Suprarenal Masses in Very Young Infants: Is It Safe to Watch and Wait? Report of a SIOPEN Observational Study Results
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MYCN Plasma Analysis
2.3. Management
2.4. Statistical Analyses
3. Results
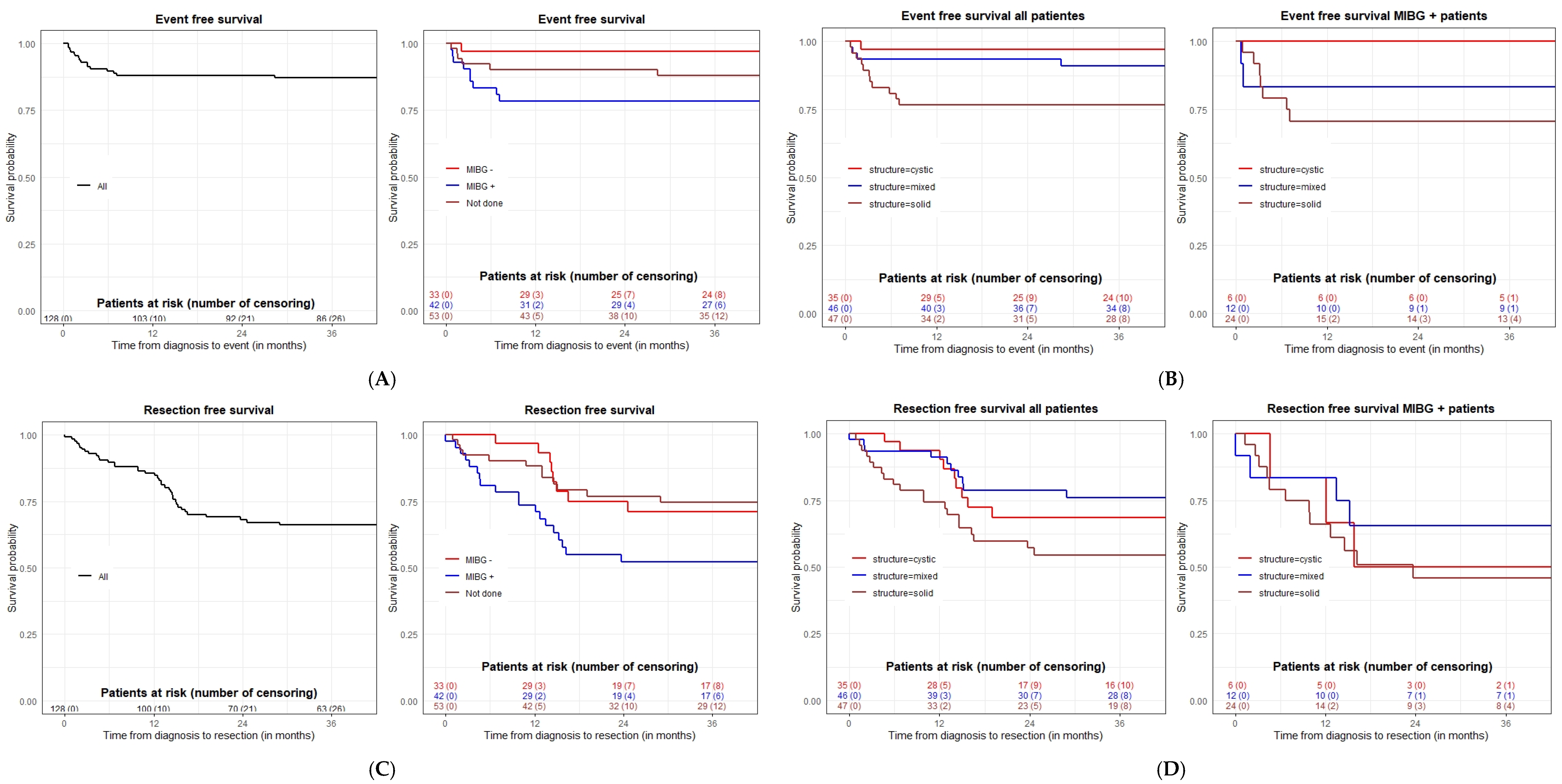
3.1. Events
3.2. Surgery
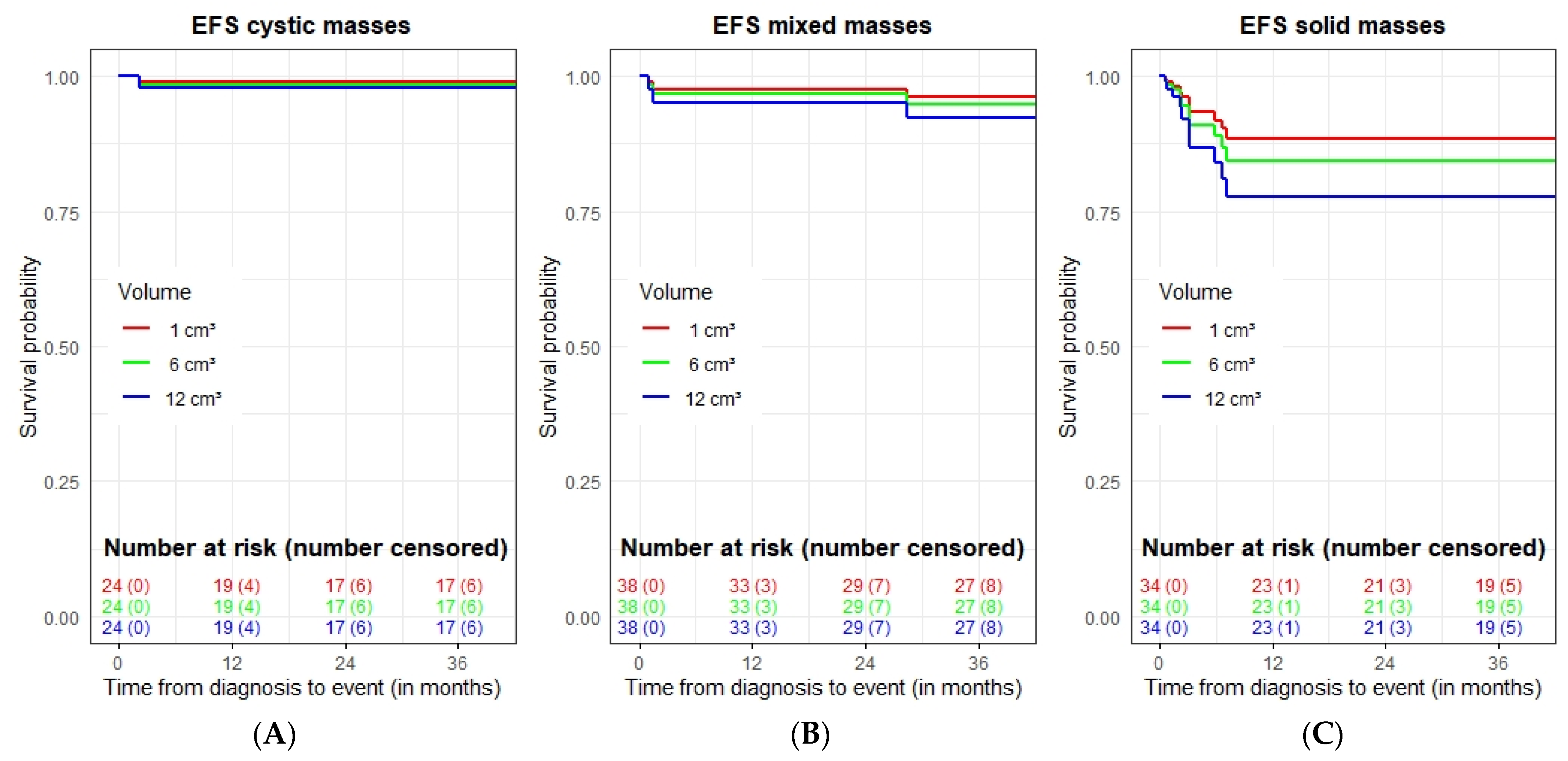
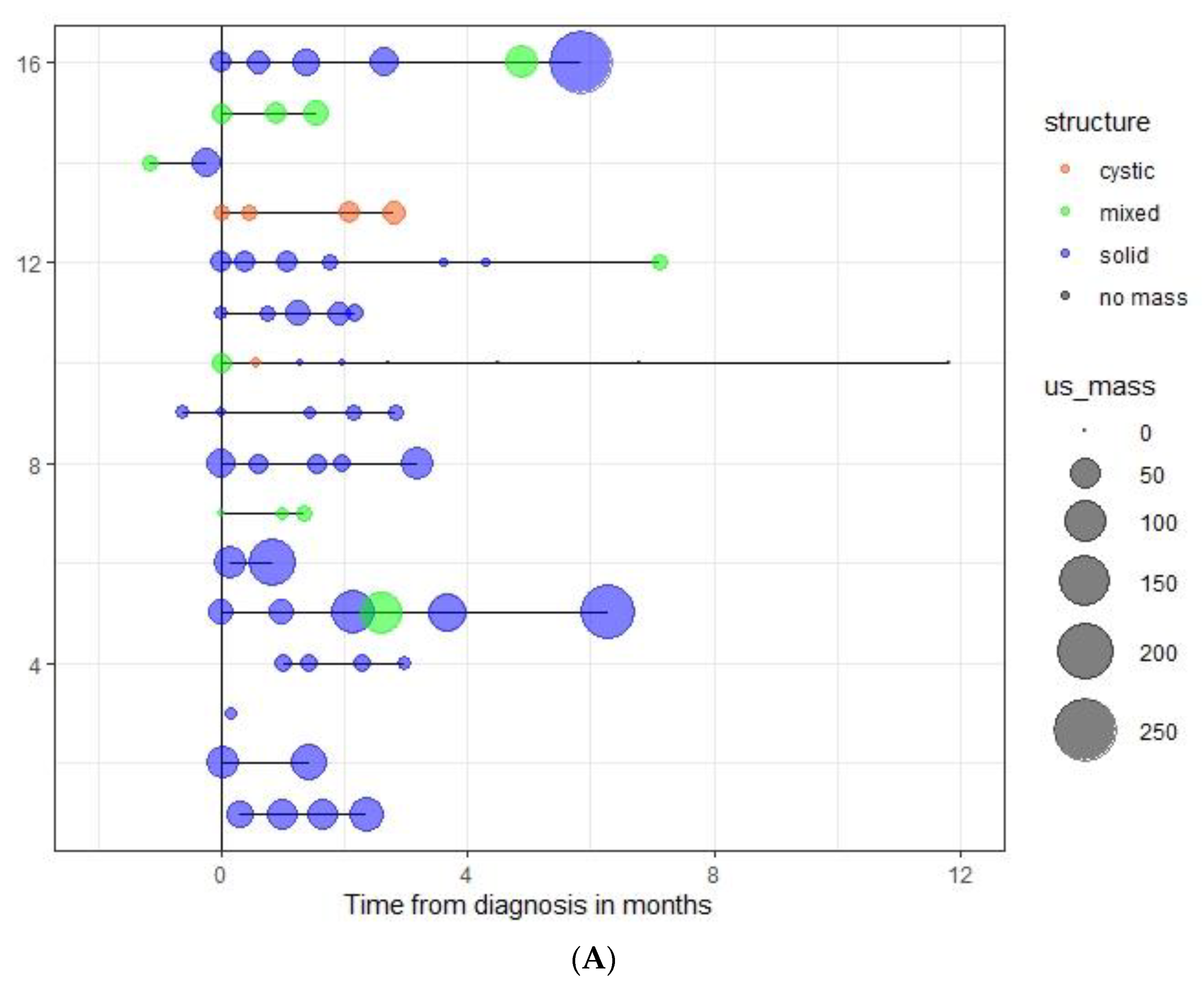
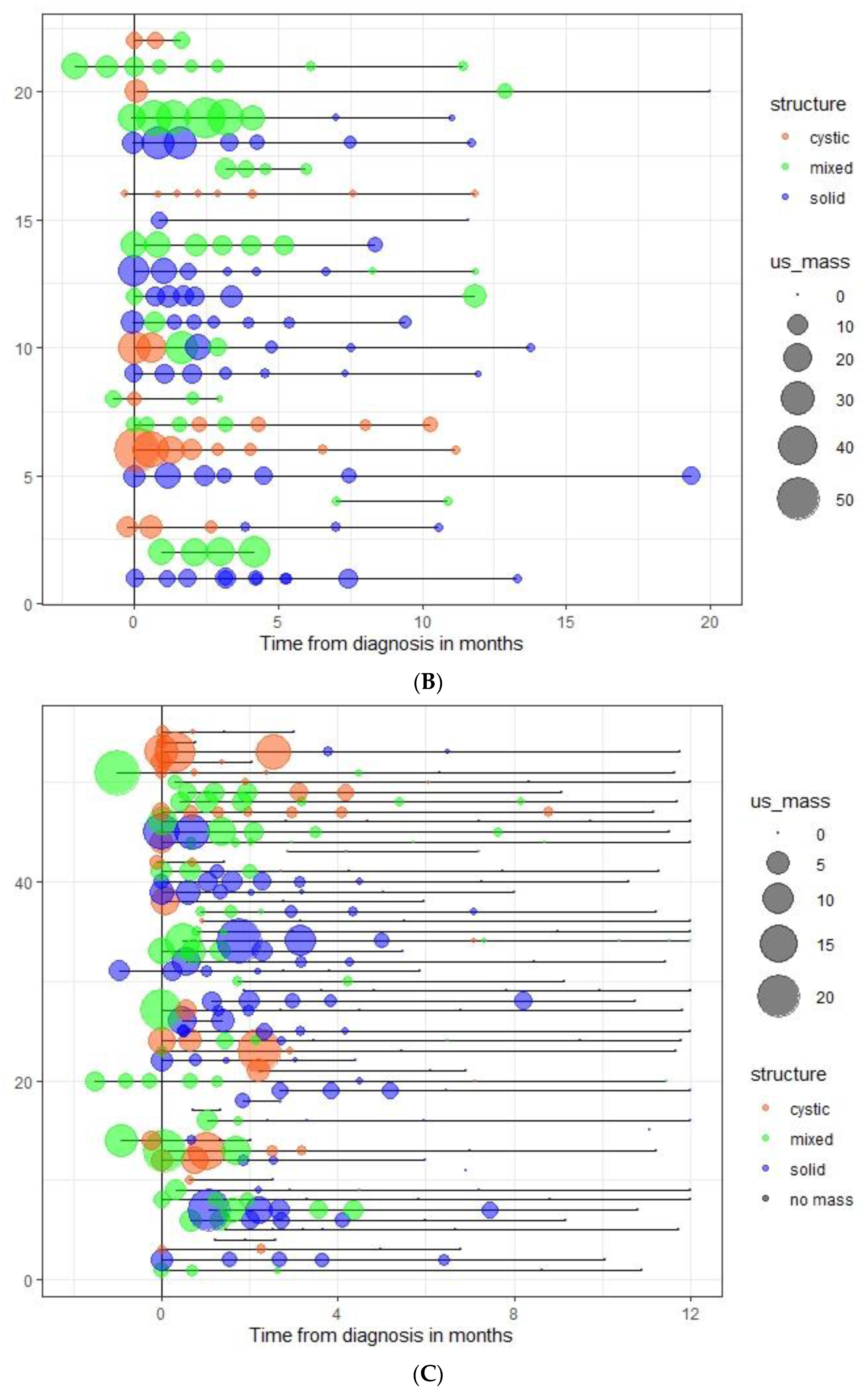
3.3. Spontaneous Evolution of sSRM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holgersen, L.O.; Subramanian, S.; Kirpekar, M.; Mootabar, H.; Marcus, J.R. Spontaneous resolution of antenatally diagnosed adrenal masses. J. Pediatr. Surg. 1996, 31, 153–155. [Google Scholar] [CrossRef]
- Kerbl, R.; Urban, C.E.; Lackner, H.; Höfler, G.; Ambros, I.M.; Ratschek, M.; Ambros, P.F. Connatal localized neuroblastoma: The case to delay treatment. Cancer 1996, 77, 1395–1401. [Google Scholar] [CrossRef]
- Kerbl, R.; Urban, C.E.; Ambros, I.M.; Dornbusch, H.J.; Schwinger, W.; Lackner, H.; Ladenstein, R.; Strenger, V.; Gadner, H.; Ambros, P.F. Neuroblastoma mass screening in late infancy: Insights into the biology of neuro-blastic tumors. J. Clin. Oncol. 2003, 21, 4228–4234. [Google Scholar] [CrossRef] [PubMed]
- Nadler, E.P.; Barksdale, E.M. Adrenal Masses in the Newborn. Semin. Pediatr. Surg. 2000, 9, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sauvat, F.; Sarnacki, S.; Brisse, H.; Medioni, J.; Rubie, H.; Aigrain, Y.; Gauthier, F.; Audry, G.; Helardot, P.; Landais, P.; et al. Outcome of suprarenal localized masses diagnosed during the perinatal period: A retrospective multicenter study. Cancer 2002, 94, 2474–2480. [Google Scholar] [CrossRef]
- Plantaz, D.; Feycon, C. Neonatal Neuroblastoma. In Neuroblastoma: Clinical and Surgical Management; Sarnacki, S., Pio, L., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 191–203. [Google Scholar]
- Schmidt, M.L.; Lal, A.; Seeger, R.C.; Maris, J.M.; Shimada, H.; O’Leary, M.; Gerbing, R.B.; Matthay, K.K. Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified MYCN neuroblastoma: A Children’s Cancer Group Study. J. Clin. Oncol. 2005, 23, 6474–6480. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- De Bernardi, B.; Conte, M.; Mancini, A.; Donfrancesco, A.; Alvisi, P.; Toma, P.; Casale, F.; Cordero Di Montezemolo, L.; Cornelli, P.E.; Carli, M. Localized resectable neuroblastoma: Results of the second study of the Italian Cooperative Group for Neuroblastoma. J. Clin. Oncol. 1995, 13, 884–893. [Google Scholar] [CrossRef]
- Evans, A.E.; Silber, J.H.; Shpilsky, A.; D’Angio, G.J. Successful management of low-stage neuroblastoma without adjuvant thera-pies: A comparison of two decades, 1972 through 1981 and 1982 through 1992, in a single institution. J. Clin. Oncol. 1996, 14, 2504–2510. [Google Scholar] [CrossRef]
- Nishihira, H.; Toyoda, Y.; Tanaka, Y.; Ijiri, R.; Aida, N.; Takeuchi, M.; Ohnuma, K.; Kigasawa, H.; Kato, K.; Nishi, T. Natural course of neuroblastoma detected by mass screening: S 5-year prospective study at a single institution. J. Clin. Oncol. 2000, 18, 3012–3017. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hanada, R.; Kikuchi, A.; Ichikawa, M.; Aihara, T.; Oguma, E.; Moritani, T.; Shimanuki, Y.; Tanimura, M.; Hayashi, Y. Spontaneous regression of localized neuroblastoma detected by mass screening. J. Clin. Oncol. 1998, 16, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Oue, T.; Imura, K.; Inoue, M.; Yagi, K.; Kawa, K.; Nishikawa, M.; Morimoto, S.; Nakayama, M. Observation of untreated patients with neuroblastoma detected by mass screening: A “wait and see” pilot study. Med. Pediatr. Oncol. 2001, 36, 160–162. [Google Scholar] [CrossRef]
- Oue, T.; Inoue, M.; Yoneda, A.; Kubota, A.; Okuyama, H.; Kawahara, H.; Nishikawa, M.; Nakayama, M.; Kawa, K. Profile of neuroblastoma detected by mass screening, resected after observation without treatment: Results of the Wait and See pilot study. J. Pediatr. Surg. 2005, 40, 359–363. [Google Scholar] [CrossRef]
- Fritsch, P.; Kerbl, R.; Lackner, H.; Urban, C. “Wait and see” strategy in localized neuroblastoma in infants: An option not only for cases detected by mass screening. Pediatr. Blood Cancer 2004, 43, 679–682. [Google Scholar] [CrossRef]
- De Bernardi, B.; Mosseri, V.; Rubie, H.; Castel, V.; Foot, A.; Ladenstein, R.; Laureys, G.; Beck-Popovic, M.; De Lacerda, A.F.; Pearson, A.D.J.; et al. Treatment of localized resectable neuroblastoma. Results of the LNESG1 study by the SIOP Europe Neuroblastoma Group. Br. J. Cancer 2008, 99, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Hero, B.; Simon, T.; Spitz, R.; Ernestus, K.; Gnekow, A.K.; Scheel-Walter, H.G.; Schwabe, D.; Schilling, F.H.; Benz-Bohm, G.; Berthold, F. Localized infant neuroblastomas often show spontaneous regression: Results of the prospective trials NB95-S and NB97. J. Clin. Oncol. 2008, 26, 1504–1510. [Google Scholar] [CrossRef]
- Nuchtern, J.G.; London, W.B.; Barnewolt, C.E.; Naranjo, A.; McGrady, P.W.; Geiger, J.D.; Diller, L.; Schmidt, M.L.; Maris, J.M.; Cohn, S.L.; et al. A prospective study of expectant observation as primary therapy for neuro-blastoma in young infants: A Children’s Oncology Group study. Ann. Surg. 2012, 256, 573–580. [Google Scholar] [CrossRef]
- Combaret, V.; Audoynaud, C.; Iacono, I.; Favrot, M.-C.; Schell, M.; Bergeron, C.; Puisieux, A. Circulating MYCN DNA as a tumor-specific marker in neuroblastoma patients. Cancer Res. 2002, 62, 3646–3648. [Google Scholar]
- Combaret, V.; Bergeron, C.; Noguera, R.; Iacono, I.; Puisieux, A. Circulating MYCN DNA Predicts MYCN-Amplification in Neuroblastoma. J. Clin. Oncol. 2005, 23, 8919–8920. [Google Scholar] [CrossRef]
- Combaret, V.; Hogarty, M.D.; London, W.B.; McGrady, P.; Iacono, I.; Brejon, S.; Swerts, K.; Noguera, R.; Gross, N.; Rousseau, R. Influence of neuroblastoma stage on serum-based detection of MYCN ampli-fication. Pediatr. Blood Cancer 2009, 53, 329–331. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Seeger, R.C.; Barrett, A.; Berthold, F.; Castleberry, R.P.; D’Angio, G.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Freeman, A.I. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J. Clin. Oncol. 1988, 6, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Interiano, R.B.; Davidoff, A.M. Current Management of Neonatal Neuroblastoma. Curr. Pediatr. Rev. 2015, 11, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chicard, M.; Boyault, S.; Colmet Daage, L.; Richer, W.; Gentien, D.; Pierron, G.; Lapouble, E.; Bellini, A.; Clement, N.; Iacono, I.; et al. Genomic Copy Number Profiling Using Circulating Free Tumor DNA High-lights Heterogeneity in Neuroblastoma. Clin. Cancer Res. 2006, 22, 5564–5573. [Google Scholar] [CrossRef] [PubMed]
- Andropoulos, D.B.; Greene, M.F. Anesthesia and Developing Brains—Implications of the FDA Warning. N. Engl. J. Med. 2017, 376, 905–907. [Google Scholar] [CrossRef]
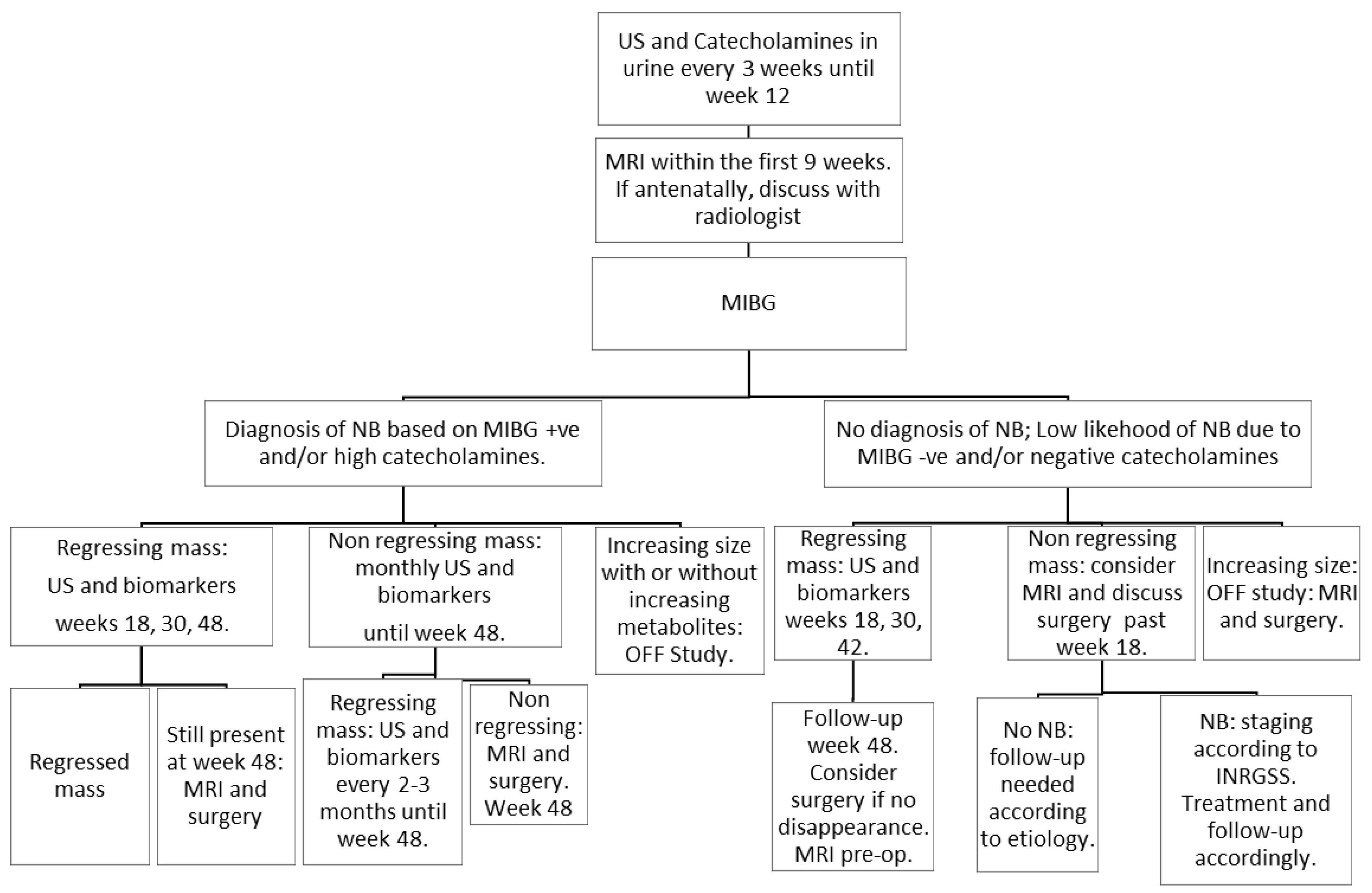
| Follow-Up (Weeks) | 0 | 3 | 6 | 9 | 12 | 18 | 30 | 48 |
|---|---|---|---|---|---|---|---|---|
| US | + | + | + | + | + | + | + | + |
| Catecholamines | + | + | + | + | + | + | + | + |
| MRI |  | + | + | |||||
| MIBG |  | |||||||
| Number of Patients | % of Total | |
|---|---|---|
| Total | 128 | |
| Males | 78 | 60.9 |
| Females | 50 | 39.1 |
| Median age at diagnosis (days) | 3 | |
| Range | 0–87 | |
| Time of detection | ||
| Prenatal | 54 | 42.2 |
| Postnatal | 74 | 57.8 |
| Location of mass by MRI (N = 82) | ||
| Suprarenal | 75 | 91.5 |
| Abdominal non-suprarenal | 2 | 2.4 |
| Regressed * | 5 | 6.1 |
| Structure of mass by MRI (N = 77) | ||
| Cystic | 22 | 28.6 |
| Solid | 28 | 36.4 |
| Mixed | 27 | 35 |
| Structure of mass by US (N = 128) | ||
| Cystic | 35 | 27.3 |
| Solid | 47 | 36.7 |
| Mixed | 46 | 35.9 |
| Vascularization of mass by US (N = 128) | ||
| Present | 35 | 37.3 |
| Absent | 55 | 43 |
| Not evaluable | 36 | 28.1 |
| Not evaluated | 2 | 1.6 |
| Patient Nº | Sex | Time to Event | Age at Diagnosis | Type of Event | Time to Surgery | Surgery | Final Diagnosis | MIGB at Diagnosis | MYCN * | Mass Structure at Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| A | F | 22 | 0 | MS | 28 | Complete excision | NB | ND | ND | Solid |
| B | F | 26 | 2 | Size increase | 40 | Complete excision | NB | Positive | Negative | Solid |
| C | F | 30 | 1 | Size increase | 62 | Complete excision | NB | Positive | ND | Mixed |
| D | M | 43 | 18 | Size increase | 50 | Complete excision | NB | Positive | Negative | Mixed |
| E | F | 47 | 0 | Size increase | 51 | Complete excision | NB | ND | ND | Solid |
| F | M | 47 | 39 | MS | 60 | Biopsy only > 50% residual tumor | NB | ND | ND | Mixed |
| G | F | 64 | 0 | Size increase | 205 | Complete excision | Bronchogenic cyst | Negative | Negative | Cystic |
| H | M | 66 | 3 | MS | 72 | Complete excision | NB | ND | ND | Solid |
| I | F | 72 | 72 | Size increase | 83 | Complete excision | NB | Positive | Negative | Solid |
| J | F | 97 | 44 | Size increase | 131 | Complete excision | NB | Positive | Negative | Solid |
| K | F | 98 | 79 | Size increase | 98 | Excision with minimal residual tumor (<5% or <5 mL) | NB | Positive | Negative | Solid |
| L | F | 109 | 0 | MS | 140 | Complete excision | NB | Positive | ND | Solid |
| M | F | 178 | 30 | MS | 179 | Biopsy only > 50% residual tumor | NB | ND | ND | Solid |
| N | M | 205 | 29 | Size increase | 205 | Complete excision | NB | Positive | Negative | Solid |
| O | M | 217 | 36 | Size increase | 302 | Complete excision | NB | Positive | Negative | Solid |
| P | F | 865 | 60 | Size increase | 882 | Complete excision | NB | ND | Negative | Mixed |
| Patient Nº | Months between Diagnosis and Surgery | MIGB at Diagnosis (Y (+, −, ND) | Urine Catecholamines and LDH at Diagnosis | Structure of the Mass at Diagnosis | Surgery Outcome | Final Diagnosis | Indication for Surgery |
|---|---|---|---|---|---|---|---|
| 1 | 0.9 | ND | ND | Solid | CR | NB | MS |
| 2 | 1.3 | (+) | LDH/VMA/HVA | Solid | CR | NB | size increased |
| 3 | 1.7 | ND | LDH/HVA/VMA | Solid | CR | NB | size increased |
| 4 | 1.7 | (+) | LDH/VMA/HVA/DOPA/COR | Mixed | CR | NB | size increased |
| 5 | 2.0 | ND | LDH/VMA/HVA | Mixed | Biopsy * | NB | MS |
| 6 | 2.1 | (+) | LDH/VMA/HVA | Mixed | CR | NB | size increased |
| 7 | 2.4 | ND | LDH/VMA | Solid | CR | NB | MS |
| 8 | 2.8 | (+) | LDH/HVA/VMA | Solid | CR | NB | size increased |
| 9 | 3.3 | (+) | LDH/VMA/HVA | Solid | Excision with residue ** | NB | size increased |
| 10 | 4.4 | (+) | VMA/HVA | Solid | CR | NB | size increased |
| 11 | 4.7 | (+) | VMA/HVA/DOPA | Solid | CR | NB | MS |
| 12 | 4.7 | (+) | LDH/VMA/HVA/DOPA | Cystic | CR | NB | size increased <40% |
| 13 | 6.0 | ND | LDH/VMA/HVA | Solid | Biopsy * | NB | MS |
| 14 | 6.8 | (−) | LDH/VMA/HVA/DOPA/COR | Cystic | CR | bronchogenis cyst | size increased |
| 15 | 6.8 | (+) | LDH/HVA/VMA | Solid | CR | NB | size increased |
| 16 | 10.0 | (+) | LDH/VMA/HVA | Solid | CR | Subdiaphragmatic extralobar pulmonary sequestration | persisting mass |
| 17 | 10.1 | (+) | LDH/VMA/HVA/DOPA/COR | Solid | CR | NB | size increased |
| 18 | 11.0 | ND | LDH/VMA/HVA | Mixed | CR | NB | persisting mass |
| 19 | 12.3 | (+) | VMA/HVA | Cystic | CR | NB | persisting mass |
| 20 | 12.7 | (−) | LDH/DOPA | Cystic | CR | NB | persisting mass |
| 21 | 12.9 | (+) | LDH | Solid | CR | suprarenal gland with nodular area composed of fibromyxoid stroma | persisting mass |
| 22 | 13.2 | ND | LDH/VMA/HVA | Solid | CR | NB | persisting mass |
| 23 | 13.2 | ND | LDH/VMA | Mixed | CR | Hemangioendothelioma of the liver | persisting mass |
| 24 | 13.7 | (+) | VMA/HVA/DOPA | Mixed | CR | NB | persisting mass |
| 25 | 14.3 | (−) | ND | Cystic | CR | NB | persisting mass |
| 26 | 14.5 | (−) | LDH/VMA/HVA/COR | Cystic | CR | NB | persisting mass |
| 27 | 14.7 | (−) | LDH | Mixed | CR | bronchogenis cyst | persisting mass |
| 28 | 14.8 | ND | VMA/HVA | Solid | CR | NB | persisting mass |
| 29 | 14.8 | (+) | LDH/VMA/DOPA/HVA | Solid | CR | NB | persisting mass |
| 30 | 15.2 | (−) | LDH/VMA/HVA/DOPA | Cystic | CR | GN | persisting mass |
| 31 | 15.3 | ND | LDH/VMA/HVA/DOPA/COR | Mixed | CR | bronchogenis cyst | persisting mass |
| 32 | 15.5 | (+) | LDH/VMA | Mixed | CR | NB | persisting mass |
| 33 | 16.0 | (+) | LDH/VMA/HVA/DOPA | Cystic | CR | Totally necrotic tumor.No malignancy | persisting mass |
| 34 | 16.5 | (+) | DOPA | Solid | CR | cicatricial lesion without viable tumor (>99.9% necrosis) | persisting mass |
| 35 | 16.8 | (−) | LDH/VMA/HVA/DOPA | Solid | CR | GNB intermixed | persisting mass |
| 36 | 19.3 | ND | VMA/HVA | Cystic | CR | NB | persisting mass |
| 37 | 24.0 | (+) | LDH/VMA/HVA | Solid | CR | NB | persisting mass |
| 38 | 24.9 | (−) | VMA | Solid | CR | NB | persisting mass |
| 39 | 29.4 | ND | LDH/DOPA/COR | Mixed | CR | NB | size increased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, V.; Segura, V.; Conte, M.; Plantaz, D.; Di Cataldo, A.; Schleiermacher, G.; Wheeler, K.; Bermúdez, J.D.; Ash, S.; Brichard, B.; et al. Suprarenal Masses in Very Young Infants: Is It Safe to Watch and Wait? Report of a SIOPEN Observational Study Results. Cancers 2022, 14, 4007. https://doi.org/10.3390/cancers14164007
Papadakis V, Segura V, Conte M, Plantaz D, Di Cataldo A, Schleiermacher G, Wheeler K, Bermúdez JD, Ash S, Brichard B, et al. Suprarenal Masses in Very Young Infants: Is It Safe to Watch and Wait? Report of a SIOPEN Observational Study Results. Cancers. 2022; 14(16):4007. https://doi.org/10.3390/cancers14164007
Chicago/Turabian StylePapadakis, Vassilios, Vanessa Segura, Massimo Conte, Dominique Plantaz, Andrea Di Cataldo, Gudrun Schleiermacher, Kate Wheeler, Jose D. Bermúdez, Shifra Ash, Bénédicte Brichard, and et al. 2022. "Suprarenal Masses in Very Young Infants: Is It Safe to Watch and Wait? Report of a SIOPEN Observational Study Results" Cancers 14, no. 16: 4007. https://doi.org/10.3390/cancers14164007
APA StylePapadakis, V., Segura, V., Conte, M., Plantaz, D., Di Cataldo, A., Schleiermacher, G., Wheeler, K., Bermúdez, J. D., Ash, S., Brichard, B., Ladenstein, R., Combaret, V., Sarnacki, S., Fagnani, A. M., Granata, C., & Cañete, A. (2022). Suprarenal Masses in Very Young Infants: Is It Safe to Watch and Wait? Report of a SIOPEN Observational Study Results. Cancers, 14(16), 4007. https://doi.org/10.3390/cancers14164007








