Significance of Dermoscopy in Association with Clinical Features in Differentiation of Basal Cell Carcinoma and Benign Trichoblastic Tumours
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
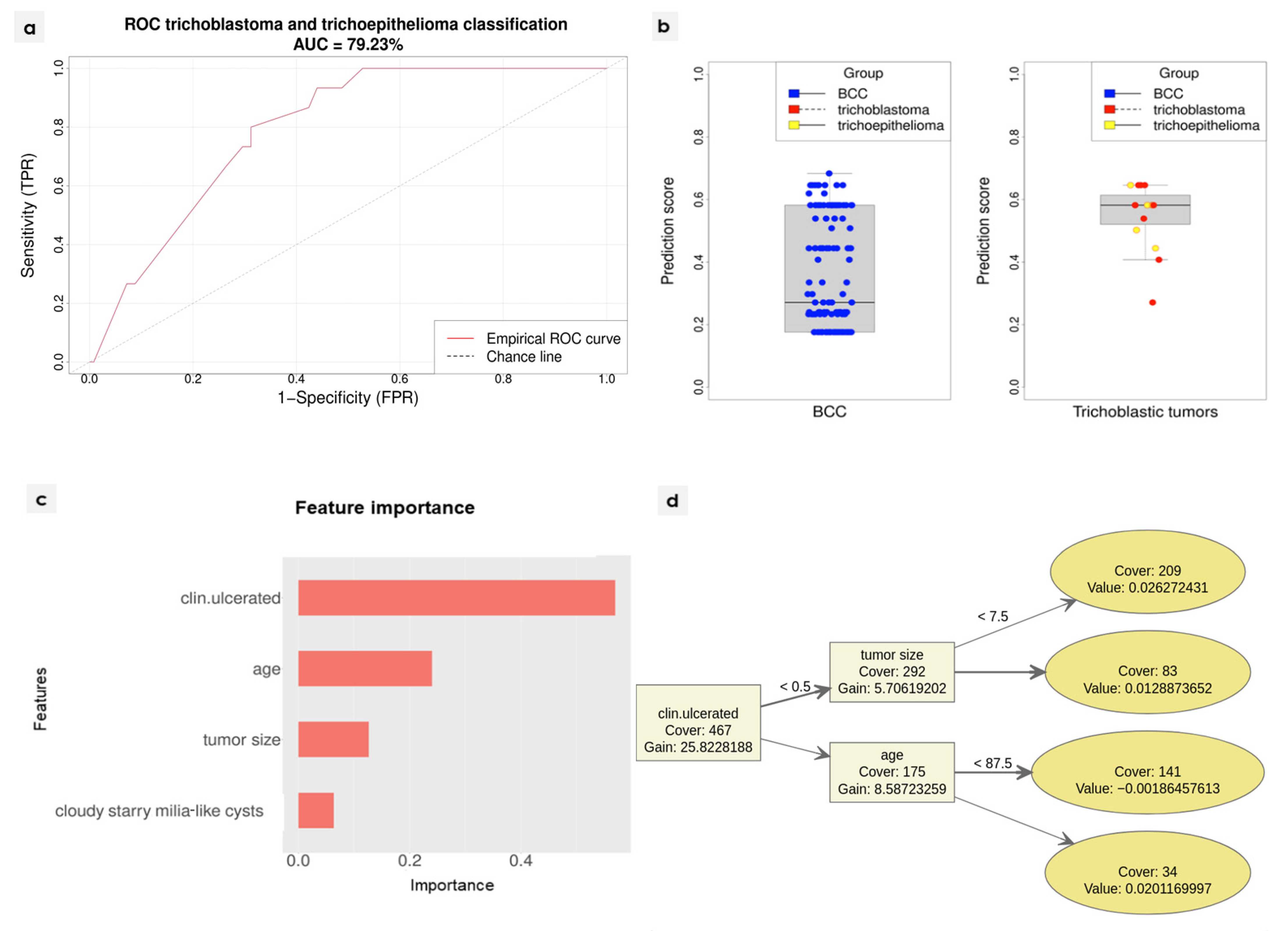
2.2. Diagnostic Algorithm Development
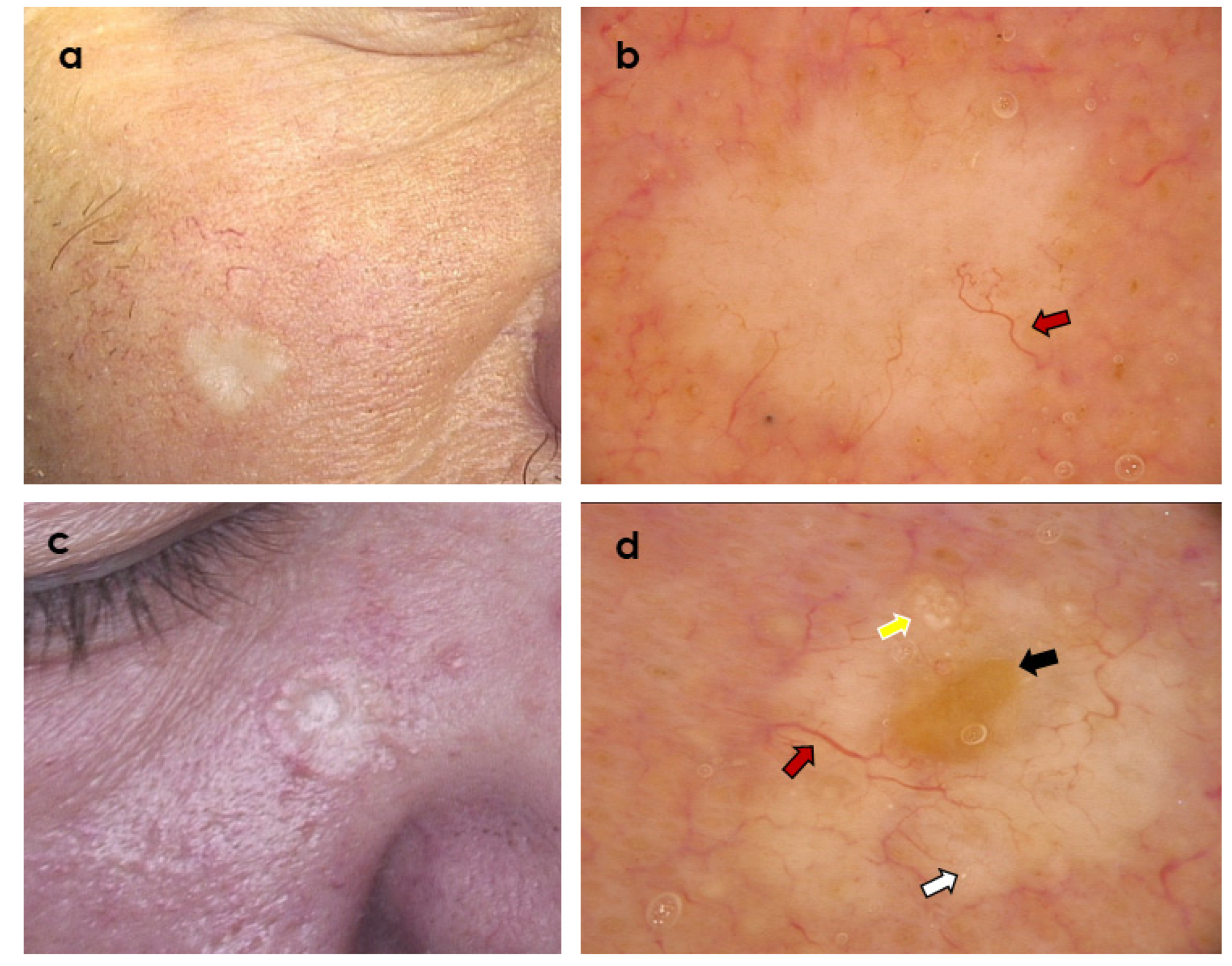
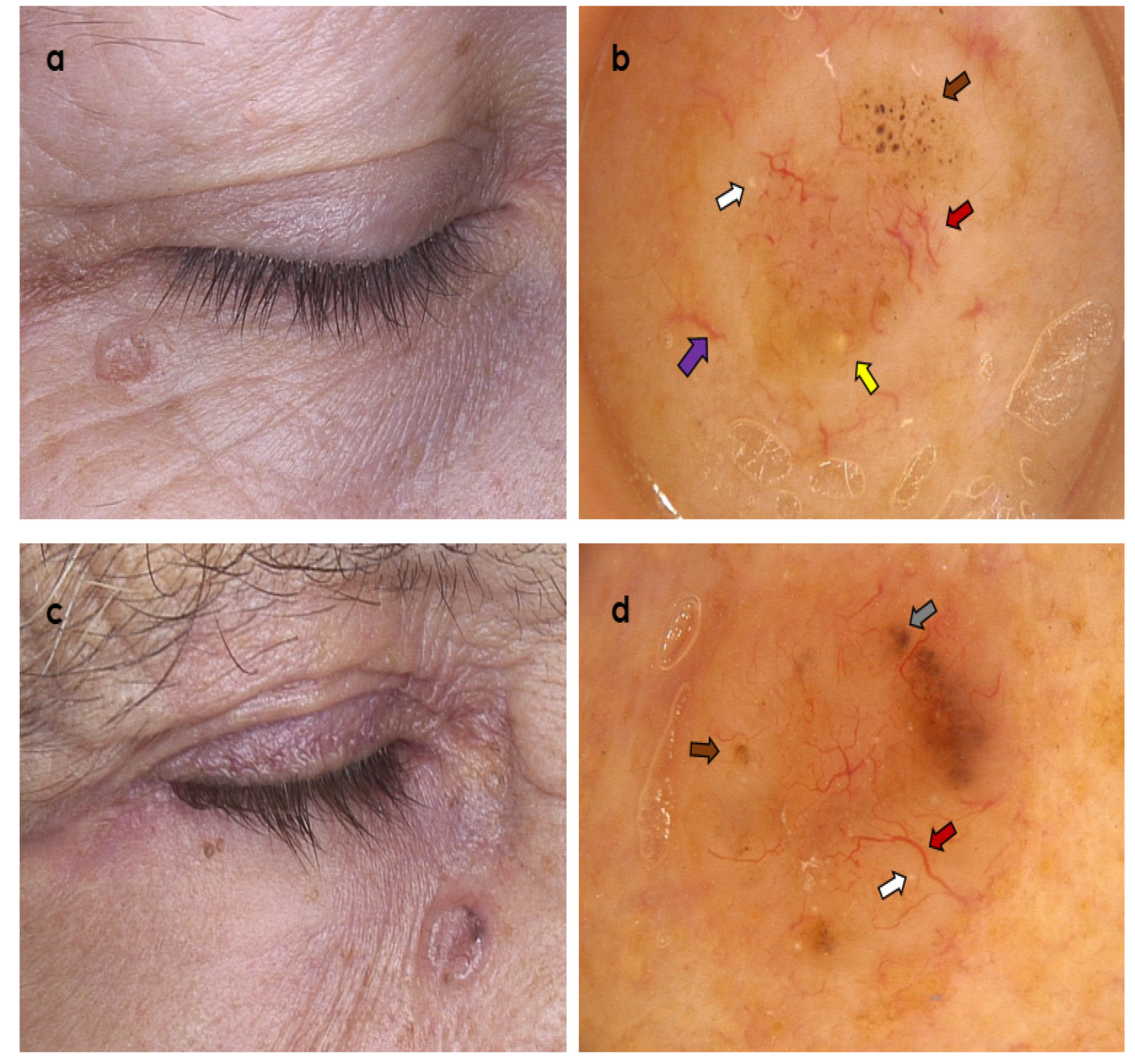
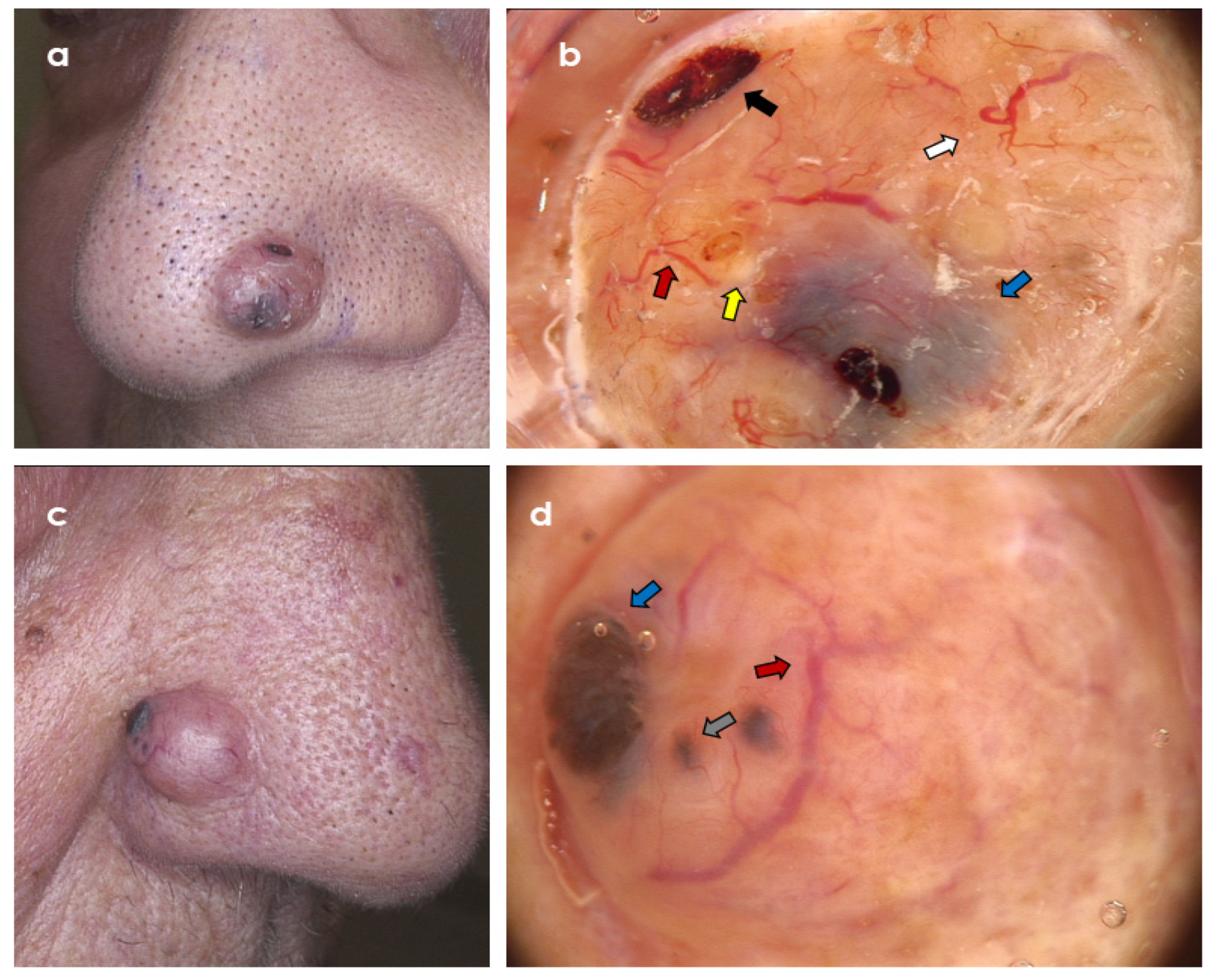
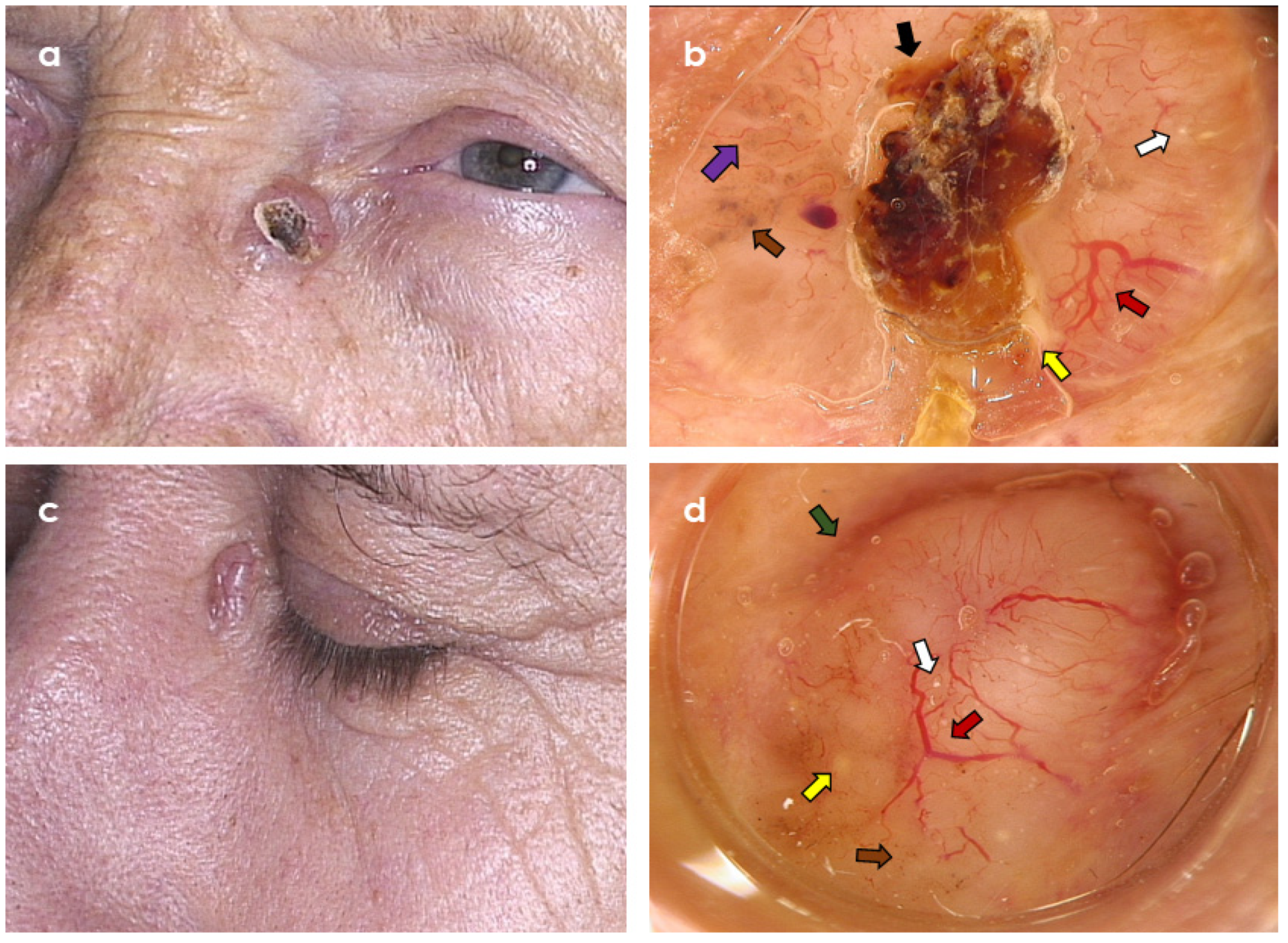
3. Results
| Clinical/Dermoscopic Variable | BCC n = 502 | Trichoblastic Tumours n = 61 | p | Concordance between Both Readers: Positive Agreement, %/Presence According to Both Readers, % | Concordance between Both Readers: Cohen Kappa (95% CI) 1 |
|---|---|---|---|---|---|
| Clinical variables | |||||
| Mean (years) | 71.4 | 64.4 | - | ||
| Median (years) (age range) | 73 (12–95) | 68 (9–94) | 0.01523 | - | |
| Females/males | 261 F/241 M (52.0% F) | 27 F/34 M (44.3% F) | 0.279 2 | - | |
| Tumour size—mean (median) (mm) 4 | 10.998 (9) | 8.197 (7) | 0.0013 | - | |
| Tumour morphology—flat | 96 (19.12%) | 9 (14.75%) | 0.1263 2 | - | |
| Tumour morphology—elevated | 406 (80.88%) | 52 (85.25 %) | 0.1263 2 | - | |
| Clinically visible tumour ulceration | 298 (59.4%) | 12 (19.7%) | <0.0012 | - | |
| Non-pigmented tumour | 437 (87.05%) | 47 (77.05%) | 0.0675 2 | 98.97–99.11% | 0.964 (0.933–0.995) |
| Pigmented structures within <25% tumour surface | 19 (3.78%) | 2 (3.28%) | 0.6888 2 | 72–98.76% | 0.831 (0.708–0.954) |
| Pigmented structures within 25–50% tumour surface | 27 (5.38%) | 6 (9.84%) | 0.2544 2 | 68.97–98.4% | 0.808 (0.685–0.93) |
| Pigmented structures within >50% tumour surface | 27 (5.38%) | 6 (9.84%) | 0.2544 2 | 86.84–99.11% | 0.925 (0.859–0.99) |
| Location—scalp | 21 (4.18%) | 1 (1.64%) | 0.4957 2 | - | |
| Location—face | 384 (76.49%) | 48 (78.69%) | 0.8725 2 | - | |
| Location—chest | 19 (3.78%) | 5 (8.2%) | 0.1663 2 | - | |
| Location—abdomen | 1 (0.2%) | 0 (0%) | 1 2 | - | |
| Location—back | 27 (5.38%) | 3 (4.92%) | 1 2 | - | |
| Location—upper limb | 19 (3.78%) | 1 (1.64%) | 0.7122 2 | - | |
| Location—lower limb | 12 (2.39%) | 0 (0%) | 0.7323 2 | - | |
| Location—limb | 31 (6.18%) | 1 (1.64%) | 0.2373 2 | ||
| Location—corpus | 47 (9.36%) | 8 (13.11%) | 0.3599 2 | ||
| Dermoscopic variables | |||||
| White-red/pink structureless areas | 424 (84.46%) | 39 (63.93%) | 0.0006 2 | 95.37–96.09% | 0.865 (0.81–0.92) |
| Monomorphic vessels 5 | 246 (49%) | 31 (50.82%) | 0.2895 2 | 100–97.51% | 1 (1–1) |
| Polymorphic vessels (2 or more vessel types) 5 | 244 (48.61%) | 29 (47.54%) | 0.2895 2 | 88.89–92.18% | 0.891 (0.853–0.929) |
| Branched (arborizing) vessels | 396 (78.88%) | 49 (80.33%) | 0.6951 2 | 97.32–97.87% | 0.937 (0.902–0.972) |
| Superficial fine telangiectasia | 294 (58.57%) | 37 (60.66%) | 0.2921 2 | 90.91–94.49% | 0.887 (0.849–0.926) |
| Dotted vessels | 33 (6.57%) | 5 (8.2%) | 0.7478 2 | 82.93–98.76% | 0.9 (0.827–0.973) |
| Coiled vessels | 34 (6.77%) | 4 (6.56%) | 1 2 | 81.08–98.76% | 0.889 (0.808–0.97) |
| Looped vessels | 42 (8.37%) | 1 (1.64%) | 0.25 2 | 88.37–99.11 % | 0.933 (0.876–0.991) |
| Helical vessels | 0 (0%) | 0 (0%) | - | 100–100% | 1 (1–1) |
| Curved vessels | 7 (1.39%) | 0 (0%) | 1 2 | 100–100% | 1 (1–1) |
| Small erosions—mean (median) | 1.034 (0) | 0.984 (0) | 0.984 3 | - | 0.862 (0.821–0.904) |
| Ulceration | 262 (52.2%) | 9 (14.8%) | <0.00012 | 93.97–96.98% | 0.94 (0.911–0.968) |
| In-focus blue-grey dots | 12 (2.39%) | 4 (6.56%) | 0.1083 2 | 61.9–98.58% | 0.757 (0.595–0.92) |
| Blue/grey globules | 61 (12.15%) | 10 (16.39%) | 0.5066 2 | 76.54–96.63% | 0.848 (0.781–0.915) |
| Large blue ovoid nests | 43 (8.57%) | 6 (9.84%) | 0.7944 2 | 81.82–98.22% | 0.89 (0.823–0.957) |
| Brown dots | 57 (11.4%) | 20 (32.8%) | 0.00012 | 81.93–97.34% | 0.885 (0.828–0.942) |
| Brown globules | 41 (8.2%) | 18 (29.5%) | <0.00012 | 84.62–98.22% | 0.907 (0.85–0.964) |
| Maple-leaf like structures | 5 (1%) | 3 (4.92%) | 0.01362 | 63.64–99.29% | 0.774 (0.56–0.989) |
| Spoke-wheel-like structures | 3 (0.6%) | 0 (0%) | 1 2 | 75–99.82% | 0.856 (0.578–1) |
| Concentric structures | 5 (1%) | 0 (0%) | 1 2 | 55.56–99.29% | 0.711 (0.44–0.982) |
| Starry milia-like cysts | 80 (15.94%) | 11 (18.03%) | 0.0839 2 | 80.58–96.45% | 0.871 (0.816–0.926) |
| Cloudy milia-like cysts | 75 (14.94%) | 11 (18.03%) | 0.2731 2 | 84.27–97.51% | 0.9 (0.849–0.952) |
| Cloudy/starry milia-like cysts | 57 (11.6%) | 16 (26.2%) | 0.00312 | 89.19–98.58% | 0.935 (0.89–0.98) |
| Yellow globules/light yellow globules | 10 (1.99%)/36 (7.2%) | 1 (1.64%)/10 (16.4%) | 0.7202 2/0.033 2 | 100–100%/82.35–98.4% | 1 (1–1)/0.895 (0.826–0.963) |
| Multiple aggregated yellow-white globules (MAY-globules) | 15 (2.99%) | 5 (8.2%) | 0.065 2 | 73.08–98.76% | 0.838 (0.72–0.956) |
| Brown structureless areas | 65 (12.95%) | 7 (11.48%) | 0.8064 2 | 93.51–99.11% | 0.961 (0.928–0.995) |
3.1. Clinical Variables
3.2. Dermoscopic Variables
3.3. Diagnostic Algorithm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lallas, A.; Apalla, Z.; Argenziano, G.; Longo, C.; Moscarella, E.; Specchio, F.; Raucci, M.; Zalaudek, I. The dermatoscopic universe of basal cell carcinoma. Dermatol. Pract. Concept. 2014, 4, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wozniak-Rito, A.; Zalaudek, I.; Rudnicka, L. Dermoscopy of basal cell carcinoma. Clin. Exp. Dermatol. 2018, 43, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordov, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Khelifa, E.; Masouyé, I.; Kaya, G.; Le Gal, F.A. Dermoscopy of desmoplastic trichoepithelioma reveals other criteria to distinguish it from basal cell carcinoma. Dermatology 2013, 226, 101–104. [Google Scholar] [CrossRef]
- Pitarch, G.; Botella-Estrada, R. Dermoscopic findings in trichoblastoma. Actas Dermosifiliogr. 2015, 106, 45–48. [Google Scholar] [CrossRef]
- Ghigliotti, G.; De Col, E.; Parodi, A.; Bombonato, C.; Argenziano, G. Trichoblastoma: Is a clinical or dermoscopic diagnosis possible? J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1978–1980. [Google Scholar] [CrossRef]
- Ardigo, M.; Zieff, J.; Scope, A.; Gill, M.; Spencer, P.; Deng, L.; Marghoob, A.A. Dermoscopic and reflectance confocal microscope findings of trichoepithelioma. Dermatology 2007, 215, 354–358. [Google Scholar] [CrossRef]
- Patel, P.; Nawrocki, S.; Hinther, K.; Khachemoune, A. Trichoblastomas Mimicking Basal Cell Carcinoma: The Importance of Identification and Differentiation. Cureus 2020, 12, e8272. [Google Scholar] [CrossRef]
- Kolm, I.; Kastnerova, L.; Konstantinova, A.M.; Michal, M.; Kazakov, D.V. Trichoblastoma: A Consecutive Series of 349 Sporadic Cases Analyzed by Ackerman Subtypes. Am. J. Dermatopathol. 2021, 43, 887–897. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Hu, S.C.; Lin, C.L.; Tsai, K.B.; Chen, G.S.; Chin, Y.Y.; Lin, C.T.; Lan, C.C.E. Trichoblastoma-like tumour with follicular, matrical and sebaceous differentiation: Involvement of hair follicle stem? Acta Derm. Venereol. 2010, 90, 436–437. [Google Scholar]
- Costello, C.M.; Han, M.Y.; Severson, K.J.; Maly, C.J.; Yonan, Y.; Nelson, S.A.; Swanson, D.L.; Mangold, A.R. Dermoscopic characteristics of microcystic adnexal carcinoma, desmoplastic trichoepithelioma, and morpheaform basal cell carcinoma. Int. J. Dermatol. 2021, 60, e83–e84. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, C.; Arginelli, F.; Bassoli, S.; Magnoni, C.; Seidenari, S. Dermoscopic yellow structures in basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 651–654. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Liopyris, K.; Rishpon, A.; Marghoob, N.G.; Cordova, M.; Dusza, S.W.; Sahu, A.; Kose, K.; Oliviero, M.; Rabinovitz, H.; et al. Association of Multiple Aggregated Yellow-White Globules With Nonpigmented Basal Cell Carcinoma. JAMA Dermatol. 2020, 156, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Pampena, R.; Peccerillo, F.; Marghoob, N.G.; Piana, S.; Longo, C. Peritumoural clefting as a key feature in differentiating basal cell carcinoma from trichoblastoma through in vivo reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e201–e203. [Google Scholar] [CrossRef]
- Rosales-Duran, S.; Ricaurte-Jiménez, M.; Ferreira, P.S.; Sangueza, M.; Lourenço, S.V.; Nico, M.M.S. In Vivo Reflectance Confocal Microscopy of Adnexal Tumors: Evaluation of Trichoepithelioma, Sebaceoma, and Fibrofolliculoma. Am. J. Dermatopathol. 2022, 44, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Zhang, S.; Li, Z.; Zhang, S.; Li, J.; Wang, G. Trichoepithelioma: Reflectance confocal microscopy features and dermoscopic features. Ski. Res. Technol. 2021, 27, 283–284. [Google Scholar] [CrossRef]
- Cüre, K.; Kocatürk, E.; Koku Aksu, A.E.; Yüksel, T.; Leblebici, C.; Özekinci, S.; Özkaya, Ö.; Değirmentepe, E. Brooke-Spiegler syndrome: Focus on reflectance confocal microscopy findings of trichoepithelioma and flat cylindroma. Clin. Exp. Dermatol. 2017, 42, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Arzberger, E.; Zalaudek, I.; Hofmann-Wellenhof, R. Desmoplastic trichoepithelioma and melanocytic nevus: Dermoscopic and reflectance confocal microscopy presentation of a rare collision tumor. J. Am. Acad. Dermatol. 2015, 72 (Suppl. S1), S13–S15. [Google Scholar] [CrossRef][Green Version]
- Fraga-Braghiroli, N.A.; Stephens, A.; Grossman, D.; Rabinovitz, H.; Castro, R.; Scope, A. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: A case series. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 933–942. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Diagnostic criteria of common benign and malignant neoplasms, dermoscopic and histopathologic correlates of key confocal criteria, and diagnostic algorithms. J. Am. Acad. Dermatol. 2021, 84, 17–31. [Google Scholar] [CrossRef]
- Oliveira, A.; Arzberger, E.; Zalaudek, I.; Hofmann-Wellenhof, R. Imaging of desmoplastic trichoepithelioma by high-definition optical coherence tomography. Dermatol. Surg. 2015, 41, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Niculet, E.; Craescu, M.; Rebegea, L.; Bobeica, C.; Nastase, F.; Lupasteanu, G.; Stan, D.J.; Chioncel, V.; Anghel, L.; Lungu, M.; et al. Basal cell carcinoma: Comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (Review). Exp. Ther. Med. 2022, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared with histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Guitera, P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br. J. Dermatol. 2015, 173, 1371–1380. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Aleissa, S.; Cordova, M.; Liopyris, K.; Sahu, A.; Rossi, A.M.; Lee, E.H.; Nehal, K.S. Management of complex head-and-neck basal cell carcinomas using a combined reflectance confocal microscopy/optical coherence tomography: A descriptive study. Arch. Dermatol. Res. 2021, 313, 193–200. [Google Scholar] [CrossRef]
- Halip, I.A.; Vâţă, D.; Statescu, L.; Salahoru, P.; Patraşcu, A.I.; Olinici, D.T.; Tarcau, B.; Popescu, I.-A.; Mocanu, M.; Constantin, A.-M.; et al. Assessment of Basal Cell Carcinoma Using Dermoscopy and High Frequency Ultrasound Examination. Diagnostics 2022, 12, 735. [Google Scholar] [CrossRef]
- Piłat, P.; Borzęcki, A.; Jazienicki, M.; Gerkowicz, A.; Krasowska, D. High-frequency ultrasound in the diagnosis of selected non-melanoma skin nodular lesions. Postepy Dermatol. Alergol. 2019, 36, 572–580. [Google Scholar] [CrossRef]
- Laverde-Saad, A.; Simard, A.; Nassim, D.; Jfri, A.; Alajmi, A.; O’Brien, E.; Wortsman, X. Performance of Ultrasound for Identifying Morphological Characteristics and Thickness of Cutaneous Basal Cell Carcinoma: A Systematic Review. Dermatology 2022, 238, 692–710. [Google Scholar] [CrossRef]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Giordano, C.N.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Contemporary approaches to diagnosis, treatment, and prevention. J. Am. Acad. Dermatol. 2019, 80, 321–339. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławińska, M.; Płaszczyńska, A.; Lakomy, J.; Pastuszak, K.; Biernat, W.; Sikorska, M.; Nowicki, R.J.; Sobjanek, M. Significance of Dermoscopy in Association with Clinical Features in Differentiation of Basal Cell Carcinoma and Benign Trichoblastic Tumours. Cancers 2022, 14, 3964. https://doi.org/10.3390/cancers14163964
Sławińska M, Płaszczyńska A, Lakomy J, Pastuszak K, Biernat W, Sikorska M, Nowicki RJ, Sobjanek M. Significance of Dermoscopy in Association with Clinical Features in Differentiation of Basal Cell Carcinoma and Benign Trichoblastic Tumours. Cancers. 2022; 14(16):3964. https://doi.org/10.3390/cancers14163964
Chicago/Turabian StyleSławińska, Martyna, Anna Płaszczyńska, Joanna Lakomy, Krzysztof Pastuszak, Wojciech Biernat, Monika Sikorska, Roman J. Nowicki, and Michał Sobjanek. 2022. "Significance of Dermoscopy in Association with Clinical Features in Differentiation of Basal Cell Carcinoma and Benign Trichoblastic Tumours" Cancers 14, no. 16: 3964. https://doi.org/10.3390/cancers14163964
APA StyleSławińska, M., Płaszczyńska, A., Lakomy, J., Pastuszak, K., Biernat, W., Sikorska, M., Nowicki, R. J., & Sobjanek, M. (2022). Significance of Dermoscopy in Association with Clinical Features in Differentiation of Basal Cell Carcinoma and Benign Trichoblastic Tumours. Cancers, 14(16), 3964. https://doi.org/10.3390/cancers14163964







