Potential of a Non-Contrast-Enhanced Abbreviated MRI Screening Protocol (NC-AMRI) in High-Risk Patients under Surveillance for HCC
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
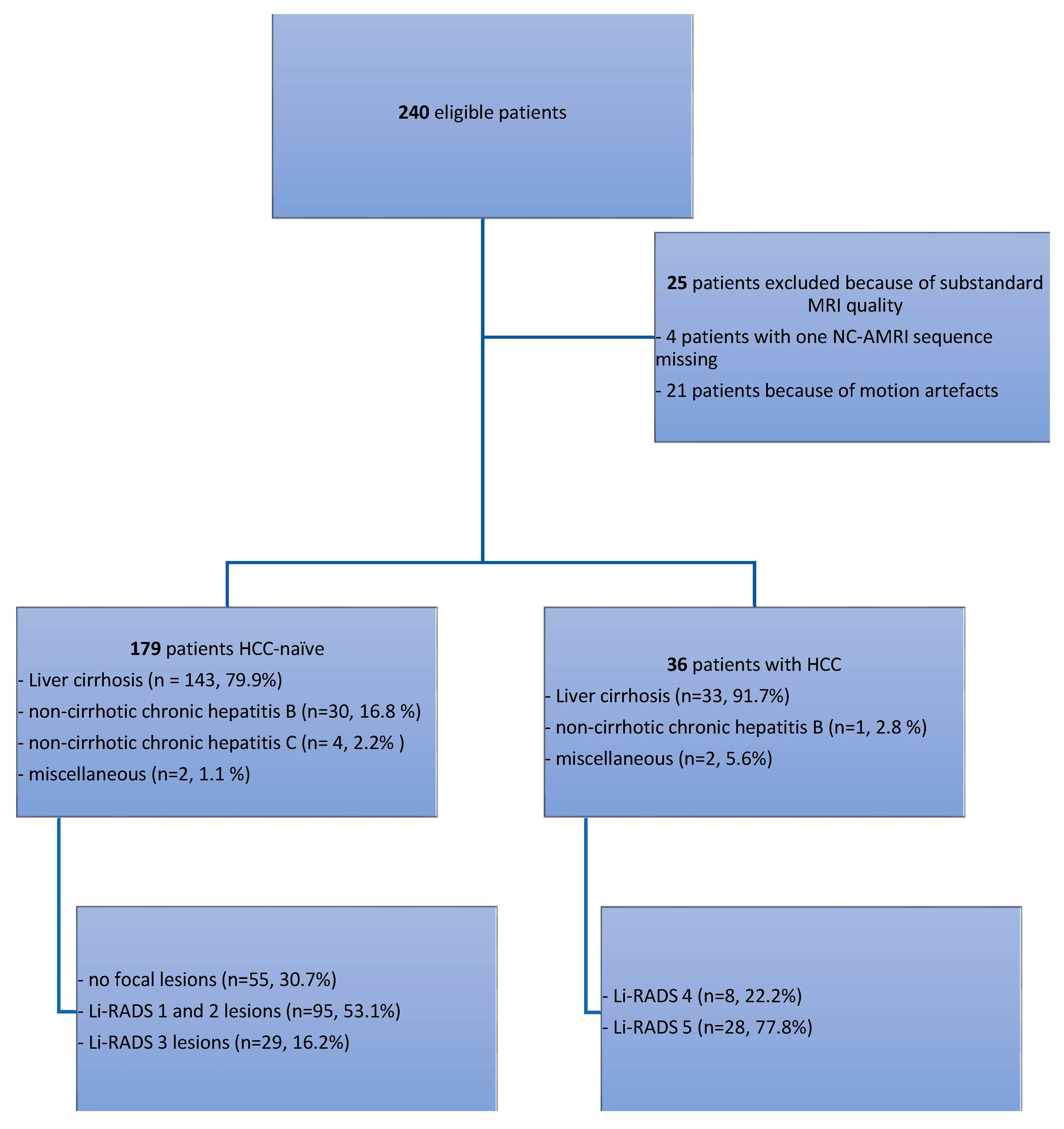
2.1. Patient Selection
2.2. Full MRI Liver Protocol and Non-Contrast-Enhanced Abbreviated MRI Surveillance Protocols
2.3. Image Interpretation of the NC-AMRI Protocol
2.4. Reference Standard
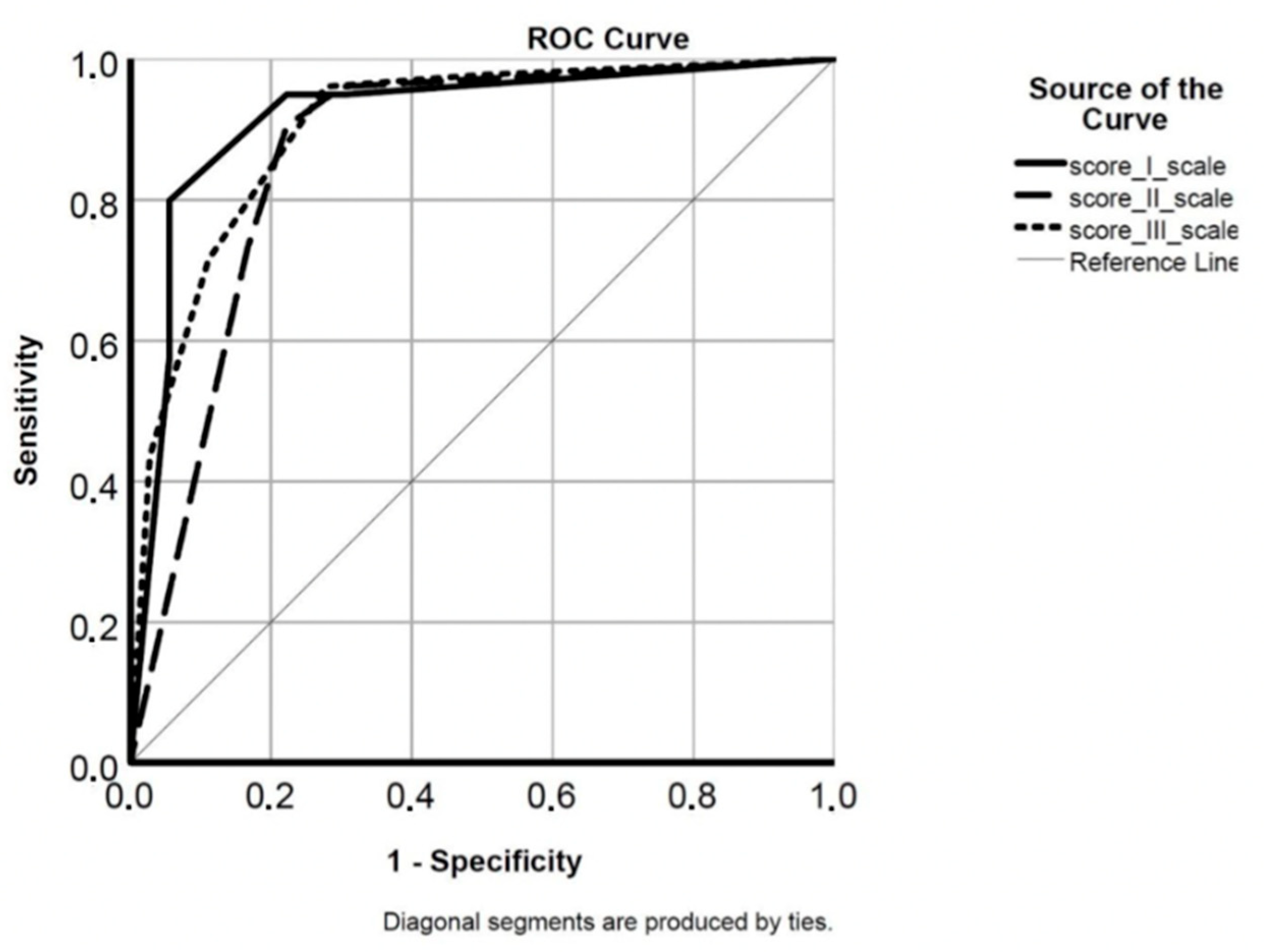
2.5. Data Analysis and Statistical Methods
2.6. Review by Consensus after Data Analysis
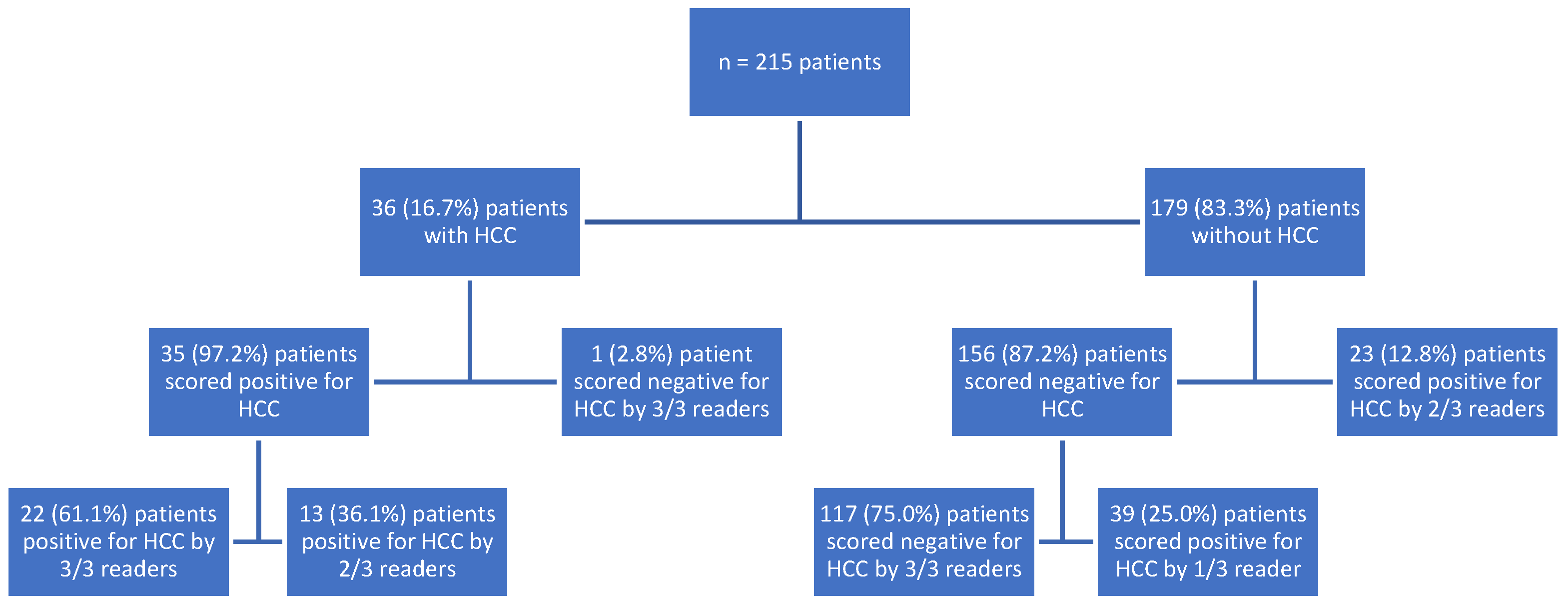
3. Results
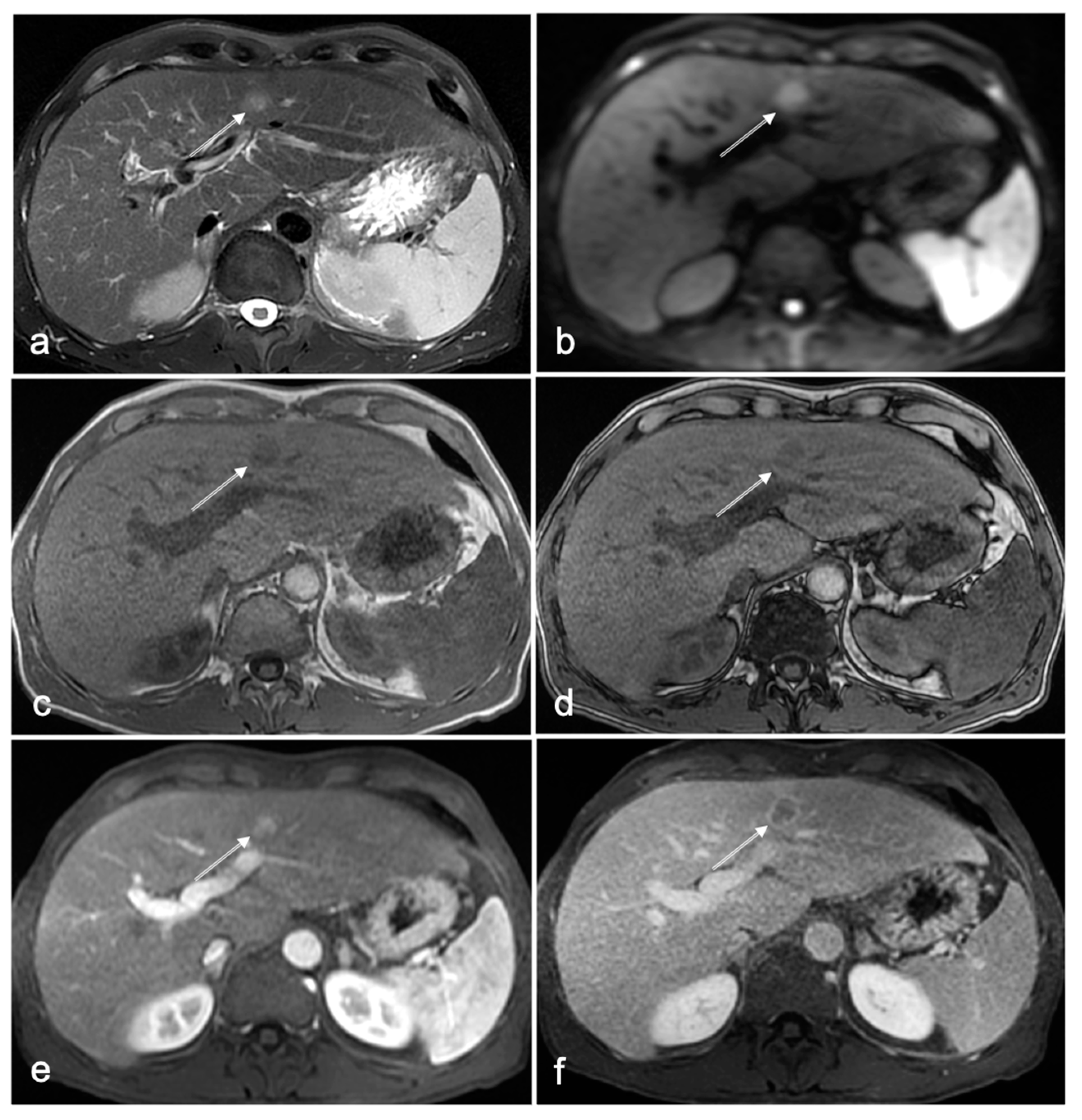
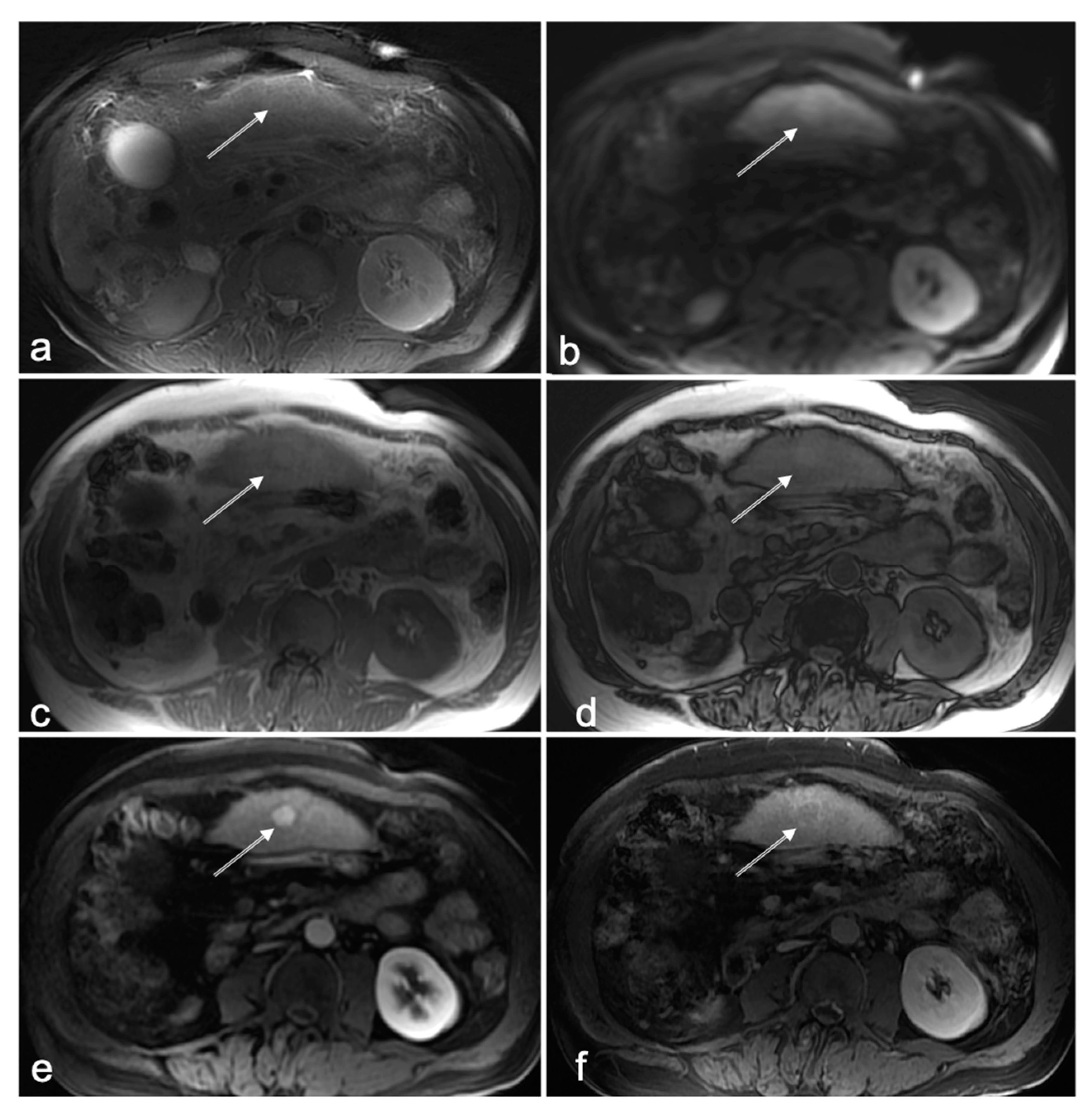
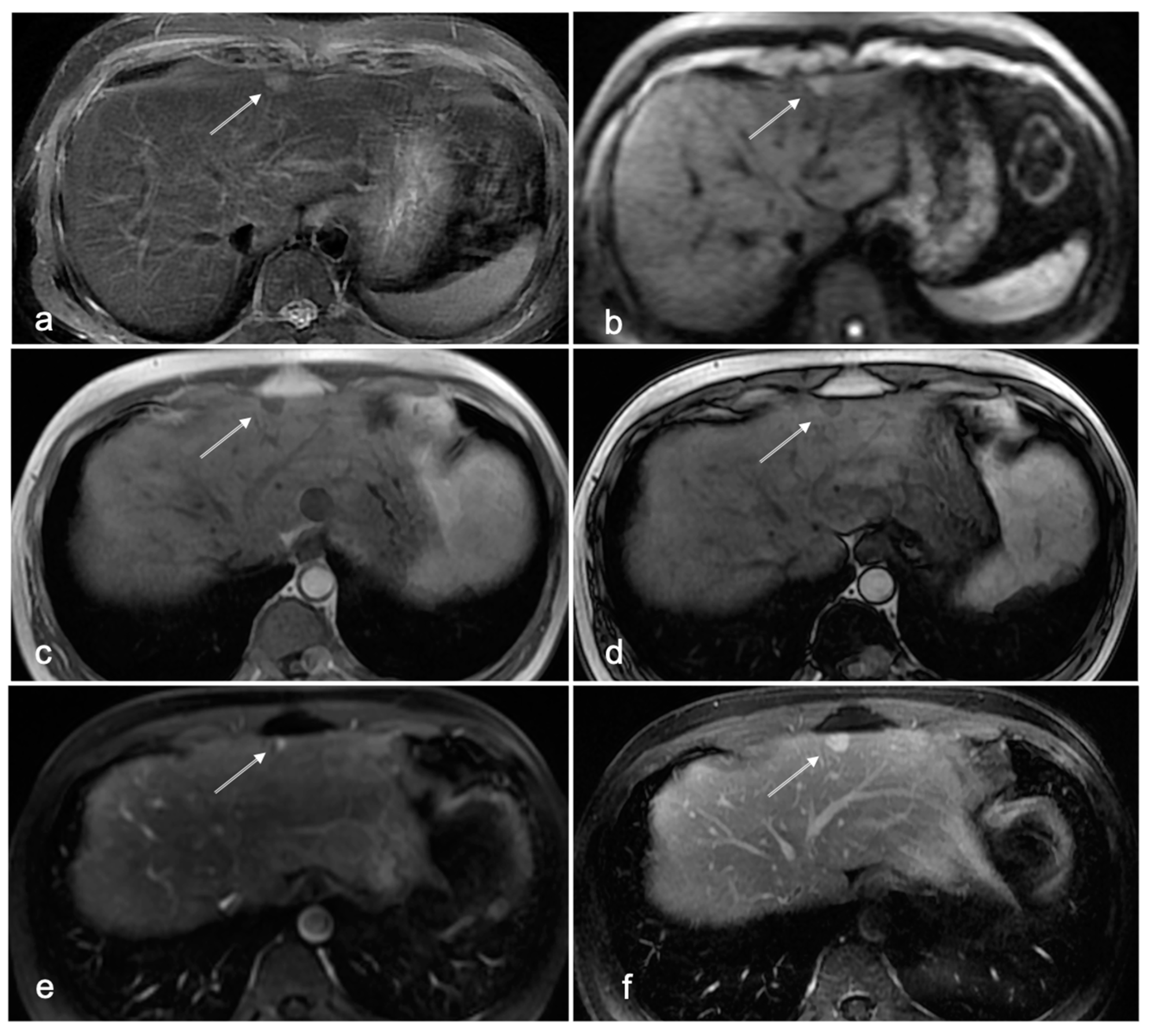
3.1. Cases with HCC
3.2. HCC-Naïve Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef] [PubMed]
- van Meer, S.; de Man, R.A.; Siersema, P.D.; van Erpecum, K.J. Surveillance for hepatocellular carcinoma in chronic liver disease: Evidence and controversies. World J. Gastroenterol. 2013, 19, 6744–6756. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Kim, Y.Y.; An, C.; Kim, D.Y.; Aljoqiman, K.S.; Choi, J.Y.; Kim, M.J. Failure of hepatocellular carcinoma surveillance: Inadequate echogenic window and macronodular parenchyma as potential culprits. Ultrasonography 2019, 38, 311–320. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.D.; Browning, T.; Singal, A.G. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef]
- Esfeh, J.M.; Hajifathalian, K.; Ansari-Gilani, K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin. Mol. Hepatol. 2020, 26, 54–59. [Google Scholar] [CrossRef]
- Lima, P.H.; Fan, B.; Berube, J.; Cerny, M.; Olivie, D.; Giard, J.M.; Beauchemin, C.; Tang, A. Cost-Utility Analysis of Imaging for Surveillance and Diagnosis of Hepatocellular Carcinoma. AJR Am. J. Roentgenol. 2019, 213, 17–25. [Google Scholar] [CrossRef]
- Perisinakis, K.; Tzedakis, A.; Pouli, S.; Spanakis, K.; Hatzidakis, A.; Damilakis, J. Comparison of patient dose from routine multi-phase and dynamic liver perfusion CT studies taking into account the effect of iodinated contrast administration. Eur. J. Radiol. 2019, 110, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.H.; Fairley, R.; Murphy, L.S.; Doss, M. The Risk of Cancer from CT Scans and Other Sources of Low-Dose Radiation: A Critical Appraisal of Methodologic Quality. Prehosp. Disaster Med. 2020, 35, 3–16. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Pena, M.A.; Cunha, G.M.; Booker, M.T.; Taouli, B.; Yokoo, T.; Sirlin, C.B.; Fowler, K.J. Abbreviated MRI for Hepatocellular Carcinoma Screening and Surveillance. Radiographics 2020, 40, 1916–1931. [Google Scholar] [CrossRef] [PubMed]
- Canellas, R.; Rosenkrantz, A.B.; Taouli, B.; Sala, E.; Saini, S.; Pedrosa, I.; Wang, Z.J.; Sahani, D.V. Abbreviated MRI Protocols for the Abdomen. Radiographics 2019, 39, 744–758. [Google Scholar] [CrossRef]
- Donato, H.; Franca, M.; Candelaria, I.; Caseiro-Alves, F. Liver MRI: From basic protocol to advanced techniques. Eur. J. Radiol. 2017, 93, 30–39. [Google Scholar] [CrossRef]
- Geertse, T.D.; Paap, E.; van der Waal, D.; Duijm, L.E.M.; Pijnappel, R.M.; Broeders, M.J.M. Utility of Supplemental Training to Improve Radiologist Performance in Breast Cancer Screening: A Literature Review. J. Am. Coll. Radiol. 2019, 16, 1528–1546. [Google Scholar] [CrossRef]
- Taylor-Phillips, S.; Stinton, C. Double reading in breast cancer screening: Considerations for policy-making. Br. J. Radiol. 2020, 93, 20190610. [Google Scholar] [CrossRef]
- Herrick, R.; Horton, W.; Olsen, T.; McKay, M.; Archie, K.A.; Marcus, D.S. XNAT Central: Open sourcing imaging research data. Neuroimage 2016, 124, 1093–1096. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.K.; Baek, S.Y.; Yun, H.I. Diagnostic performance of a minimized protocol of non-contrast MRI for hepatocellular carcinoma surveillance. Abdom. Radiol. 2020, 45, 211–219. [Google Scholar] [CrossRef]
- Sutherland, T.; Watts, J.; Ryan, M.; Galvin, A.; Temple, F.; Vuong, J.; Little, A.F. Diffusion-weighted MRI for hepatocellular carcinoma screening in chronic liver disease: Direct comparison with ultrasound screening. J. Med. Imaging Radiat. Oncol. 2017, 61, 34–39. [Google Scholar] [CrossRef]
- Cavelaars, M.; Rousseau, J.; Parlayan, C.; de Ridder, S.; Verburg, A.; Ross, R.; Visser, G.R.; Rotte, A.; Azevedo, R.; Boiten, J.-W.; et al. OpenClinica. J. Clin. Bioinform. 2015, 5, S2. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhang, L.; Jiang, G.; Wang, Q.; Liu, L.; Liu, D.; Wang, Z.; Zhu, Z.; Deng, Q.; Xiong, X.; et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J. Clin. Oncol. 2015, 33, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Benchoufi, M.; Matzner-Lober, E.; Molinari, N.; Jannot, A.S.; Soyer, P. Interobserver agreement issues in radiology. Diagn. Interv. Imaging 2020, 101, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jang, H.Y.; Kim, S.Y.; Lee, S.J.; Won, H.J.; Byun, J.H.; Choi, S.H.; Lee, S.S.; An, J.; Lim, Y.S. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J. Hepatol. 2020, 72, 718–724. [Google Scholar] [CrossRef]
- Chan, M.V.; McDonald, S.J.; Ong, Y.Y.; Mastrocostas, K.; Ho, E.; Huo, Y.R.; Santhakumar, C.; Lee, A.U.; Yang, J. HCC screening: Assessment of an abbreviated non-contrast MRI protocol. Eur. Radiol. Exp. 2019, 3, 49. [Google Scholar] [CrossRef]
- Khatri, G.; Pedrosa, I.; Ananthakrishnan, L.; de Leon, A.D.; Fetzer, D.T.; Leyendecker, J.; Singal, A.G.; Xi, Y.; Yopp, A.; Yokoo, T. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. J. Magn. Reson. Imaging 2020, 51, 415–425. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.; Kim, S.Y.; Shim, Y.S.; Kim, J.H.; Huh, J.; Kim, H.J.; Kim, K.W.; Lee, S.S. Abbreviated MRI with optional multiphasic CT as an alternative to full-sequence MRI: LI-RADS validation in a HCC-screening cohort. Eur. Radiol. 2020, 30, 2302–2311. [Google Scholar] [CrossRef]
- Gupta, P.; Soundararajan, R.; Patel, A.; Kumar, M.P.; Sharma, V.; Kalra, N. Abbreviated MRI for hepatocellular carcinoma screening: A systematic review and meta-analysis. J. Hepatol. 2021, 75, 108–119. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, S.H.; Shim, J.H.; Kim, S.Y.; Lee, S.S.; Byun, J.H.; Choi, J.I. Meta-Analysis of the Accuracy of Abbreviated Magnetic Resonance Imaging for Hepatocellular Carcinoma Surveillance: Non-Contrast versus Hepatobiliary Phase-Abbreviated Magnetic Resonance Imaging. Cancers 2021, 13, 2975. [Google Scholar] [CrossRef]
- Vietti Violi, N.; Lewis, S.; Liao, J.; Hulkower, M.; Hernandez-Meza, G.; Smith, K.; Babb, J.S.; Chin, X.; Song, J.; Said, D.; et al. Gadoxetate-enhanced abbreviated MRI is highly accurate for hepatocellular carcinoma screening. Eur. Radiol. 2020, 30, 6003–6013. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Singal, A.G.; King, L.Y.; Andersson, K.L.; Fuchs, B.C.; Besa, C.; Taouli, B.; Chung, R.T.; Hoshida, Y. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin. Transl. Gastroenterol. 2017, 8, e101. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.B.; Gowda, V.; Cheng, G. Gadolinium Deposition Disease: A New Risk Management Threat. J. Am. Coll. Radiol. 2020, 17, 546–550. [Google Scholar] [CrossRef]
- Radbruch, A.; Weberling, L.D.; Kieslich, P.J.; Eidel, O.; Burth, S.; Kickingereder, P.; Heiland, S.; Wick, W.; Schlemmer, H.P.; Bendszus, M. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015, 275, 783–791. [Google Scholar] [CrossRef]
- Shanbhogue, K.; Tong, A.; Smereka, P.; Nickel, D.; Arberet, S.; Anthopolos, R.; Chandarana, H. Accelerated single-shot T2-weighted fat-suppressed (FS) MRI of the liver with deep learning-based image reconstruction: Qualitative and quantitative comparison of image quality with conventional T2-weighted FS sequence. Eur. Radiol. 2021, 31, 8447–8457. [Google Scholar] [CrossRef]
- Anderson, E.D.; Muir, B.B.; Walsh, J.S.; Kirkpatrick, A.E. The efficacy of double reading mammograms in breast screening. Clin. Radiol. 1994, 49, 248–251. [Google Scholar] [CrossRef]
- Coolen, A.M.P.; Voogd, A.C.; Strobbe, L.J.; Louwman, M.W.J.; Tjan-Heijnen, V.C.G.; Duijm, L.E.M. Impact of the second reader on screening outcome at blinded double reading of digital screening mammograms. Br. J. Cancer 2018, 119, 503–507. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, S.H.; Lee, J.S.; Choi, J.I. Inter-reader agreement of abbreviated magnetic resonance imaging for hepatocellular carcinoma detection: A systematic review and meta-analysis. Abdom. Radiol. 2021, 47, 123–132. [Google Scholar] [CrossRef]
- Girometti, R. 3.0 Tesla magnetic resonance imaging: A new standard in liver imaging? World J. Hepatol. 2015, 7, 1894–1898. [Google Scholar] [CrossRef][Green Version]
- Park, S.H.; Kim, B. Liver Magnetic Resonance Imaging for Hepatocellular Carcinoma Surveillance. J. Liver Cancer 2020, 20, 25–31. [Google Scholar] [CrossRef]
- Hong, C.W.; Park, C.C.; Mamidipalli, A.; Hooker, J.C.; Fazeli Dehkordy, S.; Igarashi, S.; Alhumayed, M.; Kono, Y.; Loomba, R.; Wolfson, T.; et al. Longitudinal evolution of CT and MRI LI-RADS v2014 category 1, 2, 3, and 4 observations. Eur. Radiol. 2019, 29, 5073–5081. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, C.B.; Lim, C.S.; Sirlin, C.B.; McGrath, T.A.; Salameh, J.P.; Bashir, M.R.; Tang, A.; Singal, A.G.; Costa, A.F.; Fowler, K.; et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology 2019, 156, 976–986. [Google Scholar] [CrossRef] [PubMed]
| Direction | Sequence (TE/TR = ms) | Location | Slice Thickness mm | Breath Hold | Scan Time (Minutes) |
|---|---|---|---|---|---|
| Localizer | SSFSE | Abdomen | 8/0.8 | Yes | 0:40 |
| Calibration | 0:48 | ||||
| Coronal | T2SSFSE (TE/TR = 90/500) | Abdomen | 6/0.6 | Yes | 0:40 |
| Calibration | 1:52 | ||||
| Axial | T2SSFSE (TE/TR = 90/500) | Liver | 6/0.6 | Yes | 0:40 |
| Axial | T2SSFSE (TE/TR = 180/500) | Liver | 6/0.6 | Yes | 0:40 |
| Axial | 3D LAVA T1 in/opp (TE/TR = 4.2–2.1/6.0) | Liver | 5 | Yes | 0:41 |
| Axial | DWI SE EPI b = 50, 600–800 s/mm2 | Liver | 6/0.6 | No | 3:40 |
| Axial | DYN 1 3D T1 LAVA FS (TE/TR = 1.8/3.9) | Liver | 4 | 0:42 | |
| Calibration | 1:54 | ||||
| Sagittal | Test bolus | Aorta | 20 | No | 1:12 |
| Calibration | 0:42 | ||||
| Axial | DYN 2 3D T1 LAVA FS art phase | Liver | 4 | Yes | 0:42 |
| Axial | DYN 3 3D T1 LAVA FS4x ven-del phase | Liver | 4 | Yes | 3:53 |
| Coronal | LAVAARC (TE/TR = 1.8/3.9) | Liver | 4 | Yes | 0:40 |
| Axial | T2 FSE FS (TE/TR = 93/15,000) | Liver | 6/0.6 | No | 6:41 |
| Total scan time | 26:23 | ||||
| Total scan time NC-AMRI | 12:30 |
| Reader 1 | Reader 2 | Reader 3 | Readers Combined * | |
|---|---|---|---|---|
| Sensitivity | 35/36 (97.2%) | 29/36 (80.1%) | 33/36 (91.7%) | 35/36 (97.2%) |
| 95% CI | 85.5–99.9% | 64.0–91.8% | 77.5–98.3% | 85.5–99.9% |
| Specificity | 147/179 (82.1%) | 163/179 (91.1%) | 129/179 (72.1%) | 156/179 (87.2%) |
| 95% CI | 75.7–87.4% | 64.0–91.8% | 65.9–78.5% | 81.4–91.7% |
| NPV | 99.3% | 95.9% | 97.7% | 99.4% |
| 95% CI | 95.5–99.9% | 92.3–97.8% | 93.6–99.2% | 95.8–99.9% |
| PPV | 52.2% | 64.4% | 39.8% | 60.3% |
| 95% CI | 44.3–60.1% | 52.5–74.8% | 33.8–46.0% | 50.9–69.1% |
| Accuracy | 84.7% | 89.3% | 75.3% | 88.8% |
| 95% CI | 79.1–89.2% | 84.4–93.1% | 69.0–81.0% | 83.9–92.7% |
| Kappa Cohen Reader 1 + 2 | K = 0.64 | 89.5% agreement | ||
| 95% CI | 0.53–0.75 | |||
| Kappa Cohen Reader 1 + 3 | K = 0.55 | 80.0% agreement | ||
| 95% CI | 0.43–0.66 | |||
| Kappa Cohen Reader 2 + 3 | K = 0.49 | 77.7% agreement | ||
| 95% CI | 0.37–0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willemssen, F.; de Lussanet de la Sablonière, Q.; Bos, D.; IJzermans, J.; De Man, R.; Dwarkasing, R. Potential of a Non-Contrast-Enhanced Abbreviated MRI Screening Protocol (NC-AMRI) in High-Risk Patients under Surveillance for HCC. Cancers 2022, 14, 3961. https://doi.org/10.3390/cancers14163961
Willemssen F, de Lussanet de la Sablonière Q, Bos D, IJzermans J, De Man R, Dwarkasing R. Potential of a Non-Contrast-Enhanced Abbreviated MRI Screening Protocol (NC-AMRI) in High-Risk Patients under Surveillance for HCC. Cancers. 2022; 14(16):3961. https://doi.org/10.3390/cancers14163961
Chicago/Turabian StyleWillemssen, François, Quido de Lussanet de la Sablonière, Daniel Bos, Jan IJzermans, Robert De Man, and Roy Dwarkasing. 2022. "Potential of a Non-Contrast-Enhanced Abbreviated MRI Screening Protocol (NC-AMRI) in High-Risk Patients under Surveillance for HCC" Cancers 14, no. 16: 3961. https://doi.org/10.3390/cancers14163961
APA StyleWillemssen, F., de Lussanet de la Sablonière, Q., Bos, D., IJzermans, J., De Man, R., & Dwarkasing, R. (2022). Potential of a Non-Contrast-Enhanced Abbreviated MRI Screening Protocol (NC-AMRI) in High-Risk Patients under Surveillance for HCC. Cancers, 14(16), 3961. https://doi.org/10.3390/cancers14163961







