Adverse Prognostic Impact of Diagnostic Ureterorenoscopy in a Subset of Patients with High-Risk Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Survival Analyses in the Entire Cohort
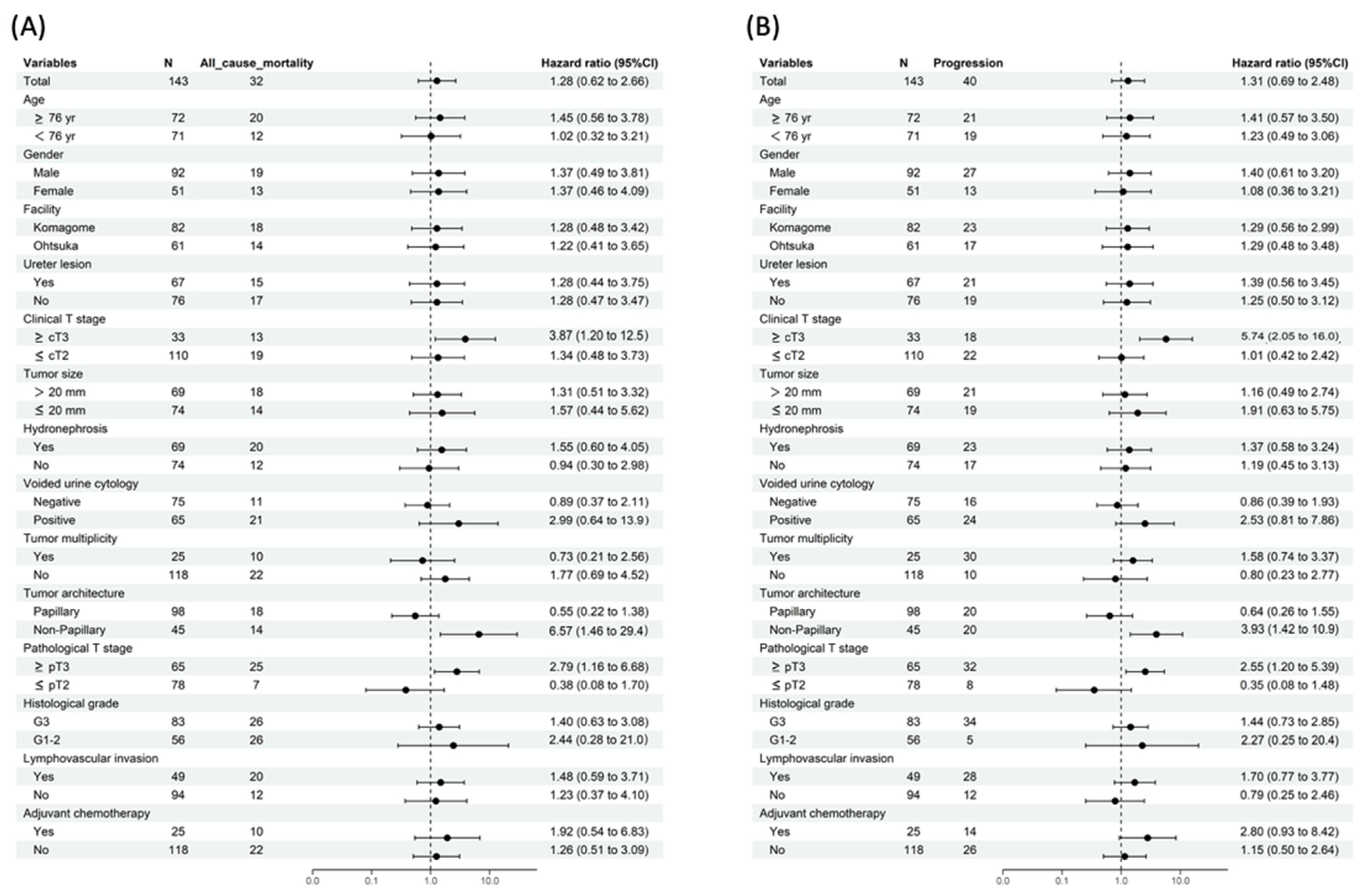
3.3. Subgroup Survival Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EAU Guidelines on Upper Urinary Tract Urothelial Carcinoma. Available online: https://uroweb.org/guidelines/upper-urinary-tract-urothelial-cell-carcinoma (accessed on 10 July 2022).
- Guo, R.Q.; Hong, P.; Xiong, G.Y.; Zhang, L.; Fang, D.; Li, X.S.; Zhang, K.; Zhou, L.Q. Impact of ureteroscopy before radical nephroureterectomy for upper tract urothelial carcinomas on oncological outcomes: A meta-analysis. BJU Int. 2018, 121, 184–193. [Google Scholar] [CrossRef]
- Rojas, C.P.; Castle, S.M.; Llanos, C.A.; Santos Cortes, J.A.; Bird, V.; Rodriguez, S.; Reis, I.M.; Zhao, W.; Gomez-Fernandez, C.; Leveillee, R.J.; et al. Low biopsy volume in ureteroscopy does not affect tumor biopsy grading in upper tract urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1696–1700. [Google Scholar] [CrossRef]
- Seisen, T.; Peyronnet, B.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Bohle, A.; Burger, M.; Comperat, E.M.; Cowan, N.C.; et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur. Urol. 2016, 70, 1052–1068. [Google Scholar] [CrossRef]
- Nowak, L.; Krajewski, W.; Chorbinska, J.; Kielb, P.; Sut, M.; Moschini, M.; Teoh, J.Y.C.; Mori, K.; Del Giudice, F.; Laukhtina, E.; et al. The Impact of Diagnostic Ureteroscopy Prior to Radical Nephroureterectomy on Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma: A Comprehensive Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4197. [Google Scholar] [CrossRef]
- Sharma, V.; Miest, T.S.; Juvet, T.S.; Toussi, A.; Packiam, V.; Chamie, K.; Matin, S.F.; Boorjian, S.A.; Thompson, R.H.; Frank, I.; et al. The Impact of Upper Tract Urothelial Carcinoma Diagnostic Modality on Intravesical Recurrence after Radical Nephroureterectomy: A Single Institution Series and Updated Meta-Analysis. J. Urol. 2021, 206, 558–567. [Google Scholar] [CrossRef]
- Sankin, A.; Tin, A.L.; Mano, R.; Chevinsky, M.; Jakubowski, C.; Sfakianos, J.P.; Cha, E.K.; Yee, A.; Friedman, F.M.; Sjoberg, D.D.; et al. Impact of Ureteroscopy Before Nephroureterectomy for Upper Tract Urothelial Carcinoma on Oncologic Outcomes. Urology 2016, 94, 148–153. [Google Scholar] [CrossRef]
- Tokas, T.; Herrmann, T.R.W.; Skolarikos, A.; Nagele, U.; Training and Research in Urological Surgery and Technology (T.R.U.S.T.) Group. Pressure matters: Intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J. Urol. 2019, 37, 125–131. [Google Scholar] [CrossRef]
- Lim, D.J.; Shattuck, M.C.; Cook, W.A. Pyelovenous lymphatic migration of transitional cell carcinoma following flexible ureterorenoscopy. J. Urol. 1993, 149, 109–111. [Google Scholar] [CrossRef]
- Barkan, G.A.; Wojcik, E.M.; Nayar, R.; Savic-Prince, S.; Quek, M.L.; Kurtycz, D.F.; Rosenthal, D.L. The Paris System for Reporting Urinary Cytology: The Quest to Develop a Standardized Terminology. Acta Cytol. 2016, 60, 185–197. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Marchioni, M.; Primiceri, G.; Cindolo, L.; Hampton, L.J.; Grob, M.B.; Guruli, G.; Schips, L.; Shariat, S.F.; Autorino, R. Impact of diagnostic ureteroscopy on intravesical recurrence in patients undergoing radical nephroureterectomy for upper tract urothelial cancer: A systematic review and meta-analysis. BJU Int. 2017, 120, 313–319. [Google Scholar] [CrossRef]
- Luo, H.L.; Kang, C.H.; Chen, Y.T.; Chuang, Y.C.; Lee, W.C.; Cheng, Y.T.; Chiang, P.H. Diagnostic ureteroscopy independently correlates with intravesical recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma. Ann. Surg. Oncol. 2013, 20, 3121–3126. [Google Scholar] [CrossRef]
- Sung, H.H.; Jeon, H.G.; Han, D.H.; Jeong, B.C.; Seo, S.I.; Lee, H.M.; Choi, H.Y.; Jeon, S.S. Diagnostic Ureterorenoscopy Is Associated with Increased Intravesical Recurrence following Radical Nephroureterectomy in Upper Tract Urothelial Carcinoma. PLoS ONE 2015, 10, e0139976. [Google Scholar] [CrossRef]
- Chung, Y.; Lee, D.H.; Lee, M.; Kim, H.; Lee, S.; Hong, S.K.; Byun, S.S.; Lee, S.E.; Oh, J.J. Impact of diagnostic ureteroscopy before radical nephroureterectomy on intravesical recurrence in patients with upper tract urothelial cancer. Investig. Clin. Urol. 2020, 61, 158–165. [Google Scholar] [CrossRef]
- Hendin, B.N.; Streem, S.B.; Levin, H.S.; Klein, E.A.; Novick, A.C. Impact of diagnostic ureteroscopy on long-term survival in patients with upper tract transitional cell carcinoma. J. Urol. 1999, 161, 783–785. [Google Scholar] [CrossRef]
- Ishikawa, S.; Abe, T.; Shinohara, N.; Harabayashi, T.; Sazawa, A.; Maruyama, S.; Kubota, K.; Matsuno, Y.; Osawa, T.; Shinno, Y.; et al. Impact of diagnostic ureteroscopy on intravesical recurrence and survival in patients with urothelial carcinoma of the upper urinary tract. J. Urol. 2010, 184, 883–887. [Google Scholar] [CrossRef]
- Nison, L.; Roupret, M.; Bozzini, G.; Ouzzane, A.; Audenet, F.; Pignot, G.; Ruffion, A.; Cornu, J.N.; Hurel, S.; Valeri, A.; et al. The oncologic impact of a delay between diagnosis and radical nephroureterectomy due to diagnostic ureteroscopy in upper urinary tract urothelial carcinomas: Results from a large collaborative database. World J. Urol. 2013, 31, 69–76. [Google Scholar] [CrossRef]
- Gurbuz, C.; Youssef, R.F.; Shariat, S.F.; Lotan, Y.; Wood, C.G.; Sagalowsky, A.I.; Zigeuner, R.; Kikuchi, E.; Weizer, A.; Raman, J.D.; et al. The impact of previous ureteroscopic tumor ablation on oncologic outcomes after radical nephrouretectomy for upper urinary tract urothelial carcinoma. J. Endourol. 2011, 25, 775–779. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yeh, H.C.; Wu, W.J.; He, J.S.; Huang, C.N.; Ke, H.L.; Li, W.M.; Li, C.F.; Li, C.C. The diagnostic ureteroscopy before radical nephroureterectomy in upper urinary tract urothelial carcinoma is not associated with higher intravesical recurrence. World J. Surg. Oncol. 2018, 16, 135. [Google Scholar] [CrossRef]
- Fukushima, H.; Koga, F.; Kawakami, S.; Fujii, Y.; Yoshida, S.; Ratovitski, E.; Trink, B.; Kihara, K. Loss of DeltaNp63alpha promotes invasion of urothelial carcinomas via N-cadherin/Src homology and collagen/extracellular signal-regulated kinase pathway. Cancer Res. 2009, 69, 9263–9270. [Google Scholar] [CrossRef]
- Bolenz, C.; Fernández, M.I.; Trojan, L.; Hoffmann, K.; Herrmann, E.; Steidler, A.; Weiss, C.; Ströbel, P.; Alken, P.; Michel, M.S. Lymphangiogenesis occurs in upper tract urothelial carcinoma and correlates with lymphatic tumour dissemination and poor prognosis. BJU Int. 2009, 103, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sagara, Y.; Watanabe, S.; Asai, A.; Matsuo, T.; Ohba, K.; Hayashi, T.; Sakai, H. CD105 is a more appropriate marker for evaluating angiogenesis in urothelial cancer of the upper urinary tract than CD31 or CD34. Virchows Arch. 2013, 463, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Mostafaei, H.; Enikeev, D.V.; Lysenko, I.; Quhal, F.; Kimura, S.; Karakiewicz, P.I.; Egawa, S.; Shariat, S.F. Differential Effect of Sex on Outcomes after Radical Surgery for Upper Tract and Bladder Urothelial Carcinoma: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Wu, W.J.; Lee, H.Y.; Lin, C.H.; Yue, C.T.; Jiang, Y.H.; Lee, Y.K.; Huang, K.H.; Tsai, Y.C. Impact of Pathology Review in Adverse Histological Characteristics and pT Stages of Upper Tract Urothelial Cancer in a Multicenter Study. Front. Oncol. 2021, 11, 757359. [Google Scholar] [CrossRef] [PubMed]

| Variables | N (%) | p Value | ||
|---|---|---|---|---|
| Total | URS+ | URS− | ||
| Total | 143 (100) | 79 (100) | 64 (100) | |
| Age * | 76 (69–79) | 76 (70–79) | 74 (68–79.75) | 0.56 |
| Gender | 0.036 | |||
| Male | 92 (64.3) | 57 (72.2) | 35 (54.7) | |
| Female | 51 (35.7) | 22 (27.8) | 29 (45.3) | |
| ECOG-PS | 0.41 | |||
| 0 | 137 (95.8) | 77 (97.5) | 60 (93.8) | |
| 1 | 6 (4.2) | 2 (2.5) | 4 (6.3) | |
| Body mass index * | 23.1 (21.2–25) | 22.9 (20.5–24.6) | 23.3 (22–26.1) | 0.052 |
| Smoking history, pack year * | 20 (0–47.7) | 21 (0–43.8) | 15.5 (0–50.8) | 0.94 |
| History of bladder cancer | 0.49 | |||
| Concurrent | 16 (11.2) | 7 (8.9) | 9 (14.1) | |
| Previous | 7(4.9) | 5 (6.3) | 2 (3.1) | |
| Never | 120 (83.9) | 67 (84.8) | 53 (82.8) | |
| Facility | 0.498 | |||
| Komagome | 82 | 43 (54) | 39 (61) | |
| Ohtsuka | 61 | 36 (46) | 25 (39) | |
| Tumor location | 0.61 | |||
| Renal pelvis | 76 (53.1) | 39 (49.4) | 37 (57.8) | |
| Ureter | 61 (42.7) | 36 (45.6) | 25 (39.1) | |
| Both | 6 (4.2) | 4 (5.1) | 2 (3.1) | |
| Clinical T stage | 0.046 | |||
| ≤cT2 | 110 (66.9) | 66 (83.5) | 44 (68.7) | |
| ≥cT3 | 33 (23.1) | 13 (16.5) | 20 (31.3) | |
| Clinical N stage | 0.038 | |||
| N0 | 139 (97.2) | 79 (100) | 60 (93.8) | |
| N1 | 4 (2.8) | 0 (0) | 4 (6.2) | |
| Tumor size (mm) * | 18 (12–25) | 15 (10–22) | 22 (15–30) | <0.001 |
| Hydronephrosis | ||||
| Yes | 69 (48.3) | 40 (50.6) | 29 (45.3) | 0.61 |
| No | 74 (51.7) | 39 (44.4) | 35 (54.7) | |
| Voided urine cytology | 1 | |||
| Positive | 65 (45.5) | 35 (44.3) | 30 (46.9) | |
| Negative | 76 (53.1) | 42 (53.2) | 34 (53.1) | |
| NA | 2 (1.4) | 2 (2.5) | 0 (0) | |
| URS biopsy | <0.001 | |||
| Yes | 61 (42.7) | 61 (77.2) | 0 (0) | |
| No | 82 (57.3) | 28 (22.8) | 64 (100) | |
| Surgical modality | 0.736 | |||
| Open | 63 (44.1) | 36 (45.6) | 27 (42.2) | |
| Minimally invasive | 80 (55.9) | 43 (54.4) | 37 (57.8) | |
| Tumor multiplicity | 1 | |||
| Yes | 25 (17.5) | 14 (17.7) | 11 (17.2) | |
| No | 118 (82.5) | 65 (82.3) | 53 (82.8) | |
| Tumor architecture | 0.59 | |||
| Papillary | 98 (68.5) | 56 (80.9) | 42 (65.6) | |
| Non-papillary | 45 (31.5) | 23 (29.1) | 22 (34.4) | |
| Pathological T stage | 0.30 | |||
| pTis/a/1 | 63 (44.1) | 38 (48.1) | 25 (39.1) | |
| pT2 | 15 (10.5) | 7 (8.9) | 8 (12.5) | |
| pT3 | 61 (42.7) | 32 (40.5) | 29 (45.3) | |
| pT4 | 4 (2.8) | 2 (2.5) | 2 (3.1) | |
| Pathological N stage | 0.41 | |||
| pN0 | 40 (28.0) | 19 (24.1) | 21 (32.8) | |
| pN1 | 6 (4.2) | 2 (2.5) | 4 (6.3) | |
| pNx | 97 (67.8) | 58 (73.4) | 39 (60.9) | |
| Histological grade | 0.30 | |||
| G1 | 8 (5.6) | 4 (5.1) | 4 (6.3) | |
| G2 | 48 (33.6) | 30 (38.0) | 18 (28.1) | |
| G3 | 83 (58.0) | 42 (53.2) | 41 (64.1) | |
| NA | 4 (2.8) | 3 (3.8) | 1 (1.6) | |
| Lymphovascular invasion | 0.60 | |||
| Yes | 49 (34.3) | 29 (36.7) | 20 (31.3) | |
| No | 94 (65.7) | 50 (63.3) | 44 (68.7) | |
| Positive surgical margin | 0.83 | |||
| Yes | 6 (4.2) | 4 (5.1) | 2 (3.1) | |
| No | 136 (95.1) | 74 (93.7) | 62 (96.9) | |
| Indeterminant | 1 (0.7) | 1 (1.3) | 0 (0) | |
| Variant histology | 0.25 | |||
| Yes | 13 (9.1) | 5 (6.3) | 8 (12.5) | |
| No | 130 (90.9) | 74 (93.7) | 56 (87.5) | |
| Platinum-based adjuvant chemotherapy | 0.51 | |||
| Yes | 25 (17.5) | 12 (15.2) | 13 (20.3) | |
| No | 118 (82.5) | 67 (84.8) | 51 (79.7) | |
| All-cause mortality | 32 (22.4) | 21 (26.6) | 11 (17.2) | |
| Progression | 40 (28.0) | 25 (31.6) | 15 (23.4) | |
| Visceral or lymph node metastasis | 35 (24.5) | 23 (29.1) | 12 (18.8) | |
| Local recurrence | 5 (3.5) | 2 (2.5) | 3 (4.7) | |
| Variables | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.05 (1.00–1.10) | 0.06 | 1.02 (0.98–1.05) | 0.44 | ||||
| Gender, male (vs. female) | 0.73 (0.36–1.47) | 0.38 | 1.14 (0.59–2.22) | 0.69 | ||||
| ECOG-PS, 1 (vs. 0) | 4.23 (1.48–12.1) | 0.007 | 2.32 (0.71–7.53) | 0.16 | ||||
| Body mass index | 1.04 (0.96–1.13) | 0.33 | 1.06 (0.99–1.14) | 0.11 | ||||
| Smoking history, pack year | 1.00 (0.99–1.01) | 0.79 | 1.00 (0.99–1.01) | 0.81 | ||||
| Facility, Komagome (vs. Ohtsuka) | 1.04 (0.52–2.09) | 0.92 | 0.99 (0.53–1.85) | 0.97 | ||||
| History of bladder cancer, yes (vs. no) | 1.638 (0.73–3.63) | 0.23 | 1.07 (0.47–2.41) | 0.88 | ||||
| Tumor location, ureter, or both (vs. renal pelvis) | 1.12 (0.56–2.24) | 0.75 | 1.44 (0.77–2.67) | 0.25 | ||||
| Clinical T stage, ≥cT3 (vs. ≤cT2) | 2.98 (1.47–6.07) | 0.003 | 3.97 (2.12–7.44) | <0.001 | ||||
| Tumor size | 1.02 (1.00–1.04) | 0.11 | 1.02 (1.00–1.04) | 0.14 | ||||
| Hydronephrosis, yes (vs. no) | 1.96 (0.96–4.00) | 0.066 | 1.55 (0.83–2.91) | 0.17 | ||||
| Voided urine cytology, positive (vs. negative) | 1.29 (0.64–2.60) | 0.48 | 1.25 (0.67–2.35) | 0.48 | ||||
| URS, yes (vs. no) | 1.28 (0.62–2.66) | 0.50 | 1.31 (0.69–2.48) | 0.41 | ||||
| URS biopsy, yes (vs. no) | 1.08 (0.54–2.16) | 0.83 | 1.19 (0.64–2.22) | 0.58 | ||||
| Tumor multiplicity, yes (vs. no) | 0.53 (0.25–1.12) | 0.095 | 0.65 (0.32–1.32) | 0.23 | ||||
| Tumor architecture, non-papillary (vs. papillary) | 2.01 (1.00–4.04) | 0.051 | 2.61 (1.40–4.86) | 0.003 | ||||
| Pathological T stage, ≥pT3 (vs. ≤pT2) | 5.28 (2.28–12.2) | <0.001 | 5.24 (2.26–12.1) | <0.001 | 6.49 (2.98–14.1) | <0.001 | 3.55 (1.47–8.55) | 0.005 |
| Pathological N stage, pN1 (vs. pN0) | 2.14 (0.47–9.71) | 0.32 | 2.81 (0.79–10.0) | 0.11 | ||||
| Histological grade, G3 (vs. G1–2) | 3.45 (1.42–8.38) | 0.006 | 5.63 (2.20–14.4) | <0.001 | ||||
| Lymphovascular invasion, yes (vs. no) | 4.11 (2.00–8.41) | <0.001 | 2.13 (0.95–4.76) | 0.06 | 6.14 (3.11–12.1) | <0.001 | 3.10 (1.43–6.70) | 0.004 |
| Positive surgical margin, yes (vs. no) | 3.68 (1.11–12.1) | 0.033 | 5.78 (2.24–14.9) | <0.001 | ||||
| Platinum-based adjuvant chemotherapy, yes (vs. no) | 2.51 (1.19–5.32) | 0.016 | 3.53 (1.82–6.85) | <0.001 | ||||
| Variant histology, yes (vs. no) | 2.45 (0.94–6.38) | 0.065 | 3.43 (1.57–7.47) | 0.002 | ||||
| Clinical T Stage | N | ||
|---|---|---|---|
| Total | ≥pT3 | ≤pT2 | |
| Total | 143 | 65 | 78 |
| ≥cT3 | 33 | 29 | 4 |
| ≤cT2 | 110 | 36 | 74 |
| Variables | N (%) | p Value | ||
|---|---|---|---|---|
| Total | URS+ | URS− | ||
| Total | 29 (100) | 16 (100) | 13 (100) | |
| Age * | 77 (69–79) | 75 (66–79) | 78 (70–83) | 0.25 |
| Gender | 0.45 | |||
| Male | 20 (69) | 10 (63) | 10 (77) | |
| Female | 9 (31) | 6 (38) | 3 (23) | |
| ECOG-PS | 0.45 | |||
| 0 | 28 (96.6) | 16 (100) | 12 (92) | |
| 1 | 1 (3.4) | 0 (0) | 1 (8) | |
| Body mass index * | 23.8 (22.3–26.4) | 23.9 (21.1–25.4) | 23.5 (22.4–26.9) | 0.68 |
| Smoking history, pack year * | 18 (0–49) | 0 (0–33) | 30 (5–57) | 0.035 |
| History of bladder cancer | 0.19 | |||
| Previous | 1 (3) | 1 (6) | 0 (0) | |
| Concurrent | 2 (7) | 0 (0) | 2 (15) | |
| No | 26 (90) | 15 (94) | 11 (85) | |
| Facility | 0.25 | |||
| Komagome | 18 (62) | 8 (50) | 10 (77) | |
| Ohtsuka | 11 (38) | 8 (50) | 3 (23) | |
| Tumor location | 0.84 | |||
| Pelvis | 13 (45) | 6 (38) | 7 (54) | |
| Ureter | 15 (52) | 9 (56) | 6 (46) | |
| Both | 1 (3) | 1 (6) | 0 (0) | |
| Clinical T stage | 0.45 | |||
| ≤cT2 | 17 (59) | 8 (50) | 9 (69) | |
| ≥cT3 | 12 (41) | 8 (50) | 4 (31) | |
| Clinical N stage | 0.45 | |||
| cN0 | 28 (97) | 16 (100) | 12 (92) | |
| cN1 | 1 (3) | 0 (0) | 1 (8) | |
| Tumor size * | 15 (10–22) | 14 (10–21) | 17 (11–24) | 0.36 |
| Hydronephrosis | 0.041 | |||
| Yes | 20 (69) | 14 (88) | 6 (46) | |
| No | 9 (31) | 2 (13) | 7 (54) | |
| Voided urine cytology | 1 | |||
| Negative | 12 (41) | 8 (50) | 4 (31) | |
| Positive | 16 (55) | 7 (44) | 9 (69) | |
| NA | 1 (3) | 1 (6) | 0 (0) | |
| URS biopsy | <0.001 | |||
| Yes | 12 (41) | 12 (75) | 0 (0) | |
| No | 17 (59) | 4 (25) | 0 (0) | |
| Surgical modality | 0.45 | |||
| Open | 12 (41) | 8 (50) | 4 (31) | |
| Minimally invasive | 17 (59) | 8 (50) | 9 (69) | |
| Tumor multiplicity | 1 | |||
| Yes | 3 (10) | 2 (13) | 1 (8) | |
| No | 26 (90) | 14 (88) | 12 (92) | |
| Pathological T stage | 1 | |||
| pT3 | 25 (86) | 14 (88) | 11 (85) | |
| pT4 | 4 (14) | 2 (13) | 2 (15) | |
| Pathological N stage | 0.57 | |||
| pN0 | 6 (21) | 4 (25) | 2 (15) | |
| pN1 | 3 (10) | 1 (6) | 2 (15) | |
| pNx | 20 (69) | 11 (69) | 9 (69) | |
| Histological grade | 0.21 | |||
| G2 | 2 (7) | 2 (13) | 0 (0) | |
| G3 | 27 (93) | 14 (88) | 13 (100) | |
| Lymphovascular invasion | 0.27 | |||
| Yes | 17 (59) | 11 (69) | 6 (46) | |
| No | 12 (41) | 5 (31) | 7 (54) | |
| Positive surgical margin | 1 | |||
| Yes | 3 (10) | 2 (13) | 1 (8) | |
| No | 25 (86) | 13 (81) | 12 (92) | |
| Indeterminant | 1 (3) | 1 (6) | 0 (0) | |
| Variant histology | 0.63 | |||
| Yes | 5 (17) | 2 (13) | 3 (23) | |
| No | 24 (83) | 14 (88) | 10 (77) | |
| Platinum-based adjuvant chemotherapy | 1 | |||
| Yes | 14 (48) | 8 (50) | 6 (46) | |
| No | 15 (52) | 8 (50) | 7 (54) | |
| All-cause mortality | 11 (38) | 10 (63) | 1 (8) | |
| Progression | 16 (55) | 12 (75) | 4 (31) | |
| Visceral or lymph node metastasis | 13 (45) | 11 (69) | 2 (15) | |
| Local recurrence | 3 (10) | 1 (6) | 2 (15) | |
| Variables | Overall Survival | Progression-Free Survival | ||||
|---|---|---|---|---|---|---|
| Univariable | Univariable | Multivariable | ||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 0.99 (0.92–1.06) | 0.71 | 0.98 (0.93–1.03) | 0.45 | ||
| Smoking history, pack year | 0.98 (0.95–1.01) | 0.21 | 1.00 (0.98–1.02) | 0.89 | ||
| Tumor location, ureter or both (vs. renal pelvis) | 0.94 (0.28–3.11) | 0.91 | 0.74 (0.28–2.00) | 0.56 | ||
| Clinical T stage, ≥cT3 (vs. ≤cT2) | 1.43 (0.41–4.95) | 0.57 | 2.25 (0.81–6.27) | 0.12 | ||
| Tumor size | 1.02 (0.94–1.11) | 0.67 | 1.03 (0.96–1.1) | 0.41 | ||
| Hydronephrosis, yes (vs. no) | 0 (0–infinity) | 1 | 2.54 (0.72–8.96) | 0.15 | 1.32 (0.32–5.39) | 0.70 |
| Voided urine cytology, positive (vs. negative) | 0.79 (0.22–2.86) | 0.72 | 1.01 (0.36–2.87) | 0.98 | ||
| URS, yes (vs. no) | 15.8 (1.97–126) | 0.009 | 4.30 (1.36–13.7) | 0.013 | 3.83 (1.06–13.9) | 0.041 |
| URS biopsy, yes (vs. no) | 5.23 (1.50–18.2) | 0.009 | 2.04 (0.75–5.54) | 0.16 | ||
| Pathological N stage, N1 (vs. N0) | 1.55 (0.16–15.1) | 0.71 | 1.66 (0.30–9.18) | 0.56 | ||
| Histological grade, G3 (vs. G2) | 0.36 (0.08–1.73) | 0.20 | 0.49 (0.11–2.23) | 0.36 | ||
| Lymphovascular invasion, yes (vs. no) | 2.06 (0.60–7.09) | 0.25 | 2.05 (0.70–5.97) | 0.19 | ||
| Positive surgical margin, yes or indeterminate (vs. no) | 2.86 (0.58–14.0) | 0.19 | 5.36 (1.36–21.2) | 0.016 | ||
| Platinum-based adjuvant chemotherapy, yes (vs. no) | 1.87 (0.56–6.21) | 0.31 | 1.83 (0.68–4.99) | 0.23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonese, I.; Ito, M.; Waseda, Y.; Kobayashi, S.; Toide, M.; Takazawa, R.; Koga, F. Adverse Prognostic Impact of Diagnostic Ureterorenoscopy in a Subset of Patients with High-Risk Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy. Cancers 2022, 14, 3962. https://doi.org/10.3390/cancers14163962
Yonese I, Ito M, Waseda Y, Kobayashi S, Toide M, Takazawa R, Koga F. Adverse Prognostic Impact of Diagnostic Ureterorenoscopy in a Subset of Patients with High-Risk Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy. Cancers. 2022; 14(16):3962. https://doi.org/10.3390/cancers14163962
Chicago/Turabian StyleYonese, Ichiro, Masaya Ito, Yuma Waseda, Shuichiro Kobayashi, Masahiro Toide, Ryoji Takazawa, and Fumitaka Koga. 2022. "Adverse Prognostic Impact of Diagnostic Ureterorenoscopy in a Subset of Patients with High-Risk Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy" Cancers 14, no. 16: 3962. https://doi.org/10.3390/cancers14163962
APA StyleYonese, I., Ito, M., Waseda, Y., Kobayashi, S., Toide, M., Takazawa, R., & Koga, F. (2022). Adverse Prognostic Impact of Diagnostic Ureterorenoscopy in a Subset of Patients with High-Risk Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy. Cancers, 14(16), 3962. https://doi.org/10.3390/cancers14163962






