Bertrand, S.; Masse, A.; Raffoux, E.; Itzykson, R.; Delord, M.; Riveiro, M.E.; et al. BET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cells. Oncotarget 2015, 6, 17698–17712. [Google Scholar] [CrossRef]
- Yeh, T.C.; O’Connor, G.; Petteruti, P.; Dulak, A.; Hattersley, M.; Barrett, J.C.; Chen, H. Identification of CCR2 and CD180 as robust pharmacodynamic tumor and blood biomarkers for clinical use with BRD4/BET inhibitors. Clin. Cancer Res. 2017, 23, 1025–1035. [Google Scholar] [CrossRef]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology—A primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar]
- Wang, L.; Wu, X.; Wang, R.; Yang, C.; Li, Z.; Wang, C.; Zhang, F.; Yang, P. BRD4 inhibition suppresses cell growth, migration and invasion of salivary adenoid cystic carcinoma. Biol. Res. 2017, 50, 19. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Sachdev, J.C.; Barve, M.; LoRusso, P.; Szmulewitz, R.; Patel, S.P.; Lara, P.N., Jr.; Chen, X.; Hu, B.; Freise, K.J.; et al. First-in-human study of mivebresib (ABBV-075), an oral pan-inhibitor of bromodomain and extra terminal proteins, in patients with relapsed/refractory solid tumors. Clin. Cancer Res. 2019, 25, 6309–6319. [Google Scholar] [CrossRef]
- NCT04817007. A Phase 1b Study of BMS-986158 Monotherapy and in Combination with Either Ruxolitinib or Fedratinib in Participants with DIPSS-Intermediate or High Risk Myelofibrosis. Available online: https://clinicaltrials.gov/ct2/show/NCT04817007 (accessed on 24 September 2021).
| Demographic Characteristics and Prior Treatments | ||||
|---|---|---|---|---|
| Schedule A (5 Days On, 2 Days Off) n = 46 | Schedule B (14 Days On, 7 Days Off) n = 8 | Schedule C (7 Days On, 14 Days Off) n = 29 | Total N = 83 | |
| Age, median (range), years | 57.0 (23–88) | 64.0 (58–75) | 57.0 (33–71) | 59.0 (23–88) |
| Sex, n (%) | ||||
| Male | 10 (21.7) | 3 (37.5) | 6 (20.7) | 19 (22.9) |
| Female | 36 (78.3) | 5 (62.5) | 23 (79.3) | 64 (77.1) |
| Race, n (%) | ||||
| White | 39 (84.8) | 8 (100.0) | 22 (75.9) | 69 (83.1) |
| Black | 2 (4.3) | 0 | 0 | 2 (2.4) |
| Asian | 2 (4.3) | 0 | 2 (6.9) | 4 (4.8) |
| Other | 3 (6.5) | 0 | 5 (17.2) | 8 (9.6) |
| Prior systemic therapies, n (%) | ||||
| 0 | 2 (4.3) | 2 (25.0) | 1 (3.4) | 5 (6.0) |
| 1 | 17 (37.0) | 0 (0) | 5 (17.2) | 22 (26.5) |
| 2 | 3 (6.5) | 2 (25.0) | 4 (13.8) | 9 (10.8) |
| 3 | 7 (15.2) | 2 (25.0) | 5 (17.2) | 14 (16.9) |
| ≥4 | 17 (37.0) | 2 (25.0) | 14 (48.3) | 33 (39.8) |
| Disease characteristics | ||||
| Current disease stage, n (%) | ||||
| 1 | 0 | 0 | 1 (3.4) | 1 (1.2) |
| 2 | 1 (2.2) | 0 | 0 | 1 (1.2) |
| 3 | 5 (10.9) | 1 (12.5) | 4 (13.8) | 10 (12.0) |
| 4 | 40 (87.0) | 7 (87.5) | 21 (82.8) | 71 (85.5) |
| Tumor type, n (%) | ||||
| Ovarian cancer | 20 (43.5) | 3 (37.5) | 18 (62.1) | 41 (49.4) |
| SCLC | 5 (10.9) | 2 (25.0) | 3 (10.3) | 10 (12.0) |
| TNBC | 6 (13.0) | 1 (12.5) | 2 (6.9) | 9 (10.8) |
| NUT carcinoma | 6 (13.0) | 0 | 1 (3.4) | 7 (8.4) |
| UM | 4 (8.7) | 0 | 1 (3.4) | 5 (6.0) |
| ACC | 1 (2.2) | 1 (12.5) | 2 (6.9) | 4 (4.8) |
| CRPC | 1 (2.2) | 0 | 1 (3.4) | 2 (2.4) |
| Ewing sarcoma | 1 (2.2) | 0 | 0 | 1 (1.2) |
| Metastatic high-grade NEC | 0 | 0 | 1 (3.4) | 1 (1.2) |
| PPSC | 1 (2.2) | 0 | 0 | 1 (1.2) |
| SpCC | 0 | 1 (12.5) | 0 | 1 (1.2) |
| UCS | 1 (2.2) | 0 | 0 | 1 (1.2) |
| BMS-986158 Monotherapy QD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Schedule A (5 Days On, 2 Days Off) n = 46 | Schedule B (14 Days On, 7 Days Off) n = 8 | Schedule C (7 Days On, 14 Days Off) n = 29 | Total N = 83 | |||||
| Any Grade n (%) | Grade ≥ 3 n (%) | Any Grade n (%) | Grade ≥ 3 n (%) | Any Grade n (%) | Grade ≥ 3 n (%) | Any Grade n (%) | Grade ≥ 3 n (%) | |
| Patients with any TRAE | 33 (71.7) | 19 (41.3) | 8 (100.0) | 4 (50.0) | 21 (72.4) | 5 (17.2) | 62 (74.7) | 28 (33.7) |
| TRAEs in ≥10% of all pts | ||||||||
| Diarrhea | 19 (41.3) | 0 | 4 (50.0) | 0 | 13 (44.8) | 0 | 36 (43.4) | 0 |
| Thrombocytopenia | 20 (43.5) | 16 (34.8) | 5 (62.5) | 2 (25.0) | 7 (24.1) | 3 (10.3) | 32 (38.6) | 21 (25.3) |
| Fatigue | 8 (17.4) | 2 (4.3) | 4 (50) | 1 (12.5) | 4 (13.8) | 0 | 16 (19.3) | 3 (3.6) |
| Nausea | 7 (15.2) | 1 (2.2) | 1 (12.5) | 1 (12.5) | 6 (20.7) | 0 | 14 (16.9) | 2 (2.4) |
| Anemia | 6 (13.0) | 2 (4.3) | 2 (25.0) | 2 (25.0) | 4 (13.8) | 1 (3.4) | 12 (14.5) | 5 (6.0) |
| Decreased appetite | 5 (10.9) | 0 | 4 (50.0) | 0 | 3 (10.3) | 0 | 12 (14.5) | 0 |
| Vomiting | 5 (10.9) | 0 | 2 (25.0) | 1 (12.5) | 3 (10.3) | 1 (3.4) | 10 (12.0) | 2 (2.4) |
| Serious TRAEs | 3 (6.5) a,d | 3 (6.5) a,d | 2 (25.0) b | 2 (25.0) b | 0 | 0 | 5 (6.0) c,d | 5 (6.0) c,d |
| Discontinuations due to TRAEs | 1 (2.2) d | 1 (2.2) d | 0 | 0 | 0 | 0 | 1 (1.2) d | 1 (1.2) d |
| Pharmacokinetic Parameter | Schedule A (5 Days On, 2 Days Off) | Schedule B (14 Days On, 7 Days Off) | Schedule C (7 Days On, 14 Days Off) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.75 mg n = 4 | 1.25 mg n = 4 | 2.0 mg n = 7 | 3.0 mg n = 8 | 4.5 mg n = 8 | 2.0 mg n = 1 | 3.0 mg n = 1 | 2.0 mg n = 5 | 3.0 mg n = 8 | 4.5 mg n = 7 | |
| BMS-986158 | ||||||||||
| Cmax (ng/mL), GM (%CV) | 136 (43) | 284 (16) | 442 (29) | 624 (44) | 898 (39) | 279 | 855 | 520 (34) | 588 (47) | 901 (62) |
| Tmax (h), median (range) | 3.14 (2.00–6.05) | 1.50 (0.50–2.00) | 2.00 (0.50–4.02) | 2.01 (0.50–2.10) | 2.00 (1.00–4.05) | 1.00 | 1.00 | 1.00 (0.83–4.00) | 1.66 (0.97–2.03) | 2.00 (0.17–2.03) |
| AUC(0–24) (h × ng/mL), GM (%CV) | 2716 (51) a | 3852 b | N/A c | 9817 (56) d | 14,551 (32) | 3430 | 13,305 | 8561 (36) | 8637 (71) | 11,286 (104) |
| AUC(0–T) (h × ng/mL), GM (%CV) | 3449 (93) | 4961 (73) | 7612 (71) | 13,378 (77) | 29,517 (36) | 6321 | 33,978 | 19,868 (45) | 18,266 (97) | 19,995 (135) |
| Effective T1/2 (h), mean (SD) | 36.0 (16.0) a | N/A c | N/A c | 25.7 (14.9) d | 27.3 (8.7) | 14.4 | 37.3 | 31.6 (10.5) | 27.3 (15.2) d | 27.6 (19.1) e |
| CL/F (mL/min), GM (%CV) | 4.60 (55) a | 5.41 b | N/Ac | 5.09 (43) d | 5.15 (29) | 9.72 | 3.76 | 3.89 (57) | 5.79 (39) | 6.65 (60) |
| Metabolite of BMS-986158 | ||||||||||
| Cmax (ng/mL), GM (%CV) | 25.8 (82) a | 31.0 (25) | 49.4 (46) | 83.3 (57) | 126 (50) | 32.7 | 127 | 80.7 (53) | 64.5 (55) | 146 (83) |
| MR Cmax, GM (%CV) | 0.17 (41) a | 0.11 (29) | 0.11 (25) | 0.13 (37) | 0.14 (18) | 0.12 | 0.15 | 0.16 (33) | 0.11 (32) | 0.16 (33) |
| Tmax (h), median (range) | 24.0 (24.0–24.0)a | 4.00 (1.50–6.00) | 2.07 (0–4.02) | 2.01 (1.00–6.10) | 4.00 (1.00–24.0) | 1.00 | 1.00 | 4.08 (3.83–24.0) | 4.00 (0–6.32) | 4.00 (1.00–27.1) |
| MR AUC(0–24) (h × ng/mL), GM (%CV) | 0.20 (37) a | 0.13 b | N/Ac | 0.18 (36) d | 0.18 (25) | 0.15 | 0.19 | 0.21 (35) | 0.15 (35) | 0.23 (45) |
| MR AUC(0–T) (h × ng/mL), GM (%CV) | 0.23 (40) a | 0.14 (18) | 0.15 (25) | 0.22 (34) | 0.23 (24) | 0.16 | 0.22 | 0.25 (43) | 0.19 (39) | 0.29 (48) |
| Effective T1/2 (h), mean (SD) | 80.9 (40.4) a | N/A c | N/A c | 72.1 (47.1) d | 80.4 (29.6) | 35.2 | 105 | 72.7 (19.4) | 63.5 (39.5) | 72.8 (74.2) |
| Schedule A n = 46 | Schedule B n = 8 | Schedule C n = 29 | |
|---|---|---|---|
| Best overall response by investigator, n (%) | |||
| Complete response | 0 | 0 | 0 |
| Partial response | 2 (4.3) a | 0 | 0 |
| Stable disease | 12 (26.1) | 3 (37.5) | 9 (31.0) |
| Progressive disease | 24 (52.2) | 4 (50.0) | 15 (51.7) |
| Unable to determine | 8 (17.4) b | 1 (12.5) c | 5 (17.2) d |
| ORR (95% CI), % | 2 (0.5–14.8) | 0 (0.0–36.9) | 0 (0.0–11.9) |
| Median PFS (95% CI), weeks | 8.29 (7.14–9.71) | 8.43 (5.57–40.14) | 9.00 (7.86–13.29) |
| PFS rate, n (95% CI) | |||
| 24 weeks | 18.1 (8.0–31.5) | 42.9 (9.8–73.4) | 13.5 (3.4–30.6) |
| 48 weeks | 10.3 (3.3–22.1) | 14.3 (0.7–46.5) | 6.8 (0.6–24.4) |
| Median OS (95% CI), months | 7.52 (4.17–11.99) | 9.23 (2.79–11.83) | 5.32 (3.52–11.83) |
| OS rate, n (95% CI) | |||
| 6 months | 52.6 (37.0–66.0) | NR e | 48.4 (27.3–66.7) |
| 12 months | 34.3 (20.8–48.2) | NR e | 30.8 (13.7–49.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilton, J.; Cristea, M.; Postel-Vinay, S.; Baldini, C.; Voskoboynik, M.; Edenfield, W.; Shapiro, G.I.; Cheng, M.L.; Vuky, J.; Corr, B.; et al. BMS-986158, a Small Molecule Inhibitor of the Bromodomain and Extraterminal Domain Proteins, in Patients with Selected Advanced Solid Tumors: Results from a Phase 1/2a Trial. Cancers 2022, 14, 4079. https://doi.org/10.3390/cancers14174079
Hilton J, Cristea M, Postel-Vinay S, Baldini C, Voskoboynik M, Edenfield W, Shapiro GI, Cheng ML, Vuky J, Corr B, et al. BMS-986158, a Small Molecule Inhibitor of the Bromodomain and Extraterminal Domain Proteins, in Patients with Selected Advanced Solid Tumors: Results from a Phase 1/2a Trial. Cancers. 2022; 14(17):4079. https://doi.org/10.3390/cancers14174079
Chicago/Turabian StyleHilton, John, Mihaela Cristea, Sophie Postel-Vinay, Capucine Baldini, Mark Voskoboynik, William Edenfield, Geoffrey I. Shapiro, Michael L. Cheng, Jacqueline Vuky, Bradley Corr, and et al. 2022. "BMS-986158, a Small Molecule Inhibitor of the Bromodomain and Extraterminal Domain Proteins, in Patients with Selected Advanced Solid Tumors: Results from a Phase 1/2a Trial" Cancers 14, no. 17: 4079. https://doi.org/10.3390/cancers14174079
APA StyleHilton, J., Cristea, M., Postel-Vinay, S., Baldini, C., Voskoboynik, M., Edenfield, W., Shapiro, G. I., Cheng, M. L., Vuky, J., Corr, B., Das, S., Apfel, A., Xu, K., Kozicki, M., Ünsal-Kaçmaz, K., Hammell, A., Wang, G., Ravindran, P., Kollia, G., ... Diamond, J. R. (2022). BMS-986158, a Small Molecule Inhibitor of the Bromodomain and Extraterminal Domain Proteins, in Patients with Selected Advanced Solid Tumors: Results from a Phase 1/2a Trial. Cancers, 14(17), 4079. https://doi.org/10.3390/cancers14174079






