HIF-1α Expression Increases Preoperative Concurrent Chemoradiotherapy Resistance in Hyperglycemic Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment Characteristics
2.2. Pathologic Evaluation, Immunohistochemical Staining, and Tunel Assay
2.3. Cell Line and Culture
2.4. Western Blotting
2.5. Irradiation Exposure and Cell Viability Assay
2.6. Colony Formation Assay
2.7. Establishment of Hyperglycemia Ectopic Rectal Cancer and Combined LW6 with Irradiation Treatment Schedule
2.8. Statistical Analysis
3. Results
3.1. Hyperglycemia Affects the Efficacy of CCRT in Patients with Rectal Cancer
3.2. HIF-1α Protein Positively Correlates with Tumor Regression Score in Rectal Cancer Patients Receiving CCRT Treatment
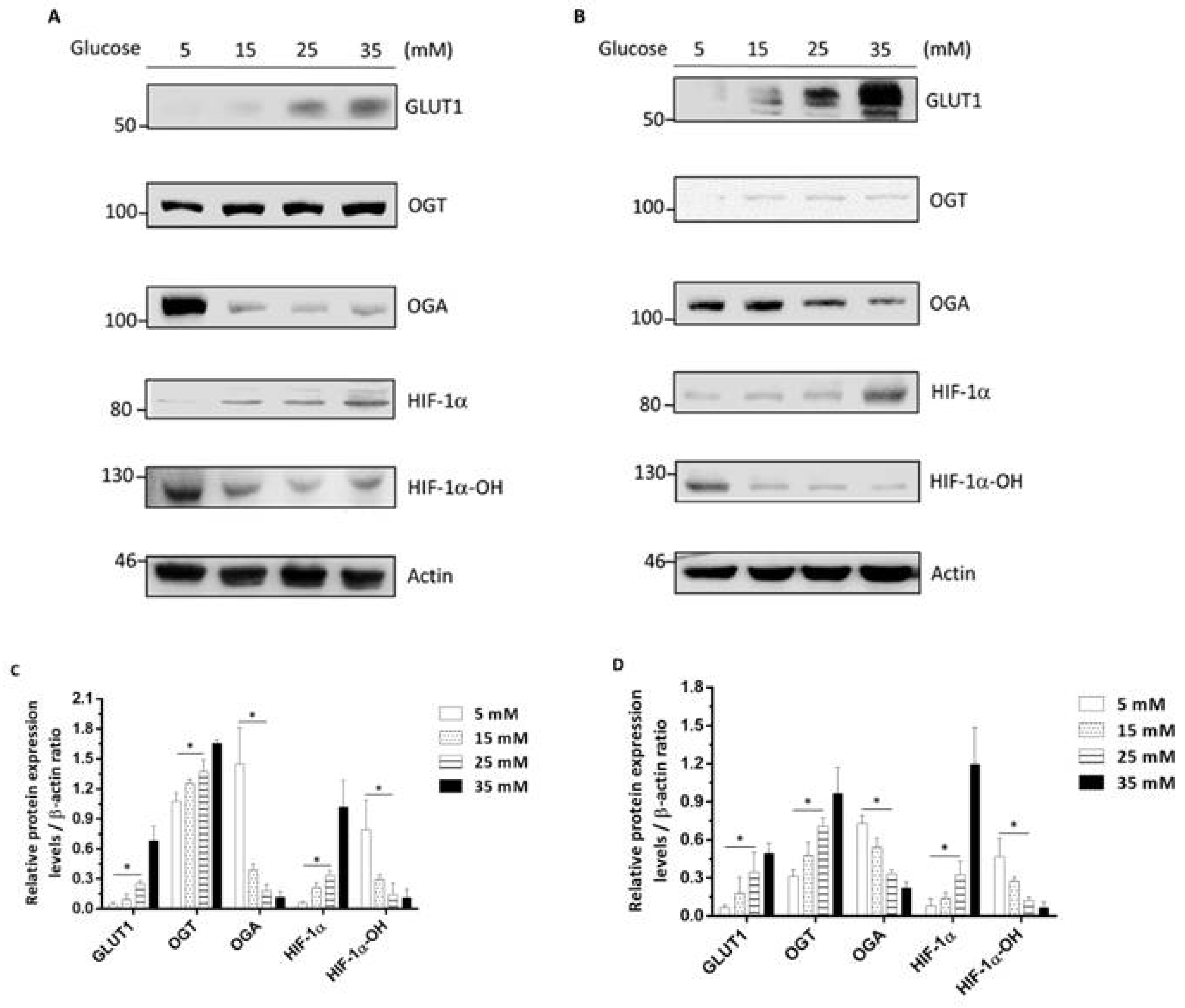
3.3. High-Glucose-Environment-Induced GLUT1-OGT-HIF-1α Signaling Pathways in Rectal Cancer Cell Lines
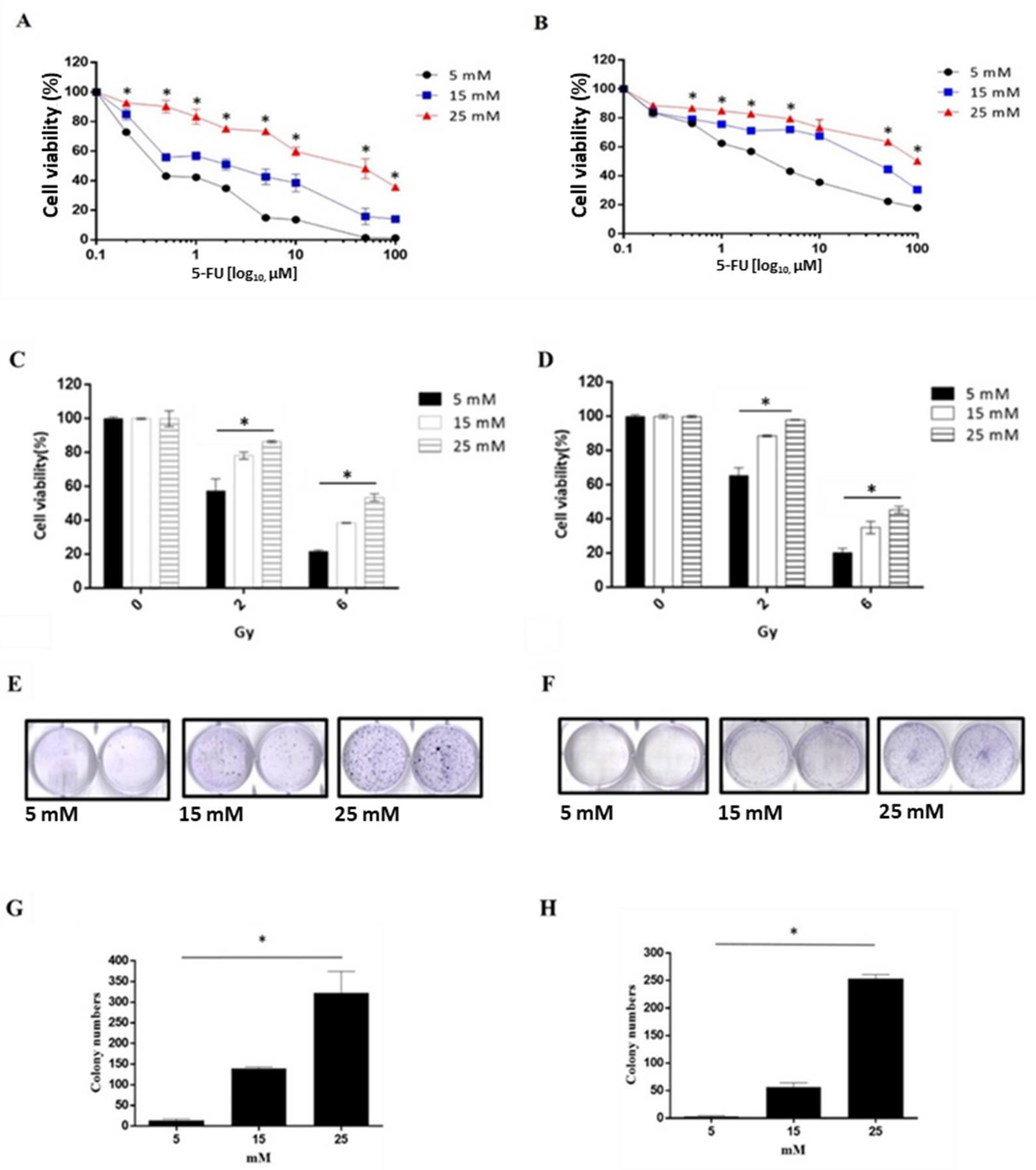
3.4. A High Glucose Environment Increases the 5-FU and Radiation Tolerance in Rectal Cancer Cell Lines
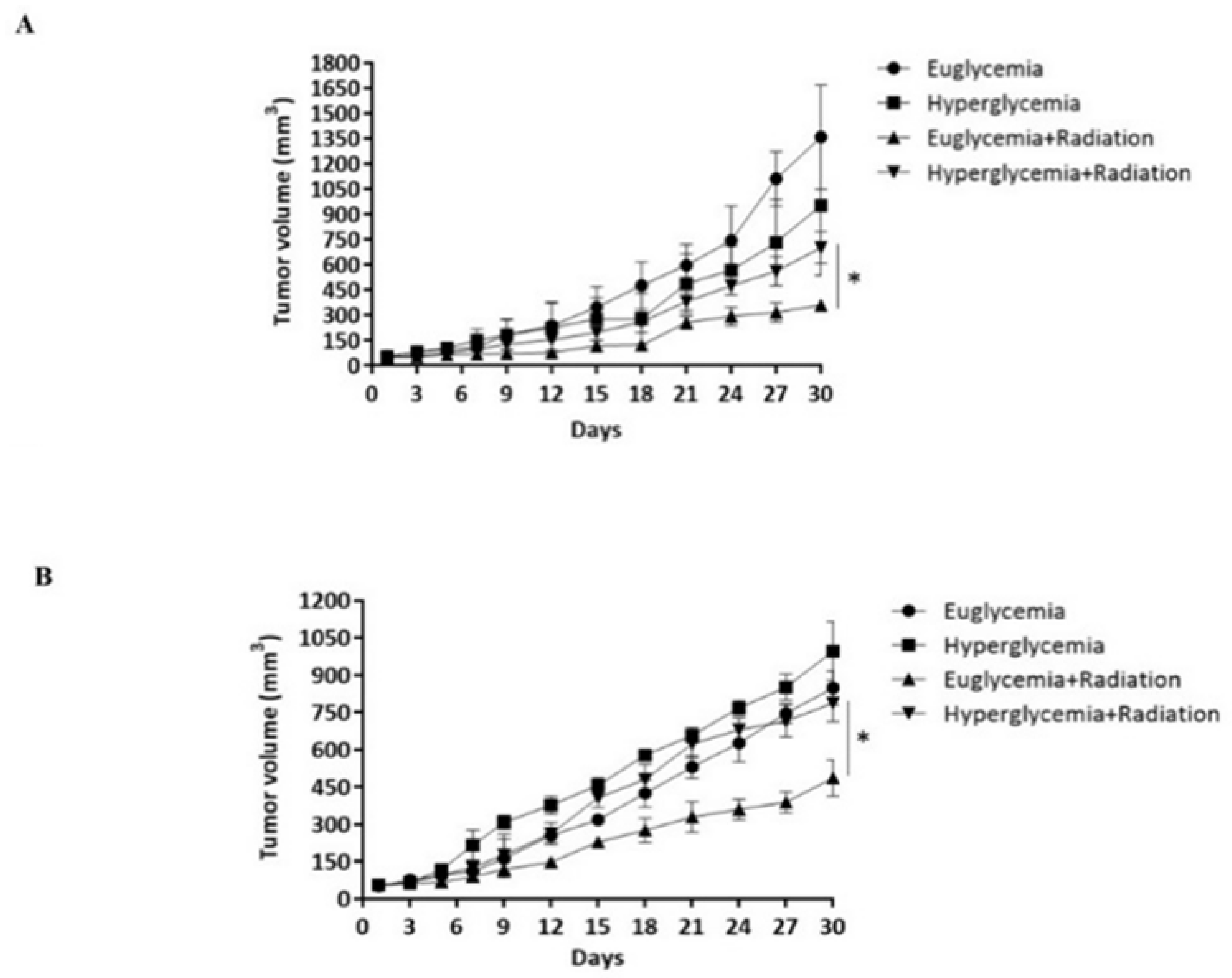
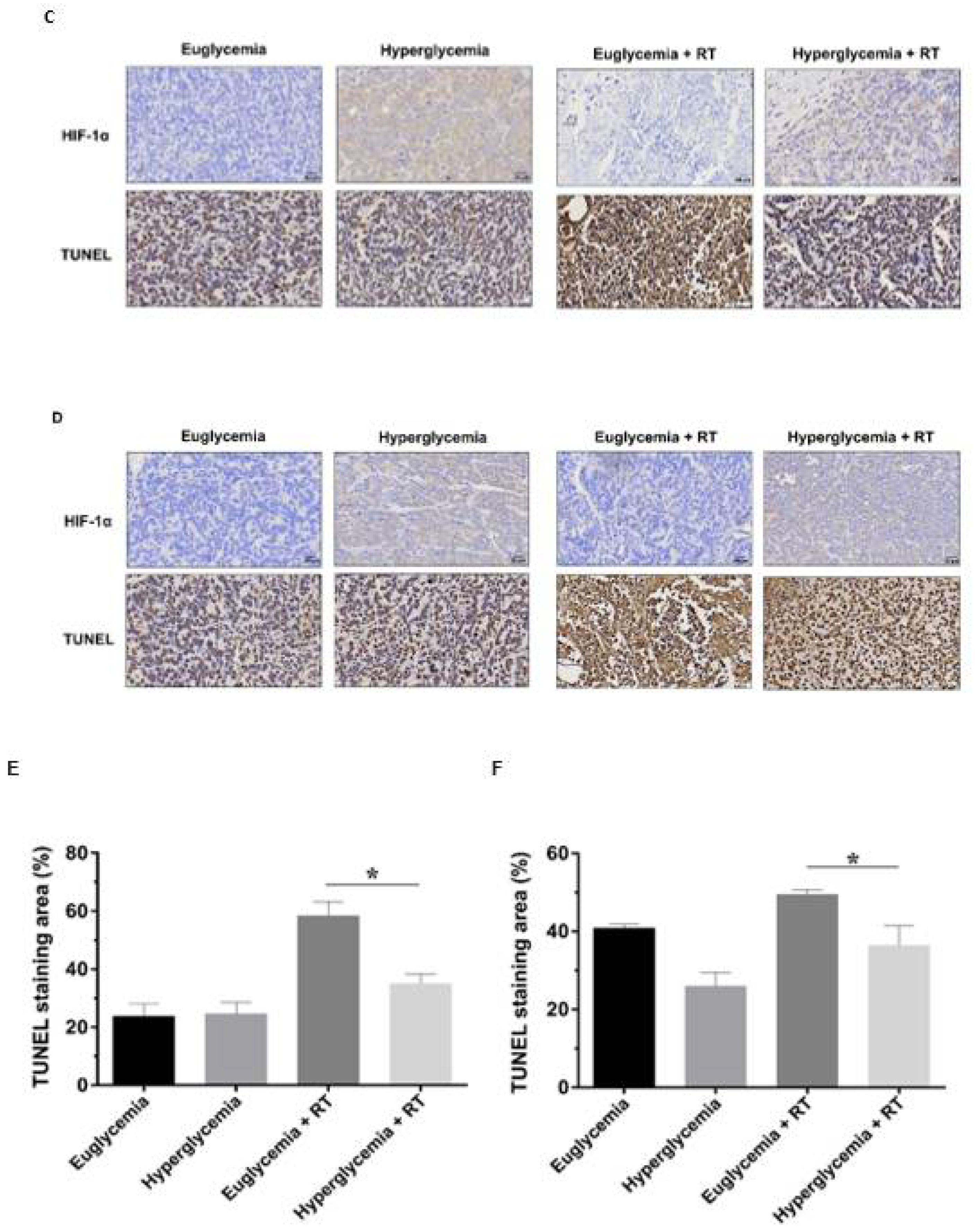
3.5. Hyperglycemia Promotes the Radioresistance of Rectal Cancer Cells by Overexpression of HIF-1α
3.6. LW6 Reduces the Radiation Tolerance of Rectal Cancer via Inhibiting the Expression of HIF-1α in a High Glucose Environment
3.7. LW6 Increases the Efficacy of Radiotherapy for Hyperglycemia Ectopic Colorectal Cancer by Inhibiting HIF-1α Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Ali Khan, U.; Fallah, M.; Sundquist, K.; Sundquist, J.; Brenner, H.; Kharazmi, E. Risk of colorectal cancer in patients with diabetes mellitus: A Swedish nationwide cohort study. PLoS Med. 2020, 17, e1003431. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with dis-ability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Whicher, C.A.; O’Neill, S.; Holt, R.I.G. Diabetes in the UK: 2019. Diabet. Med. 2020, 37, 242–247. [Google Scholar] [CrossRef]
- GBD 2017 Mortality Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes Mellitus and Risk of Colorectal Cancer: A Meta-Analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef]
- Lega, I.C.; Lipscombe, L.L. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr. Rev. 2020, 41, bnz014. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Chang, T.-K.; Su, W.-C.; Tsai, H.-L.; Wang, J.-Y. Narrative review of the influence of diabetes mellitus and hyperglycemia on colorectal cancer risk and oncological outcomes. Transl. Oncol. 2021, 14, 101089. [Google Scholar] [CrossRef]
- Brown, J.C.; Zhang, S.; Ou, F.-S.; Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; O’Neil, B.H.; E Shaw, J.; Polite, B.N.; et al. Diabetes and Clinical Outcome in Patients with Metastatic Colorectal Cancer: CALGB 80405 (Alliance). JNCI Cancer Spectr. 2019, 4, pkz078. [Google Scholar] [CrossRef]
- Tan, D.J.H.; Yaow, C.Y.L.; Mok, H.T.; Ng, C.H.; Tai, C.H.; Tham, H.Y.; Foo, F.J.; Chong, C.S. The influence of diabetes on postoperative complications following colorectal surgery. Tech. SColoproctol. 2021, 25, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Des Guetz, G.; Nicolas, P.; Perret, G.-Y.; Morere, J.-F.; Uzzan, B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur. J. Cancer 2010, 46, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.E.; Graham, L.A.; Morris, M.S.; Richman, J.S.; Hollis, R.H.; Wahl, T.S.; Copeland, L.A.; Burns, E.A.; Itani, K.M.F.; Hawn, M.T. Association between Preoperative Hemoglobin A1c Levels, Postoperative Hyperglycemia, and Readmissions Following Gastrointestinal Surgery. JAMA Surg. 2017, 152, 1031–1038. [Google Scholar] [CrossRef]
- Adamová, Z.; Bár, T. Influence of overweight and obesity on complications in colorectal surgery. Perspect. Surg. 2018, 97, 464–468. [Google Scholar]
- Ziegler, M.A.; Catto, J.A.; Riggs, T.W.; Gates, E.R.; Grodsky, M.B.; Wasvary, H.J. Risk factors for anastomotic leak and mortality in diabetic patients undergoing colectomy: Analysis from a statewide surgical quality collaborative. Arch. Surg. 2012, 147, 600–605. [Google Scholar] [CrossRef]
- Jackson, R.S.; Amdur, R.L.; White, J.C.; Macsata, R.A. Hyperglycemia Is Associated with Increased Risk of Morbidity and Mortality after Colectomy for Cancer. J. Am. Coll. Surg. 2012, 214, 68–80. [Google Scholar] [CrossRef]
- Mills, K.T.; Bellows, C.F.; Hoffman, A.E.; Kelly, T.N.; Gagliardi, G. Diabetes mellitus and colorectal cancer prognosis: A meta-analysis. Dis. Colon Rectum. 2013, 56, 1304–1319. [Google Scholar] [CrossRef]
- Yang, I.-P.; Miao, Z.-F.; Huang, C.-W.; Tsai, H.-L.; Yeh, Y.-S.; Su, W.-C.; Chang, T.-K.; Chang, S.-F.; Wang, J.-Y. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866964. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and Fluorouracil for Adjuvant Therapy of Resected Colon Carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef]
- Groehs, R.V.; Negrao, M.V.; Hajjar, L.A.; Jordão, C.P.; Carvalho, B.P.; Toschi-Dias, E.; Andrade, A.C.; Hodas, F.P.; Alves, M.J.; Sarmento, A.O.; et al. Adjuvant Treatment with 5-Fluorouracil and Oxaliplatin Does Not Influence Cardiac Function, Neurovascular Control, and Physical Capacity in Patients with Colon Cancer. Oncologist 2020, 25, e1956–e1967. [Google Scholar] [CrossRef]
- Pavelić, K.; Hrsak, I. Chemotherapy and immunotherapy of diabetic and non-diabetic mice bearing fibrosarcoma. Eur. J. Cancer (1965) 1980, 16, 1297–1301. [Google Scholar] [CrossRef]
- Andre, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; van Laethem, J.L.; et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; André, T.; Auclin, E. Refining adjuvant therapy for non-metastatic colon cancer, new standards and perspectives. Cancer Treat. Rev. 2019, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Salazar, M.C.; Rosen, J.E.; Wang, Z.; Arnold, B.N.; Thomas, D.C.; Herbst, R.S.; Kim, A.W.; Detterbeck, F.C.; Blasberg, J.D.; Boffa, D.J. Association of Delayed Adjuvant Chemotherapy With Survival After Lung Cancer Surgery. JAMA Oncol. 2017, 3, 610–619. [Google Scholar] [CrossRef]
- Gao, P.; Huang, X.-Z.; Song, Y.-X.; Sun, J.-X.; Chen, X.-W.; Sun, Y.; Jiang, Y.-M.; Wang, Z.-N. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: A population-based study. BMC Cancer 2018, 18, 234. [Google Scholar] [CrossRef]
- Turner, M.C.; Farrow, N.E.; Rhodin, K.E.; Sun, Z.; Adam, M.A.; Mantyh, C.R.; Migaly, J. Delay in Adjuvant Chemotherapy and Survival Advantage in Stage III Colon Cancer. J. Am. Coll. Surg. 2018, 226, 670–678. [Google Scholar] [CrossRef]
- Quasar Collaborative Group; Gray, R.; Barnwell, J.; McConkey, C.; Hills, R.K.; Williams, N.S.; Kerr, D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef]
- Huh, J.W.; Tanksley, J.; Chino, J.; Willett, C.G.; Dewhirst, M.W. Long-term Consequences of Pelvic Irradiation: Toxicities, Challenges, and Therapeutic Opportunities with Pharmacologic Mitigators. Clin. Cancer Res. 2020, 26, 3079–3090. [Google Scholar] [CrossRef]
- Francini, G.; Petrioli, R.; Lorenzini, L.; Mancini, S.; Armenio, S.; Tanzini, G.; Marsili, S.; Aquino, A.; Marzocca, G.; Civitelli, S.; et al. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology 1994, 106, 899–906. [Google Scholar] [CrossRef]
- André, T.; Quinaux, E.; Louvet, C.; Colin, P.; Gamelin, E.; Bouche, O.; Achille, E.; Piedbois, P.; Tubiana-Mathieu, N.; Boutan-Laroze, A.; et al. Phase III Study Comparing a Semimonthly With a Monthly Regimen of Fluorouracil and Leucovorin As Adjuvant Treatment for Stage II and III Colon Cancer Patients: Final Results of GERCOR C96.1. J. Clin. Oncol. 2007, 25, 3732–3738. [Google Scholar] [CrossRef] [PubMed]
- Twelves, C.; Wong, A.; Nowacki, M.P.; Abt, M.; Burris, H.; Carrato, A.; Cassidy, J.; Cervantes, A.; Fagerberg, J.; Georgoulias, V.; et al. Capecitabine as Adjuvant Treatment for Stage III Colon Cancer. N. Engl. J. Med. 2005, 352, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-S.; Chen, Y.-T.; Tsai, H.-L.; Huang, C.-W.; Ma, C.-J.; Su, W.-C.; Huang, C.-M.; Huang, M.-Y.; Hu, H.-M.; Lu, C.-Y.; et al. Predictive Value of ERCC1, ERCC2, and XRCC Expression for Patients with Locally Advanced or Metastatic Gastric Cancer Treated with Neoadjuvant mFOLFOX-4 Chemotherapy. Pathol. Oncol. Res. 2019, 26, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-S.; Lee, D.-H.; Kim, S.-H.; Lee, C.-H.; Shin, H.M.; Kim, H.-R.; Cho, C.-H. Hyperglycemia-induced oxidative stress promotes tumor metastasis by upregulating vWF expression in endothelial cells through the transcription factor GATA1. Oncogene 2022, 41, 1634–1646. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Sang, H.; Zhou, Y.; Shang, C.; Wang, Y.; Zhu, H. Effects of hyperglycemia on the progression of tumor diseases. J. Exp. Clin. Cancer Res. 2019, 38, 32. [Google Scholar] [CrossRef]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, L.; Zhong, T. The role of HIF-1alpha in chemo-/radioresistant tumors. Onco Targets Ther. 2018, 11, 3003–3011. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Shang, Y.; Kerndl, H.; Kumstel, S.; Gong, P.; Vollmar, B.; Zechner, D. Metformin and LW6 impairs pancreatic cancer cells and reduces nuclear localization of YAP1. J. Cancer 2020, 11, 479–487. [Google Scholar] [CrossRef]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and its Potential for Therapeutic Intervention in Malignancy and Ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Borsi, E.; Perrone, G.; Terragna, C.; Martello, M.; Dico, A.F.; Solaini, G.; Baracca, A.; Sgarbi, G.; Pasquinelli, G.; Valente, S.; et al. Hypoxia inducible factor-1 alpha as a therapeutic target in multiple myeloma. Oncotarget 2014, 5, 1779–1792. [Google Scholar] [CrossRef]
- Ferrer, C.M.; Lynch, T.P.; Sodi, V.L.; Falcone, J.N.; Schwab, L.P.; Peacock, D.L.; Vocadlo, D.J.; Seagroves, T.N.; Reginato, M.J. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol. Cell. 2014, 54, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Isoe, T.; Makino, Y.; Mizumoto, K.; Sakagami, H.; Fujita, Y.; Honjo, J.; Takiyama, Y.; Itoh, H.; Haneda, M. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbo-hydrate response element binding protein. Kidney Int. 2010, 78, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Chang, P.H.; Wang, C.H. Impact of Diabetes Mellitus on Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Sci. Rep. 2020, 10, 7702. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Tang, H.; Hu, X.; Hubert, S.M.; Li, Q.; Su, D.; Xu, H.; Fan, Y.; Yu, X.; et al. Activation of PI3K/AKT Pathway Is a Potential Mechanism of Treatment Resistance in Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 526–539. [Google Scholar] [CrossRef]
- Aas, T.; Geisler, S.; Eide, G.; Haugen, D.; Varhaug, J.; Bassøe, A.; Thorsen, T.; Berntsen, H.; Børresen-Dale, A.-L.; Akslen, L.; et al. Predictive value of tumour cell proliferation in locally advanced breast cancer treated with neoadjuvant chemotherapy. Eur. J. Cancer 2003, 39, 438–446. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.S.; Segersvärd, R.; Strömmer, L.; Sundqvist, K.-G.; Holgersson, J.; Permert, J. Hypoxia Inducible Factor-1 Mediates Effects of Insulin on Pancreatic Cancer Cells and Disturbs Host Energy Homeostasis. Am. J. Pathol. 2007, 170, 469–477. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Jia, X.; Duan, Y.; Xiao, H.; Sundqvist, K.-G.; Permert, J.; Wang, F. Excess glucose induces hypoxia-inducible factor-1alpha in pancreatic cancer cells and stimulates glucose metabolism and cell migration. Cancer Biol. Ther. 2013, 14, 428–435. [Google Scholar] [CrossRef][Green Version]
- Menini, S.; Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G. Diabetes and Pancreatic Cancer—A Dangerous Liaison Relying on Carbonyl Stress. Cancers 2021, 13, 313. [Google Scholar] [CrossRef]
- Min, J.; Zeng, T.; Roux, M.; Lazar, D.; Chen, L.; Tudzarova, S. The Role of HIF1α-PFKFB3 Pathway in Diabetic Retinopathy. J. Clin. Endocrinol. Metab. 2021, 106, 2505–2519. [Google Scholar] [CrossRef]
- van Baardwijk, A.; Dooms, C.; van Suylen, R.J.; Verbeken, E.; Hochstenbag, M.; Dehing-Oberije, C.; Rupa, D.; Pastorekova, S.; Stroobants, S.; Bue, U.; et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur. J. Cancer 2007, 43, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Pradilla, G.; Legnani, F.G.; Petrangolini, G.; Francescato, P.; Chillemi, F.; Tyler, B.M.; Gaini, S.M.; Brem, H.; Olivi, A.; DiMeco, F. Local Delivery of a Synthetic Endostatin Fragment for the Treatment of Experimental Gliomas. Neurosurgery 2005, 57, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef] [PubMed]
- Coltella, N.; Valsecchi, R.; Ponente, M.; Ponzoni, M.; Bernardi, R. Synergistic Leukemia Eradication by Combined Treatment with Retinoic Acid and HIF Inhibition by EZN-2208 (PEG-SN38) in Preclinical Models of PML-RARalpha and PLZF-RARalpha-Driven Leukemia. Clin. Cancer Res. 2015, 21, 3685–3694. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a Risk Factor for Cancer Progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef]
- Wu, C.-H.; Wu, T.-Y.; Li, C.-C.; Lui, M.-T.; Chang, K.-W.; Kao, S.-Y. Impact of Diabetes Mellitus on the Prognosis of Patients with Oral Squamous Cell Carcinoma: A Retrospective Cohort Study. Ann. Surg. Oncol. 2010, 17, 2175–2183. [Google Scholar] [CrossRef]
- Lee, K.; Kang, J.E.; Parke, S.-K.; Jin, Y.; Chung, K.-S.; Kim, H.-M.; Lee, K.; Kang, M.R.; Lee, M.K.; Song, K.B.; et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 2010, 80, 982–989. [Google Scholar] [CrossRef]




| Characteristics | HbA1C ≤ 6.5% | HbA1C > 6.5% | p | ||||
|---|---|---|---|---|---|---|---|
| Total | N | % | n = 41 | (%) | n = 13 | (%) | |
| Age (year) | 0.534 | ||||||
| <65 | 37 | 68.5 | 29 | 70.7 | 8 | 61.5 | |
| ≥65 | 17 | 31.5 | 12 | 29.3 | 5 | 38.5 | |
| Gender | 0.822 | ||||||
| Male | 36 | 66.7 | 27 | 65.9 | 9 | 69.2 | |
| Female | 18 | 33.3 | 14 | 34.1 | 4 | 30.8 | |
| Clinical tumor depth (cT) | 0.653 | ||||||
| T3 | 48 | 88.9 | 36 | 87.8 | 12 | 92.3 | |
| T4 | 6 | 11.1 | 5 | 12.2 | 1 | 7.7 | |
| Clinical lymph node metastasis (cN) | 0.536 | ||||||
| N0 | 12 | 22.2 | 10 | 24.4 | 2 | 15.4 | |
| N1 | 26 | 48.1 | 18 | 43.9 | 8 | 61.5 | |
| N2 | 16 | 29.6 | 13 | 31.7 | 3 | 23.1 | |
| Clinical tumor disease Stage | 0.496 | ||||||
| II | 12 | 22.2 | 10 | 24.4 | 2 | 15.4 | |
| III | 42 | 77.8 | 31 | 75.6 | 11 | 84.6 | |
| Pathologic tumor depth (ypT) | |||||||
| ypT0 | 16 | 29.6 | 10 | 24.4 | 6 | 46.2 | 0.338 |
| ypT1 | 6 | 11.1 | 5 | 12.2 | 1 | 7.7 | |
| ypT2 | 17 | 31.5 | 12 | 29.3 | 5 | 38.4 | |
| ypT3 | 15 | 27.8 | 14 | 34.1 | 1 | 7.7 | |
| Pathologic lymph node metastasis (ypTN) | 0.457 | ||||||
| ypN0 | 44 | 81.5 | 32 | 78 | 12 | 92.3 | |
| ypN1 | 7 | 13.0 | 6 | 14.6 | 1 | 7.7 | |
| ypN2 | 3 | 5.5 | 3 | 7.3 | 0 | 0 | |
| Pathologic tumor disease stage | 0.012 * | ||||||
| pCR | 15 | 27.8 | 14 | 34.1 | 1 | 7.7 | |
| I | 20 | 37.0 | 11 | 26.8 | 9 | 69.2 | |
| II | 9 | 16.7 | 6 | 14.6 | 3 | 23 | |
| III | 10 | 18.5 | 10 | 24.4 | 0 | 0 | |
| Lymphovascular invasion | 0.148 | ||||||
| Yes | 12 | 22.2 | 11 | 26.8 | 1 | 7.7 | |
| No | 42 | 77.8 | 30 | 73.2 | 12 | 92.3 | |
| Perineural invasion | 0.242 | ||||||
| Yes | 4 | 7.4 | 4 | 9.8 | 0 | 0 | |
| No | 50 | 92.6 | 37 | 90.2 | 13 | 100 | |
| Tumor regression score | 0.017 * | ||||||
| 0 (CR) | 16 | 29.6 | 15 | 36.6 | 1 | 7.7 | |
| 1 | 25 | 46.3 | 14 | 34.1 | 11 | 84.6 | |
| 2 | 10 | 18.5 | 9 | 22 | 1 | 7.7 | |
| 3 | 3 | 5.6 | 3 | 7.3 | 0 | 0 | |
| Pathologic complete response | 0.047 * | ||||||
| Yes | 16 | 29.6 | 15 | 36.6 | 1 | 7.7 | |
| No | 38 | 70.4 | 26 | 63.4 | 12 | 92.3 | |
| Characteristics | HIF1-α Expression | p | |||||
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| Total | N | % | 37 | 68.5 | 17 | 31.5 | 0.298 |
| Age (Year) | |||||||
| <65 | 37 | 68.5 | 27 | 73 | 10 | 58.8 | |
| ≥65 | 17 | 31.5 | 10 | 27 | 7 | 41.2 | |
| Gender | 0.407 | ||||||
| Male | 36 | 66.7 | 26 | 70 | 10 | 41.2 | |
| Female | 18 | 33.3 | 11 | 30 | 7 | 58.8 | |
| Clinical Tumor Depth (cT) | 0.078 | ||||||
| T3 | 48 | 88.9 | 31 | 83.8 | 17 | 100 | |
| T4 | 6 | 11.1 | 6 | 16.2 | 0 | 0 | |
| Clinical Lymph Node Metastasis (cN) | |||||||
| N0 | 12 | 22.2 | 10 | 27 | 2 | 11.8 | 0.081 |
| N1 | 26 | 48.1 | 14 | 37.8 | 12 | 70.6 | |
| N2 | 16 | 29.6 | 13 | 35.1 | 3 | 17.6 | |
| Clinical Tumor Disease Stage | 0.21 | ||||||
| II | 12 | 22.2 | 10 | 27 | 2 | 11.8 | |
| III | 42 | 77.8 | 27 | 73 | 15 | 88.2 | |
| Pathologic Tumor Depth (ypT) | 0.692 | ||||||
| ypT0 | 16 | 29.6 | 12 | 32.4 | 4 | 23.5 | |
| ypT1 | 6 | 11.1 | 5 | 13.5 | 1 | 5.9 | |
| ypT2 | 17 | 31.5 | 10 | 27 | 7 | 58.8 | |
| ypT3 | 15 | 27.8 | 10 | 27 | 5 | 29.4 | |
| Pathologic Lymph Node Metastasis (ypTN) | 0.778 | ||||||
| ypN0 | 44 | 81.5 | 31 | 83.8 | 13 | 76.5 | |
| ypN1 | 7 | 13.0 | 4 | 8.1 | 3 | 17.6 | |
| ypN2 | 3 | 5.5 | 2 | 8.1 | 1 | 5.9 | |
| Pathologic Tumor Disease Stage | |||||||
| pCR | 15 | 27.8 | 14 | 37.8 | 1 | 5.9 | 0.083 |
| I | 20 | 37.0 | 13 | 35.1 | 7 | 41.2 | |
| II | 9 | 16.7 | 5 | 13.5 | 4 | 23.5 | |
| III | 10 | 18.5 | 5 | 13.5 | 5 | 29.4 | |
| Lymphovascular Invasion | 0.009 * | ||||||
| Yes | 12 | 22.2 | 0 | 0 | 3 | 17.6 | |
| No | 42 | 77.8 | 37 | 100 | 14 | 82.4 | |
| Perineural Invasion | 0.002 * | ||||||
| Yes | 4 | 7.4 | 0 | 0 | 4 | 23.5 | |
| No | 50 | 92.6 | 37 | 100 | 13 | 76.5 | |
| Tumor Regression Score | <0.001 * | ||||||
| 0 (CR) | 16 | 29.6 | 15 | 40.5 | 1 | 5.9 | |
| 1 | 25 | 46.3 | 19 | 51.4 | 6 | 35.3 | |
| 2 | 10 | 18.5 | 3 | 8.1 | 7 | 41.2 | |
| 3 | 3 | 5.6 | 0 | 0 | 3 | 17.6 | |
| Pathologic Complete Response | 0.01 * | ||||||
| Yes | 16 | 29.6 | 15 | 40.5 | 1 | 5.9 | |
| No | 38 | 70.4 | 22 | 59.5 | 16 | 94.1 | |
| HbA1c | 0.046 * | ||||||
| ≤6.4 | 37 | 68.5 | 31 | 83.8 | 10 | 58.8 | |
| >6.5 | 17 | 31.5 | 6 | 16.2 | 7 | 41.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-J.; Chen, Y.-T.; Huang, C.-M.; Kuo, S.-H.; Liao, Y.-Y.; Jhang, W.-Y.; Wang, S.-H.; Ke, C.-C.; Huang, Y.-H.; Cheng, C.-M.; et al. HIF-1α Expression Increases Preoperative Concurrent Chemoradiotherapy Resistance in Hyperglycemic Rectal Cancer. Cancers 2022, 14, 4053. https://doi.org/10.3390/cancers14164053
Huang Y-J, Chen Y-T, Huang C-M, Kuo S-H, Liao Y-Y, Jhang W-Y, Wang S-H, Ke C-C, Huang Y-H, Cheng C-M, et al. HIF-1α Expression Increases Preoperative Concurrent Chemoradiotherapy Resistance in Hyperglycemic Rectal Cancer. Cancers. 2022; 14(16):4053. https://doi.org/10.3390/cancers14164053
Chicago/Turabian StyleHuang, Yi-Jung, Yi-Ting Chen, Chun-Ming Huang, Shih-Hsun Kuo, Yan-You Liao, Wun-Ya Jhang, Shuo-Hung Wang, Chien-Chih Ke, Yu-Hsiang Huang, Chiu-Min Cheng, and et al. 2022. "HIF-1α Expression Increases Preoperative Concurrent Chemoradiotherapy Resistance in Hyperglycemic Rectal Cancer" Cancers 14, no. 16: 4053. https://doi.org/10.3390/cancers14164053
APA StyleHuang, Y.-J., Chen, Y.-T., Huang, C.-M., Kuo, S.-H., Liao, Y.-Y., Jhang, W.-Y., Wang, S.-H., Ke, C.-C., Huang, Y.-H., Cheng, C.-M., Huang, M.-Y., & Chuang, C.-H. (2022). HIF-1α Expression Increases Preoperative Concurrent Chemoradiotherapy Resistance in Hyperglycemic Rectal Cancer. Cancers, 14(16), 4053. https://doi.org/10.3390/cancers14164053






