Super Enhancer-Regulated LINC00094 (SERLOC) Upregulates the Expression of MMP-1 and MMP-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Real-Time Quantitative PCR
2.3. Tissue Samples
2.4. RNA In Situ Hybridization
2.5. Adenoviral Infection
2.6. RNA-Sequencing
2.7. Western Blot Analysis
2.8. Human cSCC Xenografts
2.9. Invasion Assays
2.10. Statistical Analysis
3. Results
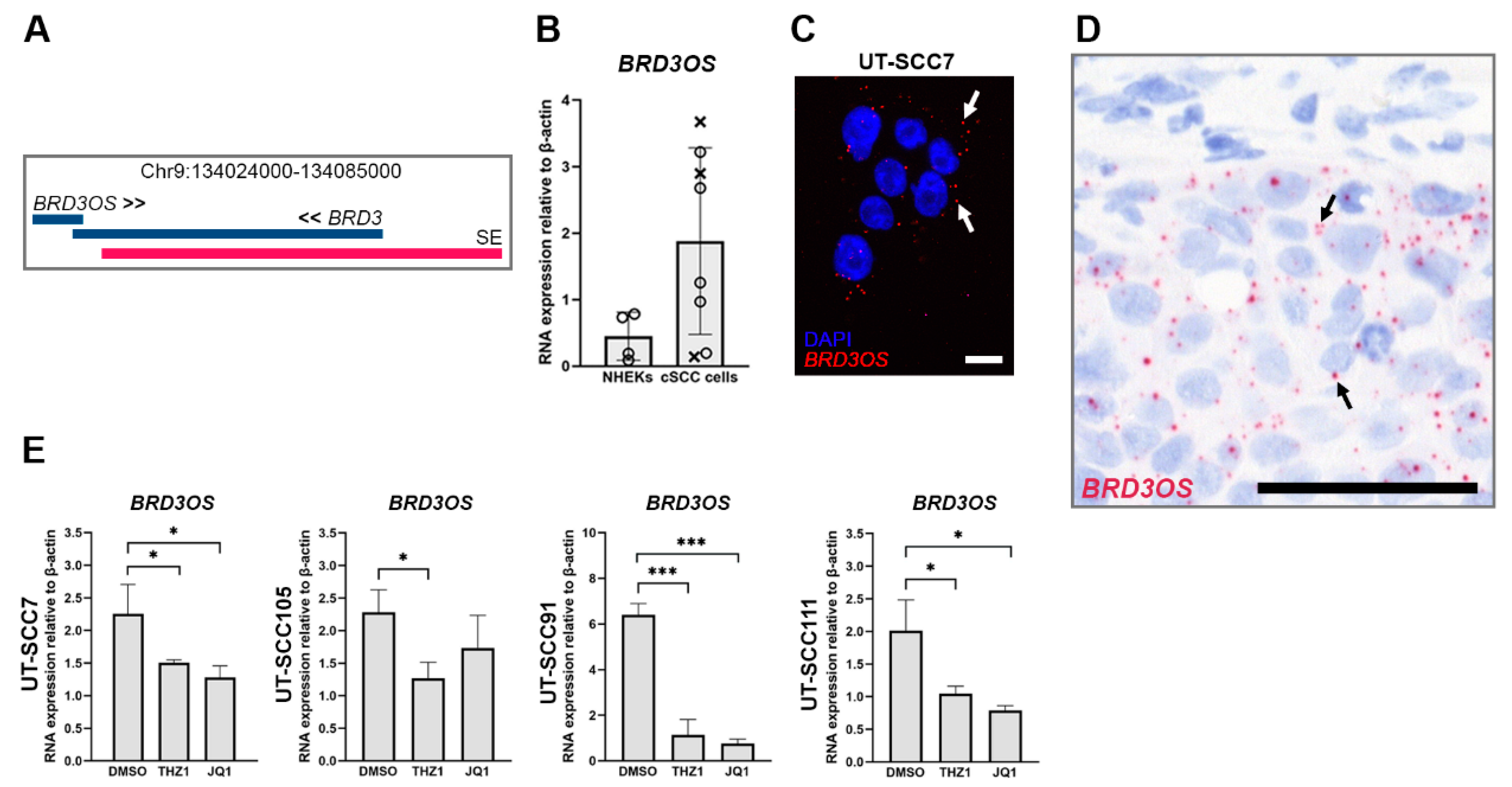
3.1. BRD3OS (LINC00094) Is Overexpressed in cSCC Cells
3.2. BRD3OS (LINC00094) Is Regulated by Super Enhancer in cSCC Cells
3.3. BRD3OS (LINC00094) Is Expressed by cSCC Tumor Cells In Vivo
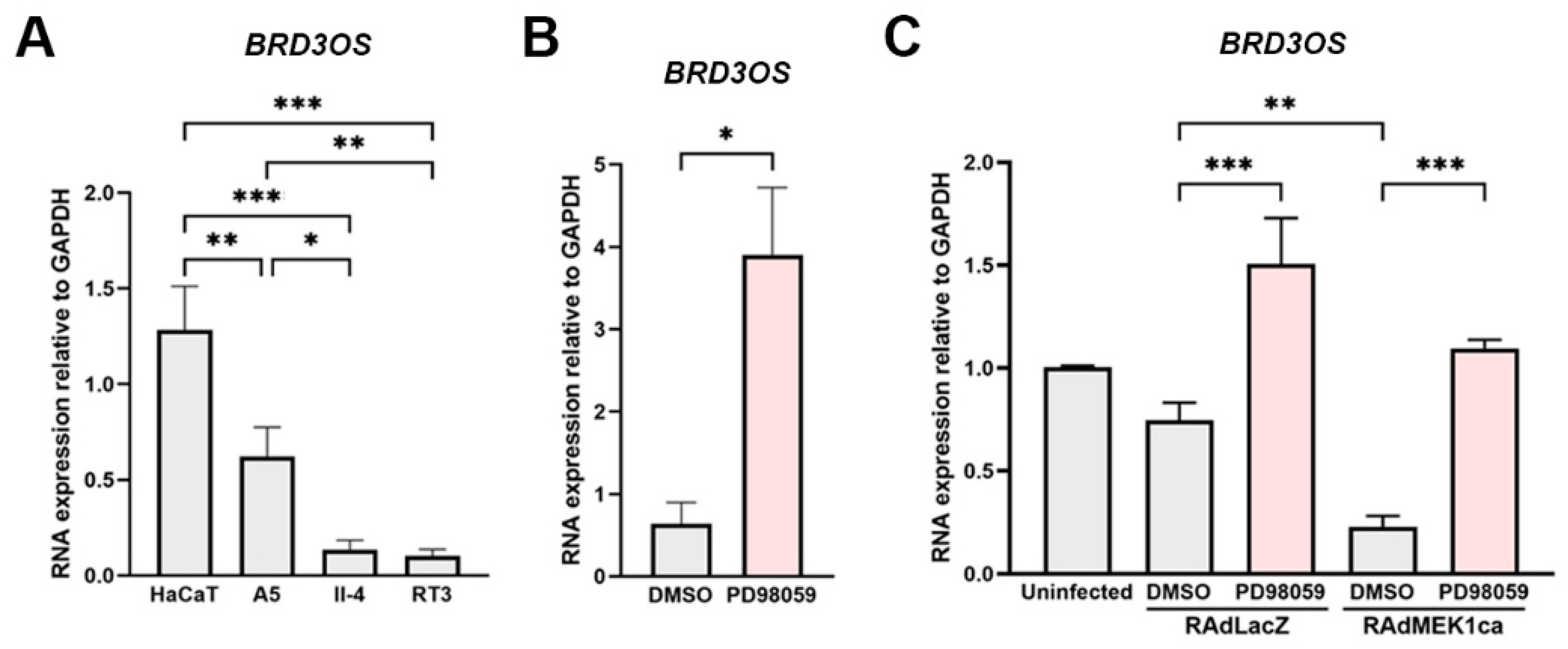
3.4. BRD3OS (LINC00094) Expression Is Regulated by ERK1/2 Pathway
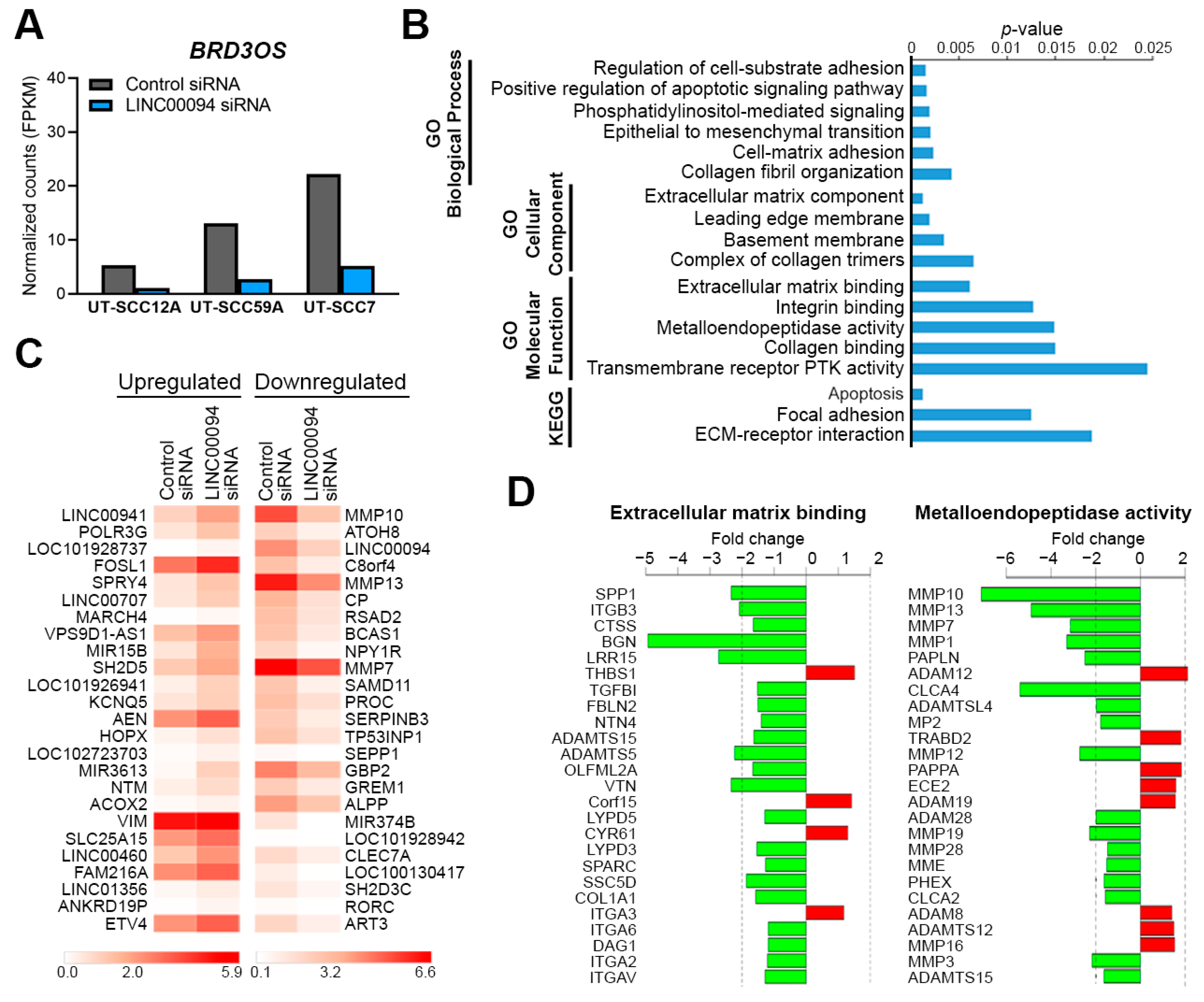
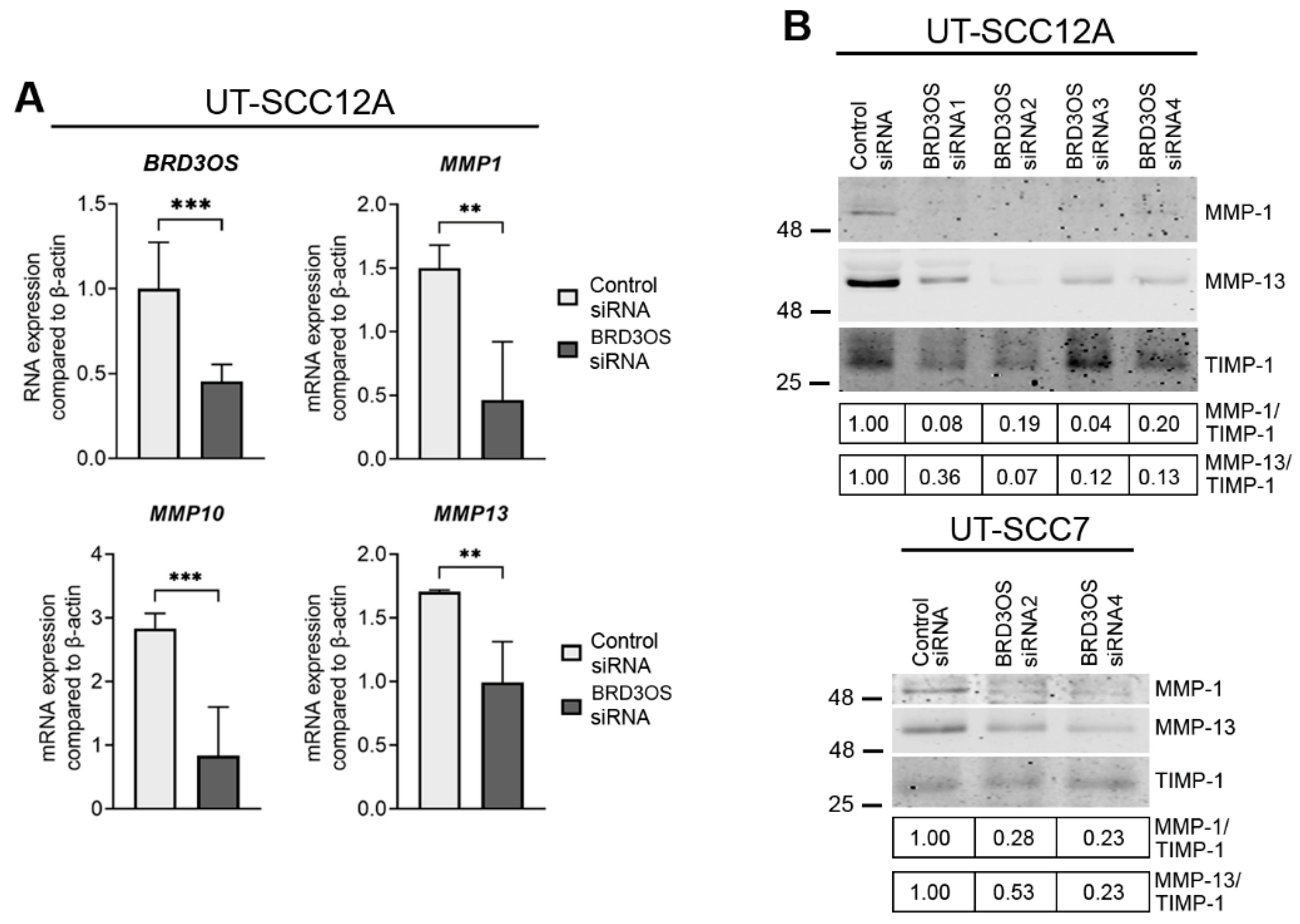
3.5. Knockdown of BRD3OS (LINC00094) Inhibits the Expression of MMP-1 and MMP-13
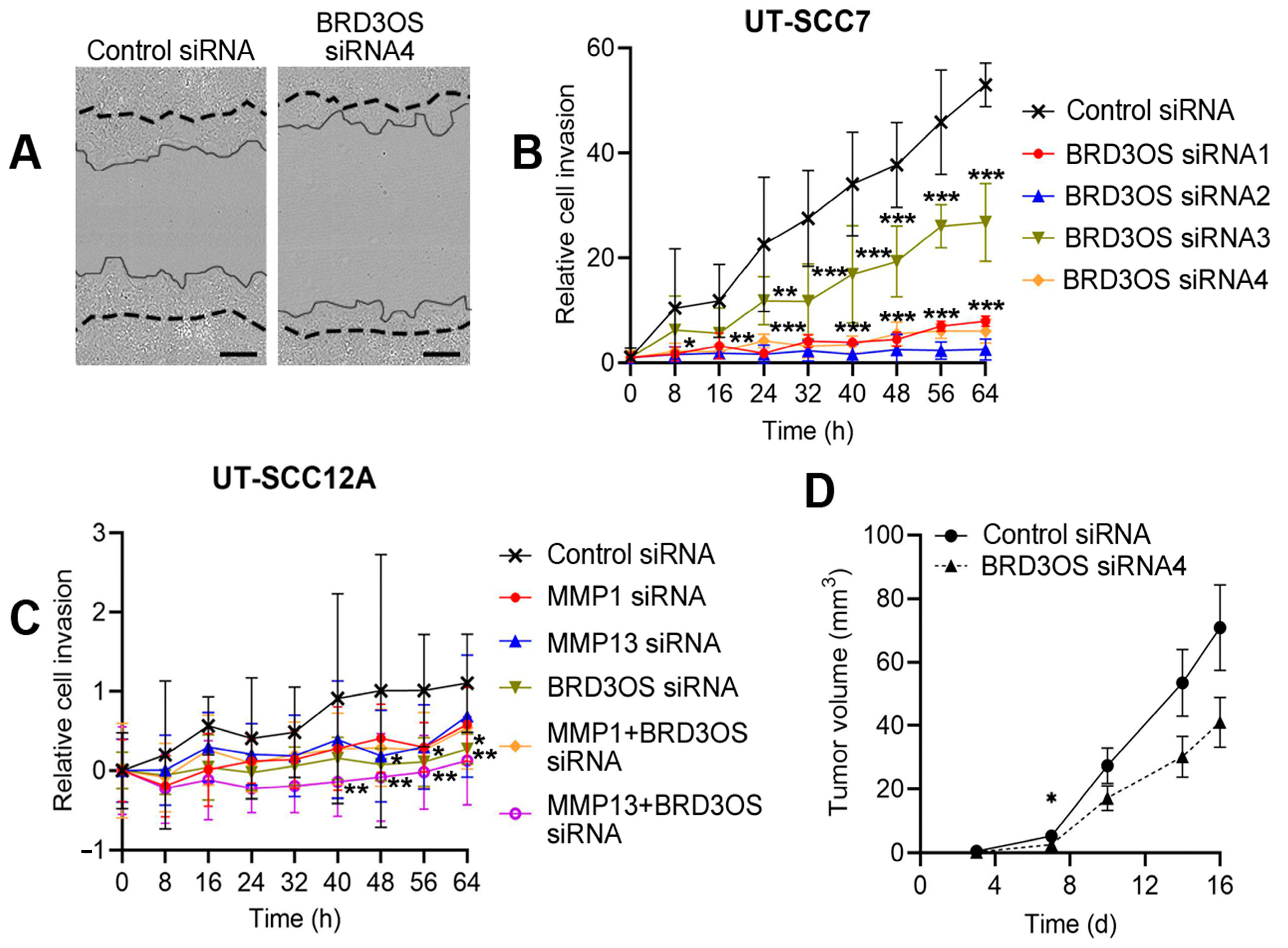
3.6. Knockdown of BRD3OS (LINC00094) Inhibits Invasion of cSCC Cells by Downregulating MMP-1 and MMP-13 Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmitt, A.M.; Chang, H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The Multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Nehal, K.S.; Bichakjian, C.K. Update on keratinocyte carcinomas. N. Engl. J. Med. 2018, 379, 363–374. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous squamous cell carcinoma: A Review of high-risk and metastatic disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Knuutila, J.S.; Riihilä, P.; Kurki, S.; Nissinen, L.; Kähäri, V.-M. Risk factors and prognosis for metastatic cutaneous squamous cell carcinoma: A cohort study. Acta Derm. Venereol. 2020, 100, adv00266. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Cho, R.J.; Alexandrov, L.B.; den Breems, N.Y.; Atanasova, V.S.; Farshchian, M.; Purdom, E.; Nguyen, T.N.; Coarfa, C.; Rajapakshe, K.; Prisco, M.; et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci. Transl. Med. 2018, 10, eaas9668. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y.; et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 2014, 20, 6582–6592. [Google Scholar] [CrossRef]
- Piipponen, M.; Riihilä, P.; Nissinen, L.; Kähäri, V.-M. The role of p53 in progression of cutaneous squamous cell carcinoma. Cancers 2021, 13, 4507. [Google Scholar] [CrossRef] [PubMed]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; den Breems, N.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.; Nissinen, L.; Kähäri, V.-M. Matrix metalloproteinases in keratinocyte carcinomas. Exp. Dermatol. 2021, 30, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, L.; Farshchian, M.; Riihilä, P.; Kähäri, V.-M. New perspectives on role of tumor microenvironment in progression of cutaneous squamous cell carcinoma. Cell Tissue Res. 2016, 365, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Nissinen, L.; Kähäri, V.-M. Long non-coding RNAs in cutaneous biology and keratinocyte carcinomas. Cell. Mol. Life Sci. 2020, 22, 4601–4614. [Google Scholar] [CrossRef]
- Piipponen, M.; Nissinen, L.; Farshchian, M.; Riihilä, P.; Kivisaari, A.; Kallajoki, M.; Peltonen, J.; Peltonen, S.; Kähäri, V.-M. Long Noncoding RNA PICSAR promotes growth of cutaneous squamous cell carcinoma by regulating ERK1/2 activity. J. Investig. Dermatol. 2016, 136, 1701–1710. [Google Scholar] [CrossRef]
- Piipponen, M.; Heino, J.; Kähäri, V.-M.; Nissinen, L. Long non-coding rna PICSAR decreases adhesion and promotes migration of squamous carcinoma cells by downregulating α2β1 and α5β1 integrin expression. Biol. Open 2018, 7, bio037044. [Google Scholar] [CrossRef]
- Piipponen, M.; Nissinen, L.; Riihilä, P.; Farshchian, M.; Kallajoki, M.; Peltonen, J.; Peltonen, S.; Kähäri, V.-M. P53-regulated long noncoding RNA PRECSIT promotes progression of cutaneous squamous cell carcinoma via STAT3 signaling. Am. J. Pathol. 2020, 190, 503–517. [Google Scholar] [CrossRef]
- Farshchian, M.; Nissinen, L.; Siljamäki, E.; Riihilä, P.; Toriseva, M.; Kivisaari, A.; Ala-Aho, R.; Kallajoki, M.; Veräjänkorva, E.; Honkanen, H.-K.; et al. EphB2 promotes progression of cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2015, 135, 1882–1892. [Google Scholar] [CrossRef]
- Farshchian, M.; Nissinen, L.; Grénman, R.; Kähäri, V.-M. Dasatinib promotes apoptosis of cutaneous squamous carcinoma cells by regulating activation of ERK1/2. Exp. Dermatol. 2017, 26, 89–92. [Google Scholar] [CrossRef]
- Boukamp, P.; Stanbridge, E.J.; Foo, D.Y.; Cerutti, P.A.; Fusenig, N.E. C-Ha-Ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990, 50, 2840–2847. [Google Scholar] [PubMed]
- Riihilä, P.M.; Nissinen, L.M.; Ala-Aho, R.; Kallajoki, M.; Grénman, R.; Meri, S.; Peltonen, S.; Peltonen, J.; Kähäri, V.-M. Complement Factor H: A biomarker for progression of cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2014, 134, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.; Joutsa, J.; Ala-Aho, R.; Pitchers, M.; Pennington, C.J.; Martin, C.; Premachandra, D.J.; Okada, Y.; Peltonen, J.; Grénman, R.; et al. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin. Cancer Res. 2010, 16, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Rahmati Nezhad, P.; Riihilä, P.; Knuutila, J.S.; Viiklepp, K.; Peltonen, S.; Kallajoki, M.; Meri, S.; Nissinen, L.; Kähäri, V.-M. Complement Factor D is a novel biomarker and putative therapeutic target in cutaneous squamous cell carcinoma. Cancers 2022, 14, 305. [Google Scholar] [CrossRef]
- Farshchian, M.; Kivisaari, A.; Ala-Aho, R.; Riihilä, P.; Kallajoki, M.; Grénman, R.; Peltonen, J.; Pihlajaniemi, T.; Heljasvaara, R.; Kähäri, V.-M. Serpin peptidase inhibitor clade a member 1 (serpina1) is a novel biomarker for progression of cutaneous squamous cell carcinoma. Am. J. Pathol. 2011, 179, 1110–1119. [Google Scholar] [CrossRef]
- Foschi, M.; Chari, S.; Dunn, M.J.; Sorokin, A. biphasic activation of p21ras by endothelin-1 sequentially activates the ERK cascade and phosphatidylinositol 3-kinase. EMBO J. 1997, 16, 6439–6451. [Google Scholar] [CrossRef]
- Wilkinson, G.W.; Akrigg, A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992, 20, 2233–2239. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Peng, L.; Chen, Y.; Liao, L.-D.; Chen, J.-X.; Li, M.; Li, Y.-Y.; Qian, F.-C.; Zhang, Y.-X.; Wang, F.; et al. Characterization of super-enhancer-associated functional lncRNAs acting as ceRNAs in escc. Mol. Oncol. 2020, 14, 2203–2230. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, D.; Gu, Y.; Wang, C.; Zhang, M.; Lin, X.; Xing, J.; Wang, H.; Zhang, Y. SEA Version 3.0: A comprehensive extension and update of the super-enhancer archive. Nucleic Acids Res. 2020, 48, D198–D203. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.D.; Zimmerman, M.W.; Dharia, N.V.; Abraham, B.J.; Iniguez, A.B.; Weichert-Leahey, N.; He, S.; Krill-Burger, J.M.; Root, D.E.; Vazquez, F.; et al. Selective gene dependencies in mycn-amplified neuroblastoma include the core transcriptional regulatory circuitry. Nat. Genet. 2018, 50, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Chipumuro, E.; Marco, E.; Christensen, C.L.; Kwiatkowski, N.; Zhang, T.; Hatheway, C.M.; Abraham, B.J.; Sharma, B.; Yeung, C.; Altabef, A.; et al. CDK7 Inhibition suppresses super-enhancer-linked oncogenic transcription in mycn-driven cancer. Cell 2014, 159, 1126–1139. [Google Scholar] [CrossRef]
- Junttila, M.R.; Ala-Aho, R.; Jokilehto, T.; Peltonen, J.; Kallajoki, M.; Grenman, R.; Jaakkola, P.; Westermarck, J.; Kähäri, V.-M. p38α and p38δ mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene 2007, 26, 5267–5279. [Google Scholar] [CrossRef]
- Kivisaari, A.K.; Kallajoki, M.; Ala-aho, R.; McGrath, J.A.; Bauer, J.W.; Königová, R.; Medvecz, M.; Beckert, W.; Grénman, R.; Kähäri, V.-M. Matrix metalloproteinase-7 activates heparin-binding epidermal growth factor-like growth factor in cutaneous squamous cell carcinoma. Br. J. Dermatol. 2010, 163, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Toriseva, M.; Ala-aho, R.; Peltonen, S.; Peltonen, J.; Grénman, R.; Kähäri, V.-M. Keratinocyte growth factor induces gene expression signature associated with suppression of malignant phenotype of cutaneous squamous carcinoma cells. PLoS ONE 2012, 7, e33041. [Google Scholar] [CrossRef]
- Siljamäki, E.; Rappu, P.; Riihilä, P.; Nissinen, L.; Kähäri, V.-M.; Heino, J. H-Ras Activation and fibroblast-induced TGF-β signaling promote laminin-332 accumulation and invasion in cutaneous squamous cell carcinoma. Matrix Biol. 2020, 87, 26–47. [Google Scholar] [CrossRef]
- Chen, J.; Hou, S.F.; Tang, F.J.; Liu, D.S.; Chen, Z.Z.; Zhang, H.L.; Wang, S.H. HOTAIR/Sp1/miR-199a critically regulates cancer stemness and malignant progression of cutaneous squamous cell carcinoma. Oncogene 2022, 41, 99–111. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, G.; Huang, C.; Zhang, J.; Ji, J. Downregulation of lncRNA NEAT1 inhibits the proliferation of human cutaneous squamous cell carcinoma in vivo and in vitro. Ann. Transl. Med. 2022, 10, 79. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Guo, L.; Peng, M. Long non-coding RNA MALAT1 regulates cell proliferation, invasion and apoptosis by modulating the Wnt signaling pathway in squamous cell carcinoma. Am. J. Transl. Res. 2021, 13, 9233–9240. [Google Scholar]
- Li, F.; Liao, J.; Duan, X.; He, Y.; Liao, Y. Upregulation of LINC00319 indicates a poor prognosis and promotes cell proliferation and invasion in cutaneous squamous cell carcinoma. J. Cell. Biochem. 2018, 119, 10393–10405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, M.; Fan, H. Targeting complexes of super-enhancers is a promising strategy for cancer therapy. Oncol. Lett. 2020, 20, 2557–2566. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Song, R.; Regev, A.; Struhl, K. Many lncRNAs, 5′UTRs, and pseudogenes are translated and some are likely to express functional proteins. eLife 2015, 4, e08890. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Long, W.; Liu, Q. Targeting super-enhancers as a therapeutic strategy for cancer treatment. Front. Pharmacol. 2019, 10, 361. [Google Scholar] [CrossRef]
- Fontanals-Cirera, B.; Hasson, D.; Vardabasso, C.; Di Micco, R.; Agrawal, P.; Chowdhury, A.; Gantz, M.; de Pablos-Aragoneses, A.; Morgenstern, A.; Wu, P.; et al. Harnessing BET inhibitor sensitivity reveals AMIGO2 as a melanoma survival gene. Mol. Cell. 2017, 68, 731–744.e9. [Google Scholar] [CrossRef]
- Christensen, C.L.; Kwiatkowski, N.; Abraham, B.J.; Carretero, J.; Al-Shahrour, F.; Zhang, T.; Chipumuro, E.; Herter-Sprie, G.S.; Akbay, E.A.; Altabef, A.; et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell. 2014, 26, 909–922. [Google Scholar] [CrossRef]
- Ujfaludi, Z.; Tuzesi, A.; Majoros, H.; Rothler, B.; Pankotai, T.; Boros, I.M. Coordinated activation of a cluster of MMP genes in response to UVB radiation. Sci. Rep. 2018, 8, 2660. [Google Scholar] [CrossRef]
- Airola, K.; Johansson, N.; Kariniemi, A.L.; Kähäri, V.-M.; Saarialho-Kere, U.K. Human collagenase-3 is expressed in malignant squamous epithelium of the skin. J. Investig. Dermatol. 1997, 109, 225–231. [Google Scholar] [CrossRef]
- Johansson, N.; Airola, K.; Grénman, R.; Kariniemi, A.L.; Saarialho-Kere, U.; Kähäri, V.M. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am. J. Pathol. 1997, 151, 499–508. [Google Scholar]
- Viiklepp, K.; Nissinen, L.; Ojalill, M.; Riihilä, P.; Kallajoki, M.; Meri, S.; Heino, J.; Kähäri, V.M. C1r upregulates production of matrix metalloproteinase-13 and promotes invasion of cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2022, 142, 1478–1488.e9. [Google Scholar] [CrossRef] [PubMed]
- Ala-aho, R.; Grénman, R.; Seth, P.; Kähäri, V.-M. Adenoviral delivery of p53 gene suppresses expression of collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene 2002, 21, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Ala-aho, R.; Ahonen, M.; George, S.J.; Heikkilä, J.; Grénman, R.; Kallajoki, M.; Kähäri, V.M. Targeted inhibition of human collagenase-3 (MMP-13) expression inhibits squamous cell carcinoma growth in vivo. Oncogene 2004, 23, 5111–5123. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piipponen, M.; Riihilä, P.; Knuutila, J.S.; Kallajoki, M.; Kähäri, V.-M.; Nissinen, L. Super Enhancer-Regulated LINC00094 (SERLOC) Upregulates the Expression of MMP-1 and MMP-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma. Cancers 2022, 14, 3980. https://doi.org/10.3390/cancers14163980
Piipponen M, Riihilä P, Knuutila JS, Kallajoki M, Kähäri V-M, Nissinen L. Super Enhancer-Regulated LINC00094 (SERLOC) Upregulates the Expression of MMP-1 and MMP-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma. Cancers. 2022; 14(16):3980. https://doi.org/10.3390/cancers14163980
Chicago/Turabian StylePiipponen, Minna, Pilvi Riihilä, Jaakko S. Knuutila, Markku Kallajoki, Veli-Matti Kähäri, and Liisa Nissinen. 2022. "Super Enhancer-Regulated LINC00094 (SERLOC) Upregulates the Expression of MMP-1 and MMP-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma" Cancers 14, no. 16: 3980. https://doi.org/10.3390/cancers14163980
APA StylePiipponen, M., Riihilä, P., Knuutila, J. S., Kallajoki, M., Kähäri, V.-M., & Nissinen, L. (2022). Super Enhancer-Regulated LINC00094 (SERLOC) Upregulates the Expression of MMP-1 and MMP-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma. Cancers, 14(16), 3980. https://doi.org/10.3390/cancers14163980






