Detection and Yield of Colorectal Cancer Surveillance in Adults with PTEN Hamartoma Tumour Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Setting
2.2. Data Collection and Outcomes
2.3. Statistical Analyses
3. Results
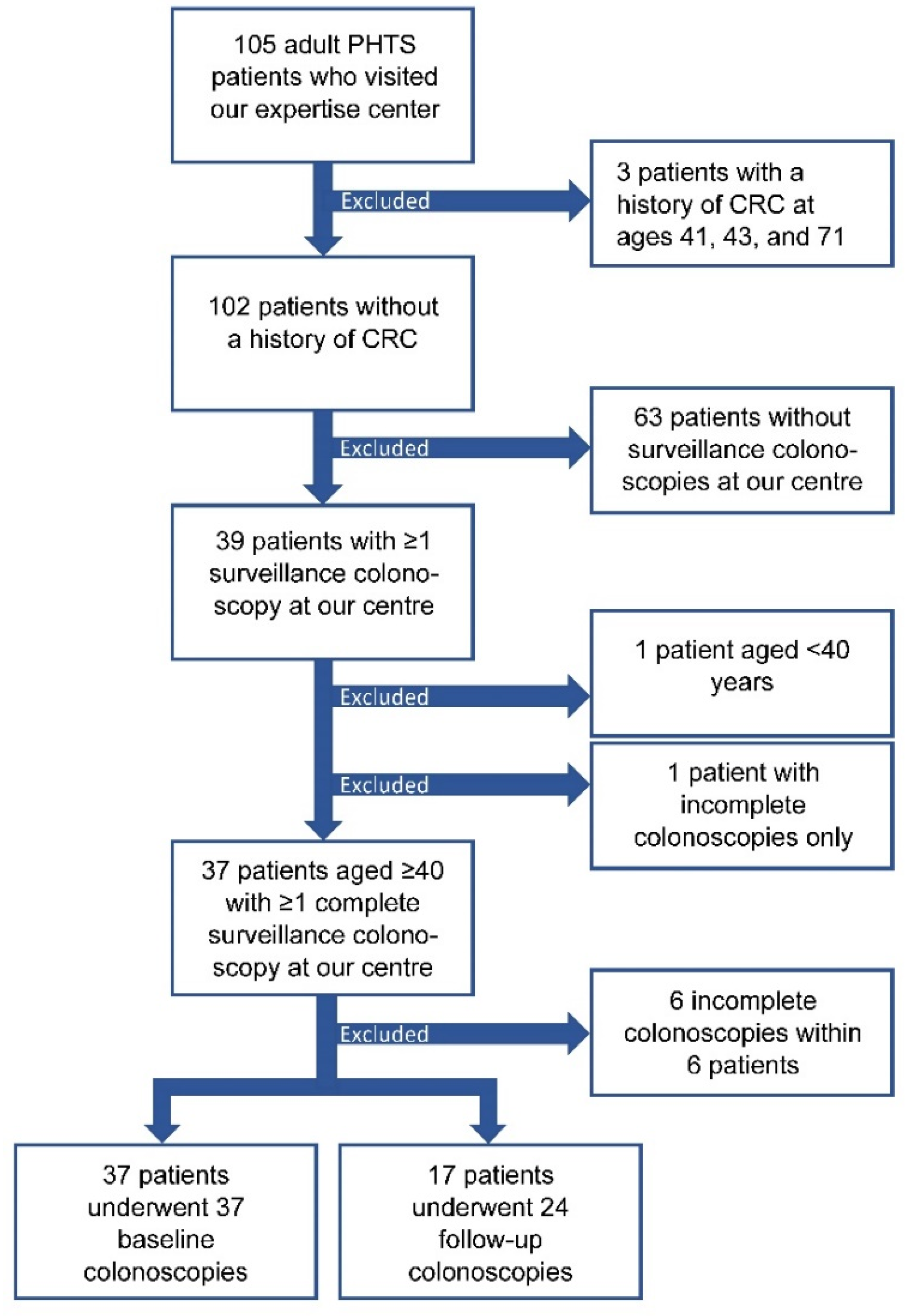
3.1. Study Participants
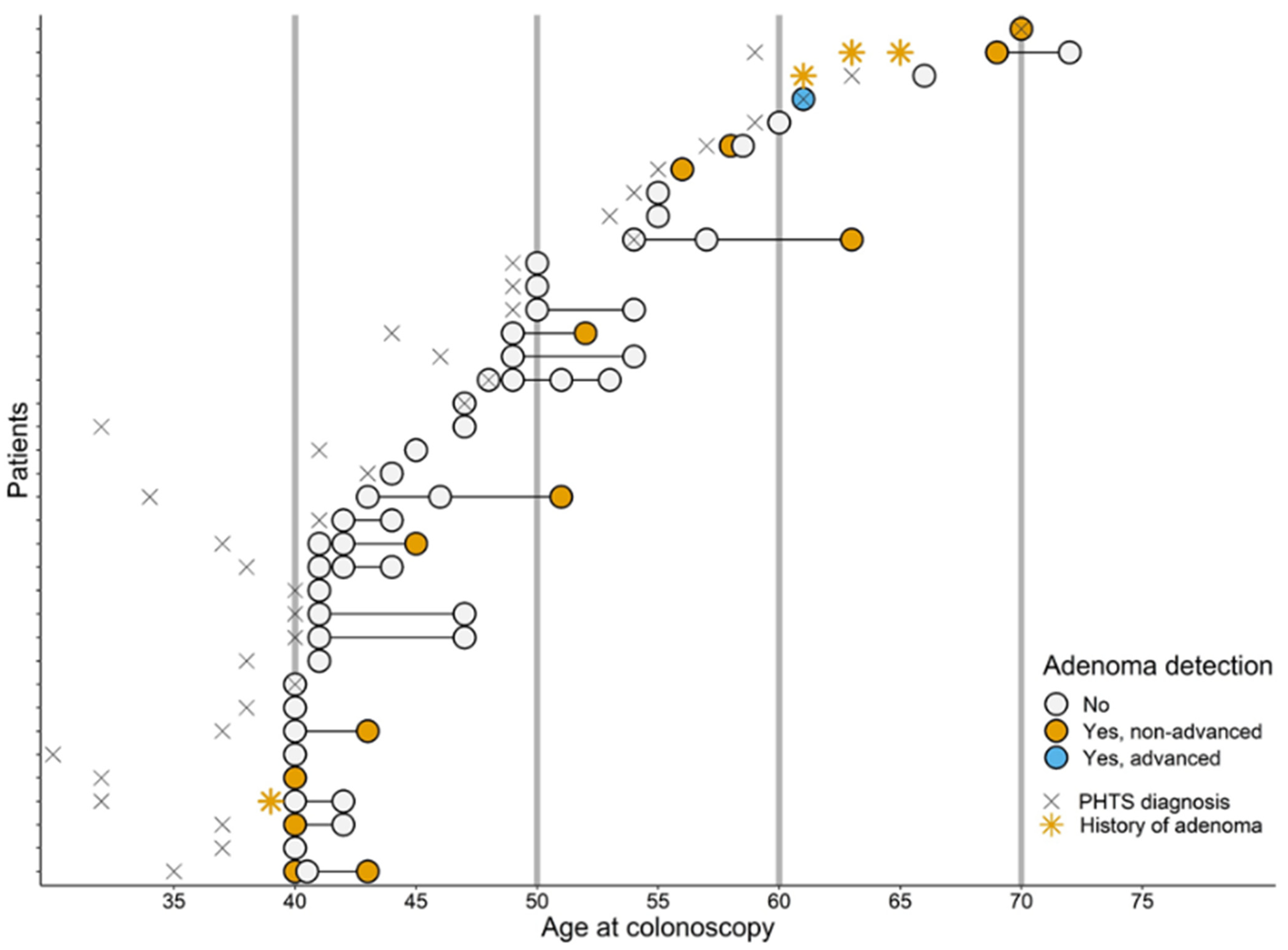
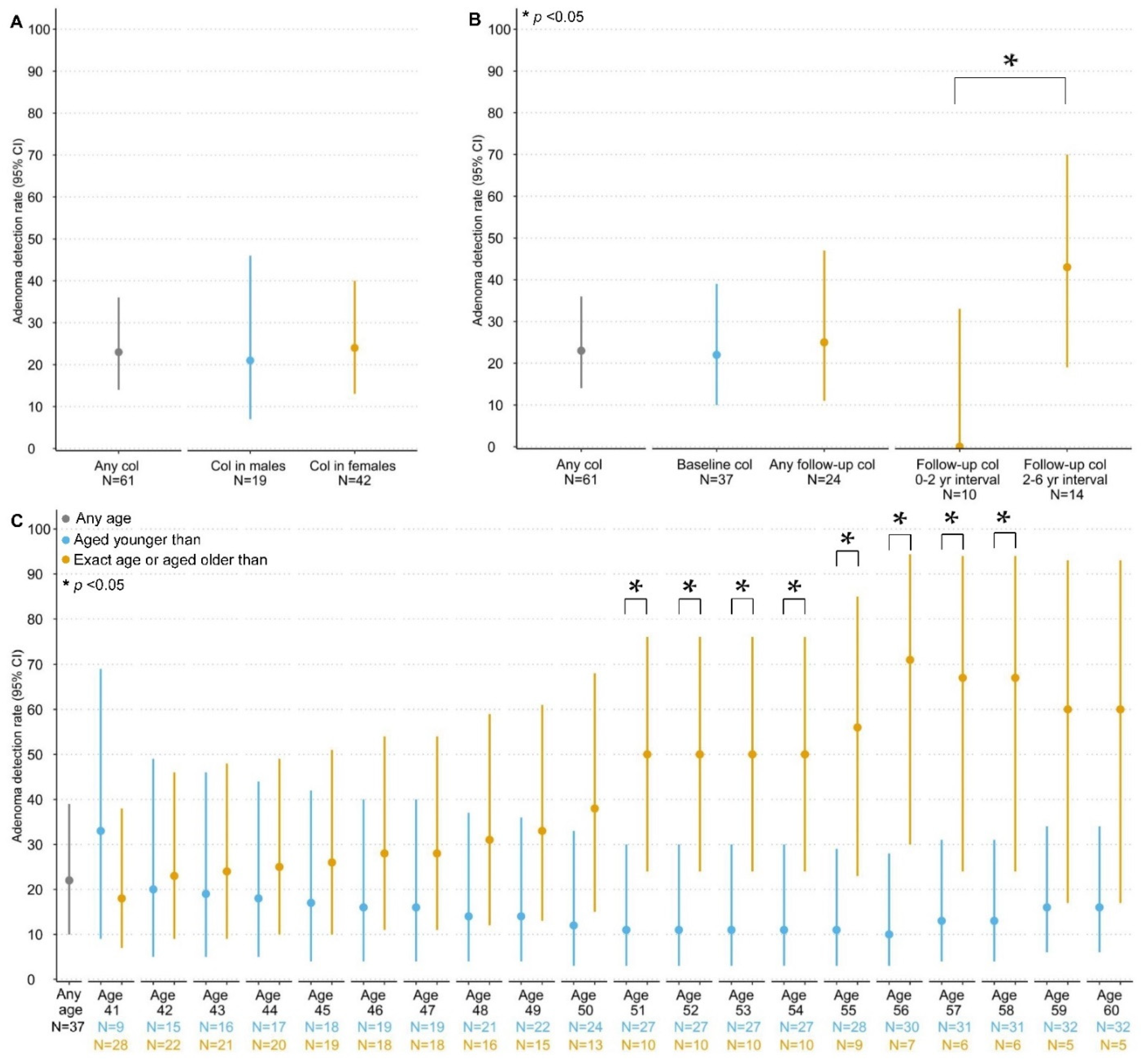
3.2. Detection and Yield of CCS
3.2.1. Colorectal Carcinomas and Adenomas
3.2.2. Other Colorectal Lesions
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelen, M.R.; Kremer, H.; Konings, I.B.; Schoute, F.; van Essen, A.J.; Koch, R.; Woods, C.G.; Fryns, J.P.; Hamel, B.; Hoefsloot, L.H.; et al. Novel PTEN mutations in patients with Cowden disease: Absence of clear genotype-phenotype correlations. Eur. J. Hum. Genet. 1999, 7, 267–273. [Google Scholar] [CrossRef]
- Hendricks, L.A.J.; Hoogerbrugge, N.; Schuurs-Hoeijmakers, J.H.M.; Vos, J.R. A review on age-related cancer risks in PTEN hamartoma tumor syndrome. Clin. Genet. 2021, 99, 219–225. [Google Scholar] [CrossRef]
- Yehia, L.; Keel, E.; Eng, C. The Clinical Spectrum of PTEN Mutations. Annu. Rev. Med. 2020, 71, 103–116. [Google Scholar] [CrossRef]
- Pilarski, R. PTEN Hamartoma Tumor Syndrome: A Clinical Overview. Cancers 2019, 11, 844. [Google Scholar] [CrossRef]
- Heald, B.; Mester, J.; Rybicki, L.; Orloff, M.S.; Burke, C.A.; Eng, C. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterology 2010, 139, 1927–1933. [Google Scholar] [CrossRef]
- Khare, A.; Burke, C.A.; Heald, B.; O’Malley, M.; LaGuardia, L.; Milicia, S.; Cruise, M.; Eng, C.; Mankaney, G. Endoscopic Findings in Patients With PTEN Hamartoma Tumor Syndrome Undergoing Surveillance. J. Clin. Gastroenterol. 2021, 56, e183–e188. [Google Scholar] [CrossRef]
- Stanich, P.P.; Pilarski, R.; Rock, J.; Frankel, W.L.; El-Dika, S.; Meyer, M.M. Colonic manifestations of PTEN hamartoma tumor syndrome: Case series and systematic review. World J. Gastroenterol. 2014, 20, 1833–1838. [Google Scholar] [CrossRef]
- The Dutch Population Cancer Screening Program. Explanation for Registration of Colonoscopy Reports in the Context of the National Screening Program for Colorectal Cancer, Version 7.0, January 2022. Available online: https://www.bevolkingsonderzoeknederland.nl/media/1717/toelichting-registratie-coloscopieverslagen-70.pdf (accessed on 17 June 2022).
- Brenner, H.; Hoffmeister, M.; Stegmaier, C.; Brenner, G.; Altenhofen, L.; Haug, U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007, 56, 1585–1589. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Schoen, R.E. Contribution of Surveillance Colonoscopy to Colorectal Cancer Prevention. Clin. Gastroenterol. Hepatol. 2020, 18, 2937–2944.e2931. [Google Scholar] [CrossRef]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Shussman, N.; Wexner, S.D. Colorectal polyps and polyposis syndromes. Gastroenterol. Rep. 2014, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dutch society of Clinical Genetics (VKGN). National Guideline PTEN Hamartoma Tumour Syndrome. Version 1.0. 2015. Available online: https://richtlijnendatabase.nl/gerelateerde_documenten/f/22306/IKNL%20richtlijn%20PTEN%20Hamartoom%20Tumor%20Syndroom.pdf (accessed on 1 June 2022).
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed on 1 June 2022).
- Boland, C.R.; Idos, G.E.; Durno, C.; Giardiello, F.M.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gross, S.; Gupta, S.; Jacobson, B.C.; et al. Diagnosis and Management of Cancer Risk in the Gastrointestinal Hamartomatous Polyposis Syndromes: Recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022, 162, 2063–2085. [Google Scholar] [CrossRef] [PubMed]
- Tischkowitz, M.; Colas, C.; Pouwels, S.; Hoogerbrugge, N.; Bisseling, T.; Bubien, V.; Caux, F.; Chabbert-Buffet, N.; Colas, C.; Da Mota Gomes, S.; et al. Cancer Surveillance Guideline for individuals with PTEN hamartoma tumour syndrome. Eur. J. Hum. Genet. 2020, 28, 1387–1393. [Google Scholar] [CrossRef]
- UK Cancer Genetic Group. UK Guidelines for Management for Tumour Risk in PTEN Hamartoma Syndrome. Version 1.0 May 2017. Available online: https://www.ukcgg.org/media/10879/pten_management_-_cgg_4may2017.pdf (accessed on 1 June 2022).
- Vos, J.R.; Hsu, L.; Brohet, R.M.; Mourits, M.J.; de Vries, J.; Malone, K.E.; Oosterwijk, J.C.; de Bock, G.H. Bias Correction Methods Explain Much of the Variation Seen in Breast Cancer Risks of BRCA1/2 Mutation Carriers. J. Clin. Oncol. 2015, 33, 2553–2562. [Google Scholar] [CrossRef]
- Raj, K.P.; Taylor, T.H.; Wray, C.; Stamos, M.J.; Zell, J.A. Risk of second primary colorectal cancer among colorectal cancer cases: A population-based analysis. J. Carcinog. 2011, 10, 6. [Google Scholar] [CrossRef]
- Phipps, A.I.; Chan, A.T.; Ogino, S. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer 2013, 119, 3140–3147. [Google Scholar] [CrossRef]
- Wallis, Y.; Payne, S.; McAnulty, C.; Bodmer, D.; Sistermans, E.; Robertson, K.; Moore, D.; Abbs, S.; Deans, Z.; Devereau, A. Practice Guidelines for the Evaluation of Pathogenicity and the Reporting of Sequence Variants in Clinical Molecular Genetics. Association for Clinical Genetic Science and the Dutch Society of Clinical Genetic Laboratory Specialists. 2013. Available online: https://www.acgs.uk.com/media/10791/evaluation_and_reporting_of_sequence_variants_bpgs_june_2013_-_finalpdf.pdf (accessed on 1 June 2022).
- Protocol for Admission and Auditing of Colonoscopy Centers and Colonoscopists. National Bowel Cancer Screening Program. 2020 (Version 9.0). Available online: https://www.bevolkingsonderzoeknederland.nl/media/1493/20201207-protocol-toelating-en-auditing-cc-90.pdf (accessed on 1 June 2022).
- Dutch Society of Gastroenterologists and Pathologists. Dutch Guideline Colonoscopy Surveillance. 2013. Available online: https://www.mdl.nl/files/richlijnen/Richtlijn_Coloscopie_Surveillance_definitief_2013.pdf (accessed on 1 June 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016; Available online: http://www.rstudio.com/ (accessed on 1 June 2022).
- Bubien, V.; Bonnet, F.; Brouste, V.; Hoppe, S.; Barouk-Simonet, E.; David, A.; Edery, P.; Bottani, A.; Layet, V.; Caron, O.; et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J. Med. Genet. 2013, 50, 255–263. [Google Scholar] [CrossRef]
- Tan, M.-H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef]
- Riegert-Johnson, D.L.; Gleeson, F.C.; Roberts, M.; Tholen, K.; Youngborg, L.; Bullock, M.; Boardman, L.A. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered. Cancer Clin. Pract. 2010, 8, 6. [Google Scholar] [CrossRef]
- Shaco-Levy, R.; Jasperson, K.W.; Martin, K.; Samadder, N.J.; Burt, R.W.; Ying, J.; Bronner, M.P. Gastrointestinal Polyposis in Cowden Syndrome. J. Clin. Gastroenterol. 2017, 51, e60–e67. [Google Scholar] [CrossRef]
- Levi, Z.; Baris, H.N.; Kedar, I.; Niv, Y.; Geller, A.; Gal, E.; Gingold, R.; Morgenstern, S.; Baruch, Y.; Leach, B.H.; et al. Upper and Lower Gastrointestinal Findings in PTEN Mutation-Positive Cowden Syndrome Patients Participating in an Active Surveillance Program. Clin. Transl. Gastroenterol. 2011, 2, e5. [Google Scholar] [CrossRef] [PubMed]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, Z.; Ma, Y.; He, C.; Lu, Y.; Zhao, Y.; Chang, X.; Zhang, Y.; Bai, Y.; Cheng, N. Effectiveness of Screening Modalities in Colorectal Cancer: A Network Meta-Analysis. Clin. Colorectal Cancer 2017, 16, 252–263. [Google Scholar] [CrossRef]
- Barrow, P.; Khan, M.; Lalloo, F.; Evans, D.G.; Hill, J. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br. J. Surg. 2013, 100, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Kaminski, M.F.; Løberg, M.; Zauber, A.G.; Regula, J.; Kuipers, E.J.; Hernán, M.A.; McFadden, E.; Sunde, A.; Kalager, M.; et al. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 894–902. [Google Scholar] [CrossRef]
- Diamond, S.J.; Enestvedt, B.K.; Jiang, Z.; Holub, J.L.; Gupta, M.; Lieberman, D.A.; Eisen, G.M. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest. Endosc. 2011, 74, 135–140. [Google Scholar] [CrossRef]
- Mehrkhani, F.; Nasiri, S.; Donboli, K.; Meysamie, A.; Hedayat, A. Prognostic factors in survival of colorectal cancer patients after surgery. Colorectal Dis. 2009, 11, 157–161. [Google Scholar] [CrossRef]
- Brenner, H.; Jansen, L.; Ulrich, A.; Chang-Claude, J.; Hoffmeister, M. Survival of patients with symptom- and screening-detected colorectal cancer. Oncotarget 2016, 7, 44695–44704. [Google Scholar] [CrossRef]
- Friedrich, K.; Grüter, L.; Gotthardt, D.; Eisenbach, C.; Stremmel, W.; Scholl, S.G.; Rex, D.K.; Sieg, A. Survival in patients with colorectal cancer diagnosed by screening colonoscopy. Gastrointest. Endosc. 2015, 82, 133–137. [Google Scholar] [CrossRef]
- Lieberman, D.; Sullivan, B.A.; Hauser, E.R.; Qin, X.; Musselwhite, L.W.; O’Leary, M.C.; Redding, T.S.T.; Madison, A.N.; Bullard, A.J.; Thomas, R.; et al. Baseline Colonoscopy Findings Associated With 10-Year Outcomes in a Screening Cohort Undergoing Colonoscopy Surveillance. Gastroenterology 2020, 158, 862–874.e868. [Google Scholar] [CrossRef]
- Lee, J.K.; Jensen, C.D.; Levin, T.R.; Doubeni, C.A.; Zauber, A.G.; Chubak, J.; Kamineni, A.S.; Schottinger, J.E.; Ghai, N.R.; Udaltsova, N.; et al. Long-term Risk of Colorectal Cancer and Related Death After Adenoma Removal in a Large, Community-based Population. Gastroenterology 2020, 158, 884–894.e885. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Antonelli, G.; Dumonceau, J.M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline–Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar] [CrossRef]
- Hassan, C.; Wysocki, P.T.; Fuccio, L.; Seufferlein, T.; Dinis-Ribeiro, M.; Brandão, C.; Regula, J.; Frazzoni, L.; Pellise, M.; Alfieri, S.; et al. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) Guideline. Endoscopy 2019, 51, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Jover, R.; Bretthauer, M.; Dekker, E.; Holme, Ø.; Kaminski, M.F.; Løberg, M.; Zauber, A.G.; Hernán, M.A.; Lansdorp-Vogelaar, I.; Sunde, A.; et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy 2016, 48, 571–578. [Google Scholar] [CrossRef]
- Marsh, V.; Winton, D.J.; Williams, G.T.; Dubois, N.; Trumpp, A.; Sansom, O.J.; Clarke, A.R. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat. Genet. 2008, 40, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
| Patients (N = 37) | % | |
|---|---|---|
| General | ||
| Female gender, n/N (%) | 24/37 | 65% |
| Index patient, n/N (%) | 15/37 | 41% |
| Age at PHTS diagnosis, median (IQR) | 41 (37–53) | |
| Age at first colonoscopy, median (IQR) | 45 (41–54) | |
| Females | 44 (40–50) | |
| Males | 49 (41–60) | |
| Age at last colonoscopy, median (IQR) | 47 (43–55) | |
| Females | 46 (42–54) | |
| Males | 50 (44–60) | |
| CCS follow-up time 1 (years), median (IQR) | 3 (2–5) | |
| Females | 4 (3–5) | |
| Males | 3 (2–3) | |
| Colorectal surgery prior to the start of CCS, n/N (%) | 2/37 | 5% |
| PHTS-related clinical signs | ||
| Macrocephaly, n/N (%) | 23/34 | 68% |
| Multiple oral lesions 2, n/N (%) | 26/26 | 100% |
| Lhermitte-Duclos disease, n/N (%) | 4/26 | 15% |
| Multiple skin lesions 3, n/N (%) | 25/27 | 93% |
| Arteriovenous malformations, n/N (%) | 6/26 | 23% |
| Colorectal lesions 4, n/N (%) | 35/37 | 95% |
| Cancer, n/N (%) | 12/37 * | 32% |
| Thyroid | 2/37 | 5% |
| Breast | 9/24 | 38% |
| Colorectal | 0/37 ** | 0% |
| Endometrial | 2/24 | 8% |
| Melanoma | 2/37 | 5% |
| Kidney | 0/37 | 0% |
| Patients with Follow-Up Colonoscopies (N = 17) | Follow-Up Colonoscopies (N = 24) | |
|---|---|---|
| Surveillance with 5-year interval, n/N (%) | ||
| No, shortened interval | 14 (82%) | 19 (79%) |
| No, lengthened interval | 2 (12%) | 2 (8%) |
| Yes | 1 (6%) | 3 (13%) |
| Reasons for shortened interval, n/N (%) | ||
| Gastroenterologist’s advice 1 | 12 (86%) | 15 (79%) |
| Patient’s choice 2 | 2 (14%) | 4 (21%) |
| Reasons for lengthened interval, n/N (%) | ||
| Gastroenterologist’s advice 1 | 0 (0%) | 0 (0%) |
| Patient’s choice 2 | 2 (100%) | 2 (100%) |
| Patients (N = 37) 3 | Colonoscopies (N = 61) | |
|---|---|---|
| Findings at colonoscopy | ||
| Colorectal lesion(s) * present | 35 (95%) | 54 (89%) |
| Number of lesions * present | ||
| 1–10 | 21 (57%) | 25 (41%) |
| 1–20 | 3 (8%) | 6 (10%) |
| 21–50 | 7 (19%) | 10 (16%) |
| >50 | 3 (8%) | 4 (7%) |
| Multiple unspecified | 1 (3%) | 9 (15%) |
| Lesions * left in situ | 22 (59%) | 37 (61%) |
| Findings at pathology revision | ||
| Any colorectal lesion * | ||
| Presence, N (%) | 33 (89%) | 48 (79%) |
| Detection rate (95% CI) 1 | 79 (66–88) | |
| Colorectal carcinomas | ||
| Presence, N (%) | 0 (0%) | 0 (0%) |
| Detection rate (95% CI) 1 | 0 (0–7) | |
| Adenomas | ||
| Presence, N (%) | 13 (35%) | 14 (23%) |
| Detection rate (95% CI) 1 | 23 (14–36) | |
| Age at first detection, median (IQR) | 52 (43–60) | |
| Location, N (%) | n.a. | |
| Proximal | 11 (79%) | |
| Distal | 2 (14%) | |
| Both proximal and distal | 1 (7%) | |
| Advanced adenoma, N (%) 2 | 1 (7%) | |
| Number of adenomas, median (IQR) | 1 (1–2) | |
| Time to detection (years), median (IQR) | 3 (3–5) | |
| Hamartomas | ||
| Hamartomas of no special type | ||
| Presence, N (%) | 16 (43%) | 18 (30%) |
| Detection rate (95% CI) 1 | 30 (19–43) | |
| Age at first detection, median (IQR) | 51 (44–59) | |
| Location, N (%) | n.a. | |
| Proximal | 2 (12%) | |
| Distal | 6 (35%) | |
| Both proximal and distal | 9 (53%) | |
| Ganglioneuromas | ||
| Presence, N (%) | 15 (41%) | 20 (33%) |
| Detection rate (95% CI) 1 | 33 (22–46) | |
| Age at first detection, median (IQR) | 44 (41–52) | |
| Location, N (%) | n.a. | |
| Proximal | 10 (50%) | |
| Distal | 6 (30%) | |
| Both proximal and distal | 4 (20%) | |
| Juvenile polyps | ||
| Presence, N (%) | 1 (3%) | 1 (2%) |
| Detection rate (95% CI) 1 | 2 (0–10) | |
| Age at first detection | 40 | |
| Location, N (%) | n.a. | |
| Proximal | 0 (0%) | |
| Distal | 1 (100%) | |
| Both proximal and distal | 0 (0%) | |
| Lymphoid polyps | ||
| Presence, N (%) | 3 (8%) | 3 (5%) |
| Detection rate (95% CI) 1 | 5 (1–15) | |
| Age at first detection, median (IQR) | 47 (46–48) | |
| Location, N (%) | n.a. | |
| Proximal | 1 (33%) | |
| Distal | 1 (33%) | |
| Both proximal and distal | 1 (33%) | |
| Inflammatory polyps | ||
| Presence, N (%) | 7 (19%) | 7 (11%) |
| Detection rate (95% CI) 1 | 11 (5–23) | |
| Age at first detection, median (IQR) | 42 (42–52) | |
| Location, N (%) | n.a. | |
| Proximal | 4 (57%) | |
| Distal | 2 (29%) | |
| Both proximal and distal | 1 (14%) | |
| Hyperplastic polyps | ||
| Presence, N (%) | 7 (19%) | 8 (13%) |
| Detection rate (95% CI) 1 | 13 (6–25) | |
| Age at first detection, median (IQR) | 49 (43–52) | |
| Location, N (%) | n.a. | |
| Proximal | 2 (25%) | |
| Distal | 6 (75%) | |
| Both proximal and distal | 0 (0%) | |
| Sessile serrated lesions | ||
| Presence, N (%) | 7 (19%) | 8 (13%) |
| Detection rate (95% CI) 1 | 13 (6–25) | |
| Age at first detection, median (IQR) | 46 (42–48) | |
| Location, N (%) | n.a. | |
| Proximal | 6 (75%) | |
| Distal | 1 (13%) | |
| Both proximal and distal | 1 (13%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drissen, M.M.C.M.; Vos, J.R.; van der Biessen-van Beek, D.T.J.; van der Post, R.S.; Nagtegaal, I.D.; van Kouwen, M.C.A.; Bisseling, T.M.; Hoogerbrugge, N. Detection and Yield of Colorectal Cancer Surveillance in Adults with PTEN Hamartoma Tumour Syndrome. Cancers 2022, 14, 4005. https://doi.org/10.3390/cancers14164005
Drissen MMCM, Vos JR, van der Biessen-van Beek DTJ, van der Post RS, Nagtegaal ID, van Kouwen MCA, Bisseling TM, Hoogerbrugge N. Detection and Yield of Colorectal Cancer Surveillance in Adults with PTEN Hamartoma Tumour Syndrome. Cancers. 2022; 14(16):4005. https://doi.org/10.3390/cancers14164005
Chicago/Turabian StyleDrissen, Meggie M. C. M., Janet R. Vos, Dorien T. J. van der Biessen-van Beek, Rachel S. van der Post, Iris D. Nagtegaal, Mariëtte C. A. van Kouwen, Tanya M. Bisseling, and Nicoline Hoogerbrugge. 2022. "Detection and Yield of Colorectal Cancer Surveillance in Adults with PTEN Hamartoma Tumour Syndrome" Cancers 14, no. 16: 4005. https://doi.org/10.3390/cancers14164005
APA StyleDrissen, M. M. C. M., Vos, J. R., van der Biessen-van Beek, D. T. J., van der Post, R. S., Nagtegaal, I. D., van Kouwen, M. C. A., Bisseling, T. M., & Hoogerbrugge, N. (2022). Detection and Yield of Colorectal Cancer Surveillance in Adults with PTEN Hamartoma Tumour Syndrome. Cancers, 14(16), 4005. https://doi.org/10.3390/cancers14164005






