Bisphenols A and S Alter the Bioenergetics and Behaviours of Normal Urothelial and Bladder Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Seahorse Energy Metabolism Measurements
2.3. Proliferation
2.4. Migration
2.5. Flow Cytometry
2.6. Statistical Analysis
3. Results
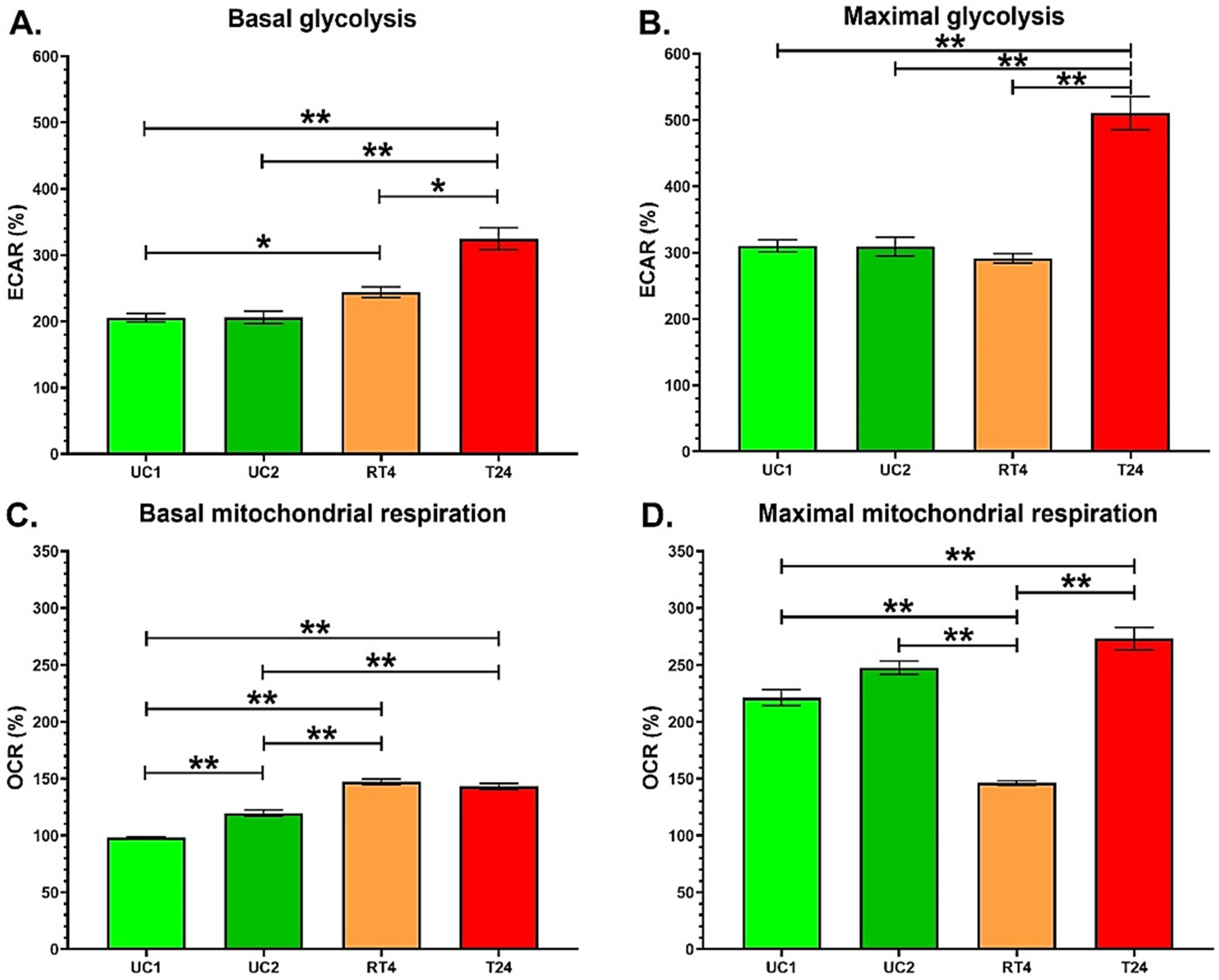
3.1. T24 Invasive Bladder Cancer Cells Exhibit an Increased Glycolytic Capacity Compared with RT4 Non-Invasive Bladder Cancer Cells
3.2. Chronic Exposure to Physiological Concentrations of BPA or BPS Does Not Modulate the Glycolysis and Mitochondrial Respiration of Normal Urothelial Cells
3.3. RT4 Non-Invasive Bladder Cancer Cells Chronically Exposed to Physiological Concentrations of BPs Exhibit Increased Bioenergetics
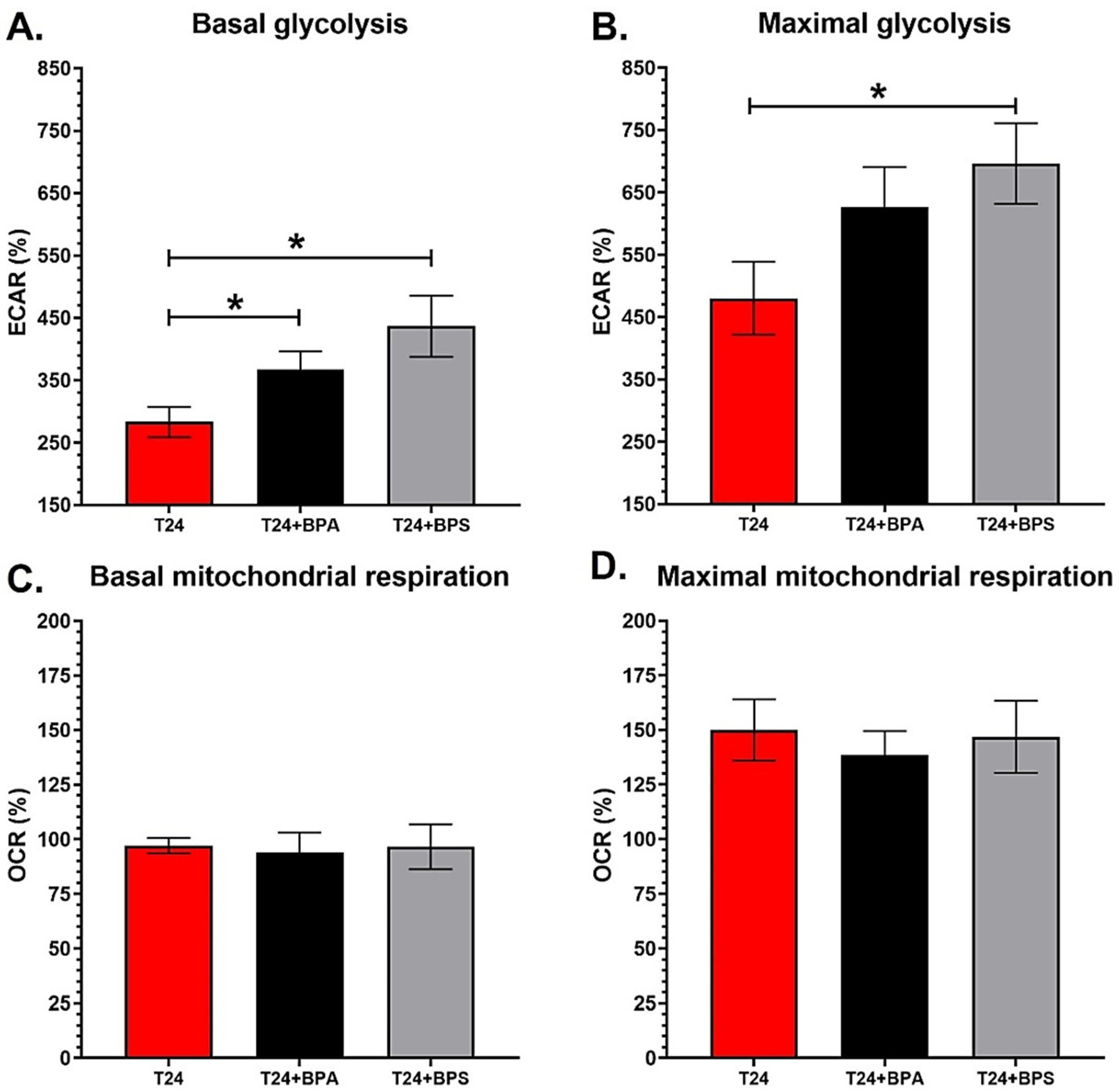
3.4. T24 Invasive Bladder Cancer Cells Chronically Exposed to Physiological Concentrations of BPA or BPS Exhibit an Increased Glycolytic Metabolism
3.5. Chronic Exposure to Physiological Concentrations of BPA or BPS Increases the Proliferation Rate of RT4 Non-Invasive Bladder Cancer Cells and Induces an Initial Boost of Proliferation for UCs and T24 Cells
3.6. Chronic Exposure to Physiological Concentrations of BPA or BPS Decreases the Migration of Normal Urothelial Cells While Increasing the Migration Speed of Bladder Cancer Cells
3.7. RT4 Non-Invasive Bladder Cancer Cells Chronically Exposed to Physiological Concentrations of BPA Exhibit an Increased Expression of α-SMA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakken, K.; Alsaker, E.; Eggen, A.E.; Lund, E. Hormone replacement therapy and incidence of hormone-dependent cancers in the Norwegian Women and Cancer study. Int. J. Cancer 2004, 112, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.P.; Anderson, W.F.; Rosenberg, P.S.; Cook, M.B. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. Eur. Urol. Focus 2018, 4, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, H. Endocrine disruptors (EDs) and hormone-dependent cancers: Correlation or causal relationship? Comptes Rendus Biol. 2017, 340, 439–445. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.; Ramzy, M.M.; Hegazy, A.I.; Husseny, A.K.; El-Hadary, U.G.; Taha, M.M.; Mosa, A.A. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene 2018, 647, 235–243. [Google Scholar] [CrossRef]
- Di Donato, M.; Cernera, G.; Giovannelli, P.; Galasso, G.; Bilancio, A.; Migliaccio, A.; Castoria, G. Recent advances on bisphenol-A and endocrine disruptor effects on human prostate cancer. Mol. Cell. Endocrinol. 2017, 457, 35–42. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, N.; Weng, S.F.; Wang, H.S. Bisphenol A Increases the Migration and Invasion of Triple-Negative Breast Cancer Cells via Oestrogen-related Receptor Gamma. Basic Clin. Pharmacol. Toxicol. 2016, 119, 389–395. [Google Scholar] [CrossRef]
- Derouiche, S.; Warnier, M.; Mariot, P.; Gosset, P.; Mauroy, B.; Bonnal, J.L.; Slomianny, C.; Delcourt, P.; Prevarskaya, N.; Roudbaraki, M. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. Springerplus 2013, 2, 54. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, R.; Ding, X.; Cao, C.; Yang, X. Bisphenol S Triggers the Migration and Invasion of Pheochromocytoma PC12 Cells via Estrogen-Related Receptor alpha. J. Mol. Neurosci. 2018, 66, 188–196. [Google Scholar] [CrossRef]
- Li, S.; Wang, B.; Tang, Q.; Liu, J.; Yang, X. Bisphenol A triggers proliferation and migration of laryngeal squamous cell carcinoma via GPER mediated upregulation of IL-6. Cell Biochem. Funct. 2017, 35, 209–216. [Google Scholar] [CrossRef]
- Song, P.; Fan, K.; Tian, X.; Wen, J. Bisphenol S (BPS) triggers the migration of human non-small cell lung cancer cells via upregulation of TGF-beta. Toxicol. Vitr. 2019, 54, 224–231. [Google Scholar] [CrossRef]
- Lewis, S.A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Ren. Physiol. 2000, 278, F867–F874. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Liu, S. Low-Dose Bisphenol A Exposure: A Seemingly Instigating Carcinogenic Effect on Breast Cancer. Adv. Sci. 2017, 4, 1600248. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Z.Y.; Prossnitz, E.R.; Bjorling, D.E. The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation. Endocrinology 2008, 149, 4024–4034. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Miyamoto, H. Androgen Receptor Signaling in Bladder Cancer. Cancers 2017, 9, 20. [Google Scholar] [CrossRef]
- Bernardo, C.; Santos, J.; Costa, C.; Tavares, A.; Amaro, T.; Marques, I.; Gouveia, M.J.; Félix, V.; Afreixo, V.; Brindley, P.J.; et al. Estrogen receptors in urogenital schistosomiasis and bladder cancer: Estrogen receptor alpha-mediated cell proliferation. Urol. Oncol. 2020, 38, 738.e723–738.e735. [Google Scholar] [CrossRef]
- Ou, Z.; Wang, Y.; Chen, J.; Tao, L.; Zuo, L.; Sahasrabudhe, D.; Joseph, J.; Wang, L.; Yeh, S. Estrogen receptor β promotes bladder cancer growth and invasion via alteration of miR-92a/DAB2IP signals. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Ide, H.; Mizushima, T.; Jiang, G.; Goto, T.; Nagata, Y.; Teramoto, Y.; Inoue, S.; Li, Y.; Kashiwagi, E.; Baras, A.S.; et al. FOXO1 as a tumor suppressor inactivated via AR/ERβ signals in urothelial cells. Endocr. Relat. Cancer 2020, 27, 231–244. [Google Scholar] [CrossRef]
- Pellerin, E.; Caneparo, C.; Chabaud, S.; Bolduc, S.; Pelletier, M. Endocrine-disrupting effects of bisphenols on urological cancers. Environ. Res. 2021, 195, 110485. [Google Scholar] [CrossRef]
- Pellerin, È.; Chabaud, S.; Pouliot, F.; Pelletier, M.; Bolduc, S. Bisphenol A Alters the Energy Metabolism of Stromal Cells and Could Promote Bladder Cancer Progression. Cancers 2021, 13, 5461. [Google Scholar] [CrossRef]
- Young, V.J.; Brown, J.K.; Maybin, J.; Saunders, P.T.; Duncan, W.C.; Horne, A.W. Transforming growth factor-β induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, 3450–3459. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Luo, T.; Wang, H.; Xia, Y.; Xie, Z. Lactic acid in tumor invasion. Clin. Chim. Acta 2021, 522, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Magnan, M.; Berthod, F.; Champigny, M.F.; Soucy, F.; Bolduc, S. In vitro reconstruction of a tissue-engineered endothelialized bladder from a single porcine biopsy. J. Pediatr. Urol. 2006, 2, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Cattan, V.; Bernard, G.; Rousseau, A.; Bouhout, S.; Chabaud, S.; Auger, F.A.; Bolduc, S. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur. Urol. 2011, 60, 1291–1298. [Google Scholar] [CrossRef]
- Pelletier, M.; Billingham, L.K.; Ramaswamy, M.; Siegel, R.M. Extracellular flux analysis to monitor glycolytic rates and mitochondrial oxygen consumption. Methods Enzymol. 2014, 542, 125–149. [Google Scholar] [CrossRef]
- Pellerin, F.A.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Pelletier, M.; Bolduc, S. Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts. Bioengineering 2021, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef]
- Bushnik, T.; Haines, D.; Levallois, P.; Levesque, J.; Van Oostdam, J.; Viau, C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010, 21, 7–18. [Google Scholar]
- Anggorowati, N.; Ratna Kurniasari, C.; Damayanti, K.; Cahyanti, T.; Widodo, I.; Ghozali, A.; Romi, M.M.; Sari, D.C.; Arfian, N. Histochemical and Immunohistochemical Study of α-SMA, Collagen, and PCNA in Epithelial Ovarian Neoplasm. Asian Pac. J. Cancer Prev. 2017, 18, 667–671. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [PubMed]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Goetze, K.; Walenta, S.; Ksiazkiewicz, M.; Kunz-Schughart, L.A.; Mueller-Klieser, W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 2011, 39, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Vallo, S.; Michaelis, M.; Rothweiler, F.; Bartsch, G.; Gust, K.M.; Limbart, D.M.; Rödel, F.; Wezel, F.; Haferkamp, A.; Cinatl, J., Jr. Drug-Resistant Urothelial Cancer Cell Lines Display Diverse Sensitivity Profiles to Potential Second-Line Therapeutics. Transl. Oncol. 2015, 8, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.R.; Hepburn, P.J.; Walker, L.; Highman, W.J.; Trejdosiewicz, L.K.; Povey, S.; Parkar, M.; Hill, B.T.; Riddle, P.R.; Franks, L.M. Tissue culture model of transitional cell carcinoma: Characterization of twenty-two human urothelial cell lines. Cancer Res. 1986, 46, 3630–3636. [Google Scholar] [PubMed]
- Bubeník, J.; Baresová, M.; Viklický, V.; Jakoubková, J.; Sainerová, H.; Donner, J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int. J. Cancer 1973, 11, 765–773. [Google Scholar] [CrossRef]
- Lobo, I. Environmental influences on gene expression. Nat. Educ. 2008, 1, 39. [Google Scholar]
- Coradini, D.; Casarsa, C.; Oriana, S. Epithelial cell polarity and tumorigenesis: New perspectives for cancer detection and treatment. Acta Pharmacol. Sin. 2011, 32, 552–564. [Google Scholar] [CrossRef]
- Werb, Z.; Lu, P. The Role of Stroma in Tumor Development. Cancer J. 2015, 21, 250–253. [Google Scholar] [CrossRef]
- Quesnot, N.; Bucher, S.; Fromenty, B.; Robin, M.A. Modulation of metabolizing enzymes by bisphenol a in human and animal models. Chem. Res. Toxicol. 2014, 27, 1463–1473. [Google Scholar] [CrossRef]
- Khan, S.; Beigh, S.; Chaudhari, B.P.; Sharma, S.; Aliul Hasan Abdi, S.; Ahmad, S.; Ahmad, F.; Parvez, S.; Raisuddin, S. Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats. Environ. Toxicol. 2016, 31, 1922–1934. [Google Scholar] [CrossRef]
- Liu, X.; Sakai, H.; Nishigori, M.; Suyama, K.; Nawaji, T.; Ikeda, S.; Nishigouchi, M.; Okada, H.; Matsushima, A.; Nose, T.; et al. Receptor-binding affinities of bisphenol A and its next-generation analogs for human nuclear receptors. Toxicol. Appl. Pharmacol. 2019, 377, 114610. [Google Scholar] [CrossRef] [PubMed]
- Dairkee, S.H.; Luciani-Torres, M.G.; Moore, D.H.; Goodson, W.H., 3rd. Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis 2013, 34, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, T. The endocrine disruptor bisphenol A promotes nuclear ERRγ translocation, facilitating cell proliferation of Grade I endometrial cancer cells via EGF-dependent and EGF-independent pathways. Mol. Cell. Biochem. 2019, 452, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhao, C.; Zhong, H.; Zhang, S.; Xia, Y.; Cai, Z. Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ. Pollut. 2019, 246, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press© The Contributors 2011: Adelaide, Australia, 2011. [Google Scholar]
- Hecht, S.S. Human urinary carcinogen metabolites: Biomarkers for investigating tobacco and cancer. Carcinogenesis 2002, 23, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.S.; Chen, H.Q.; Chen, Y.S.; Qiu, K.F.; Zheng, X.B.; Li, G.C.; Yang, H.D.; Wen, C.J. Bisphenol A stimulates human lung cancer cell migration via upregulation of matrix metalloproteinases by GPER/EGFR/ERK1/2 signal pathway. Biomed. Pharmacother. 2014, 68, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Jiang, G.; Wu, Y.; Li, J.; Liang, W.; Chen, L.; Su, Q.; Li, W.; Du, J.; Wong, C.K.C.; et al. GPER/Hippo-YAP signal is involved in Bisphenol S induced migration of triple negative breast cancer (TNBC) cells. J. Hazard. Mater. 2018, 355, 1–9. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.M.; Lee, S.J.; Cho, H.J.; Kim, D.H.; Lee, J.I.; Kang, M.S.; Seol, H.J.; Shim, Y.M.; Nam, D.H.; et al. Alpha-smooth muscle actin (ACTA2) is required for metastatic potential of human lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 5879–5889. [Google Scholar] [CrossRef]
- Jeon, M.; You, D.; Bae, S.Y.; Kim, S.W.; Nam, S.J.; Kim, H.H.; Kim, S.; Lee, J.E. Dimerization of EGFR and HER2 induces breast cancer cell motility through STAT1-dependent ACTA2 induction. Oncotarget 2017, 8, 50570–50581. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Welshons, W.V. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol. Cell. Endocrinol. 2014, 398, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ringuette Goulet, C.; Bernard, G.; Chabaud, S.; Couture, A.; Langlois, A.; Neveu, B.; Pouliot, F.; Bolduc, S. Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials 2017, 145, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Birkholz, D.; Lobo, R.A. Human excretion of bisphenol A: Blood, urine, and sweat (BUS) study. J. Environ. Public Health 2012, 2012, 185731. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]





| Cell Types | Parameters | BPA | BPS | |
|---|---|---|---|---|
| UCs | Glycolysis | Basal | ↓ | ↓ |
| Maximal | ↓ | ↓ | ||
| Mitochondrial respiration | Basal | Ø | ↓ | |
| Maximal | Ø | Ø | ||
| Proliferation | ↑ | ↑ | ||
| Migration | ↓↓ | ↓ | ||
| RT4 cells | Glycolysis | Basal | Ø | ↑↑ |
| Maximal | Ø | ↑ | ||
| Mitochondrial respiration | Basal | ↑↑ | ↑ | |
| Maximal | ↑↑ | ↑ | ||
| Proliferation | ↑↑ | ↑↑ | ||
| Migration | ↑ | Ø | ||
| T24 cells | Glycolysis | Basal | ↑↑ | ↑↑ |
| Maximal | ↑ | ↑↑ | ||
| Mitochondrial respiration | Basal | Ø | Ø | |
| Maximal | Ø | Ø | ||
| Proliferation | Ø | Ø | ||
| Migration | ↑↑ | ↑↑ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellerin, È.; Pellerin, F.-A.; Chabaud, S.; Pouliot, F.; Bolduc, S.; Pelletier, M. Bisphenols A and S Alter the Bioenergetics and Behaviours of Normal Urothelial and Bladder Cancer Cells. Cancers 2022, 14, 4011. https://doi.org/10.3390/cancers14164011
Pellerin È, Pellerin F-A, Chabaud S, Pouliot F, Bolduc S, Pelletier M. Bisphenols A and S Alter the Bioenergetics and Behaviours of Normal Urothelial and Bladder Cancer Cells. Cancers. 2022; 14(16):4011. https://doi.org/10.3390/cancers14164011
Chicago/Turabian StylePellerin, Ève, Félix-Antoine Pellerin, Stéphane Chabaud, Frédéric Pouliot, Stéphane Bolduc, and Martin Pelletier. 2022. "Bisphenols A and S Alter the Bioenergetics and Behaviours of Normal Urothelial and Bladder Cancer Cells" Cancers 14, no. 16: 4011. https://doi.org/10.3390/cancers14164011
APA StylePellerin, È., Pellerin, F.-A., Chabaud, S., Pouliot, F., Bolduc, S., & Pelletier, M. (2022). Bisphenols A and S Alter the Bioenergetics and Behaviours of Normal Urothelial and Bladder Cancer Cells. Cancers, 14(16), 4011. https://doi.org/10.3390/cancers14164011







