Clinical Outcomes of Adults with Systemic Mastocytosis: A 15-Year Multidisciplinary Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
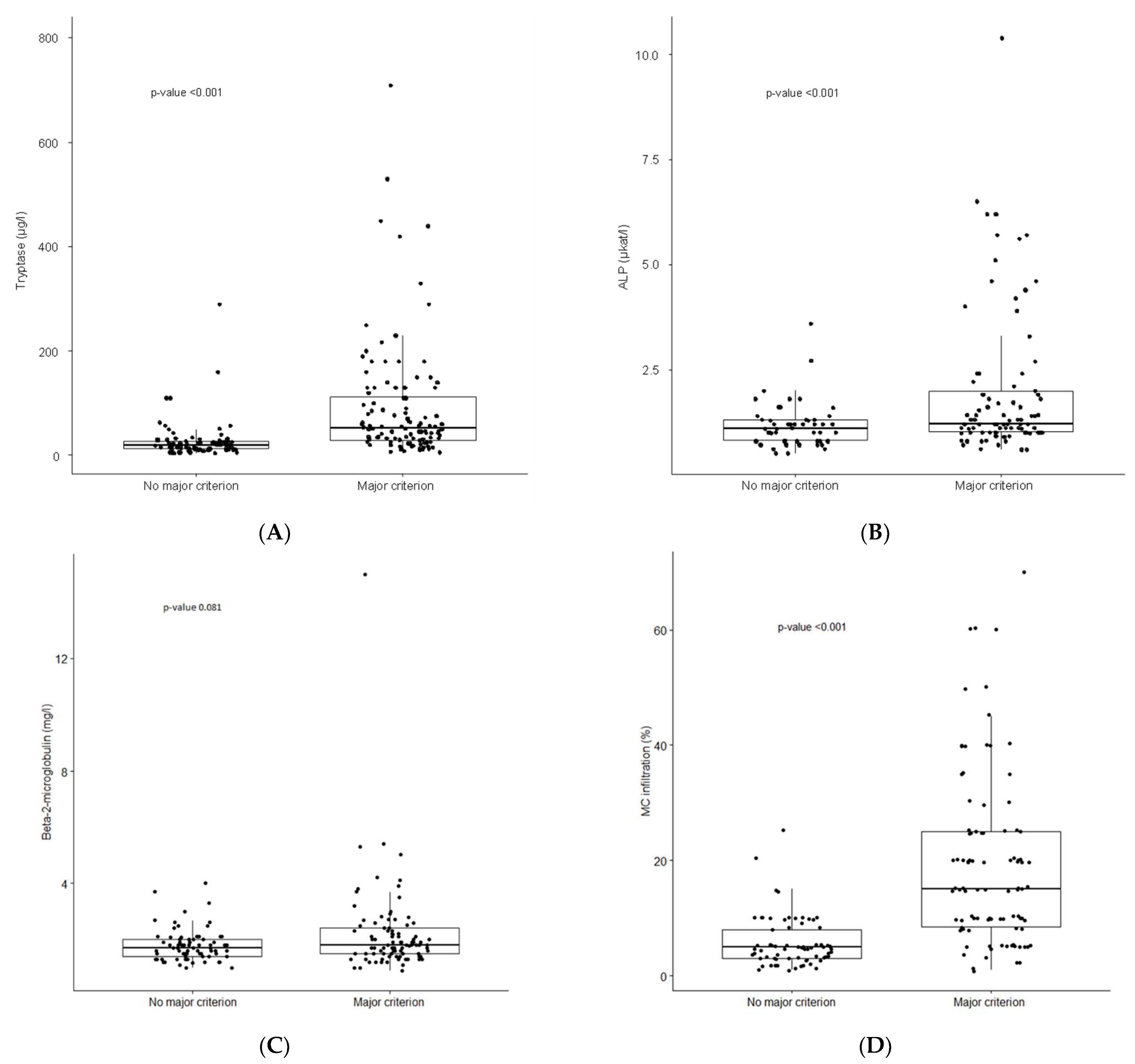
3.2. Laboratory Findings
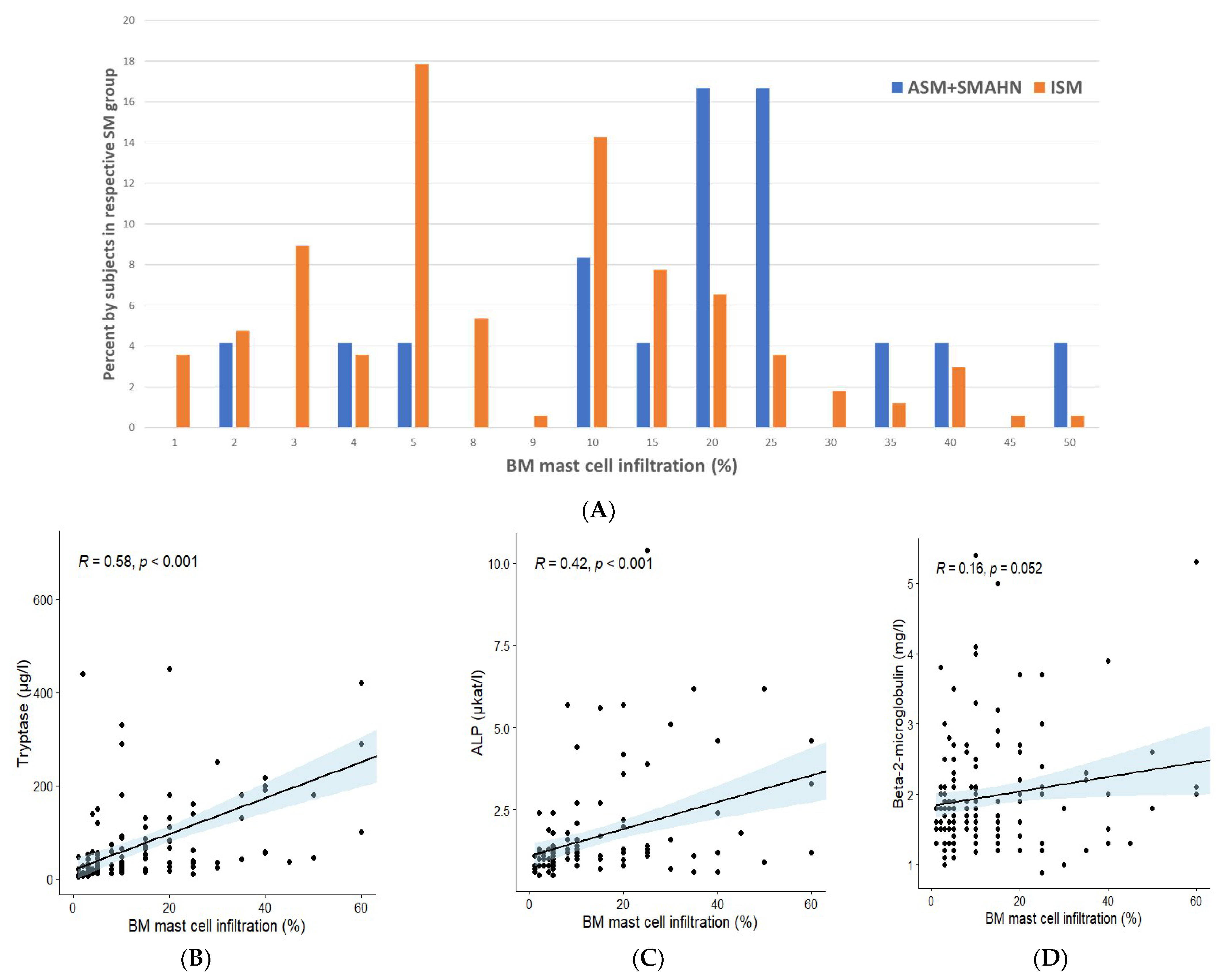
3.3. Bone Marrow Findings
3.4. Bone Mineral Density Abnormalities
3.5. Therapeutic Strategies: Treatment of Advanced SM
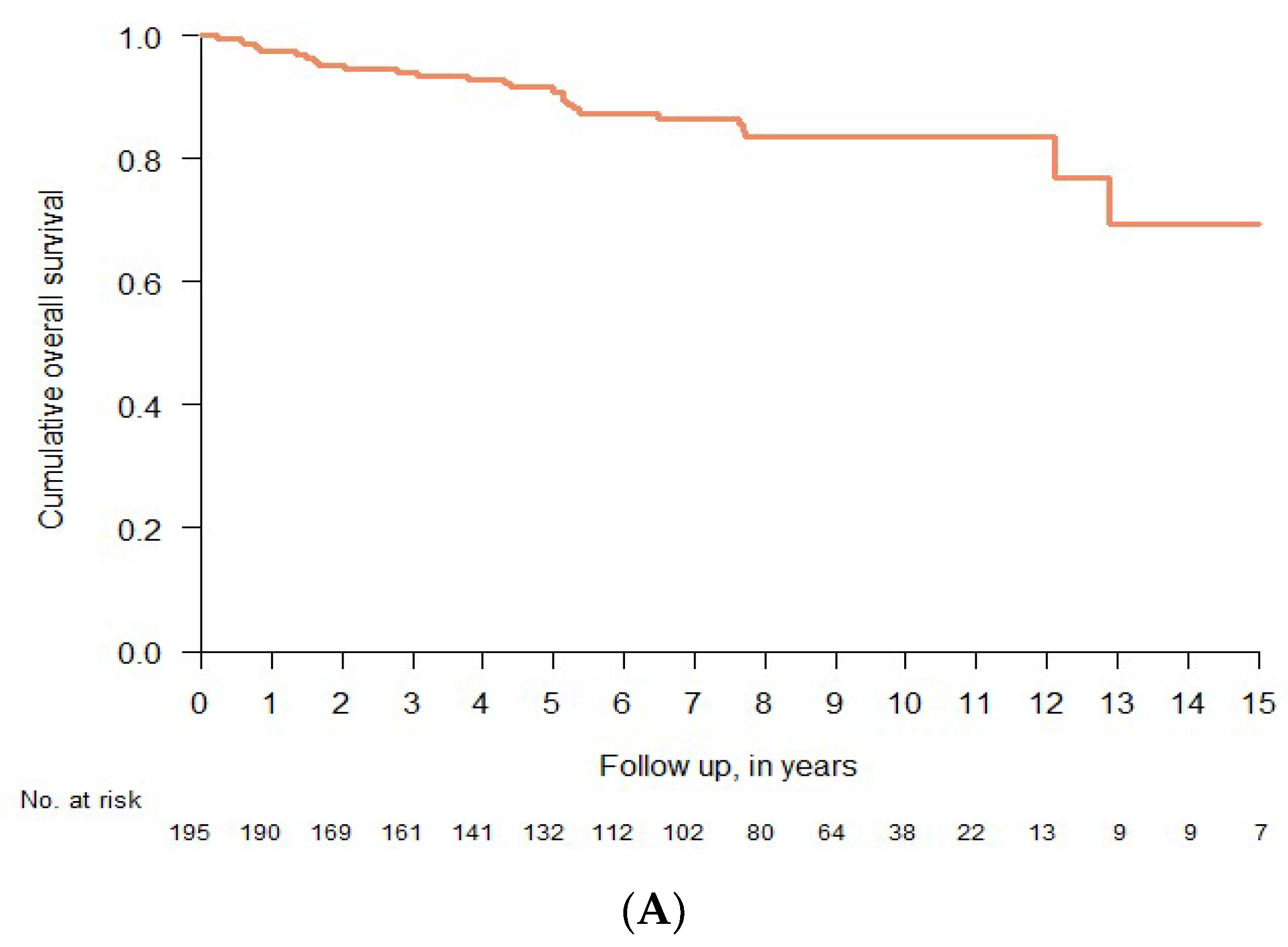
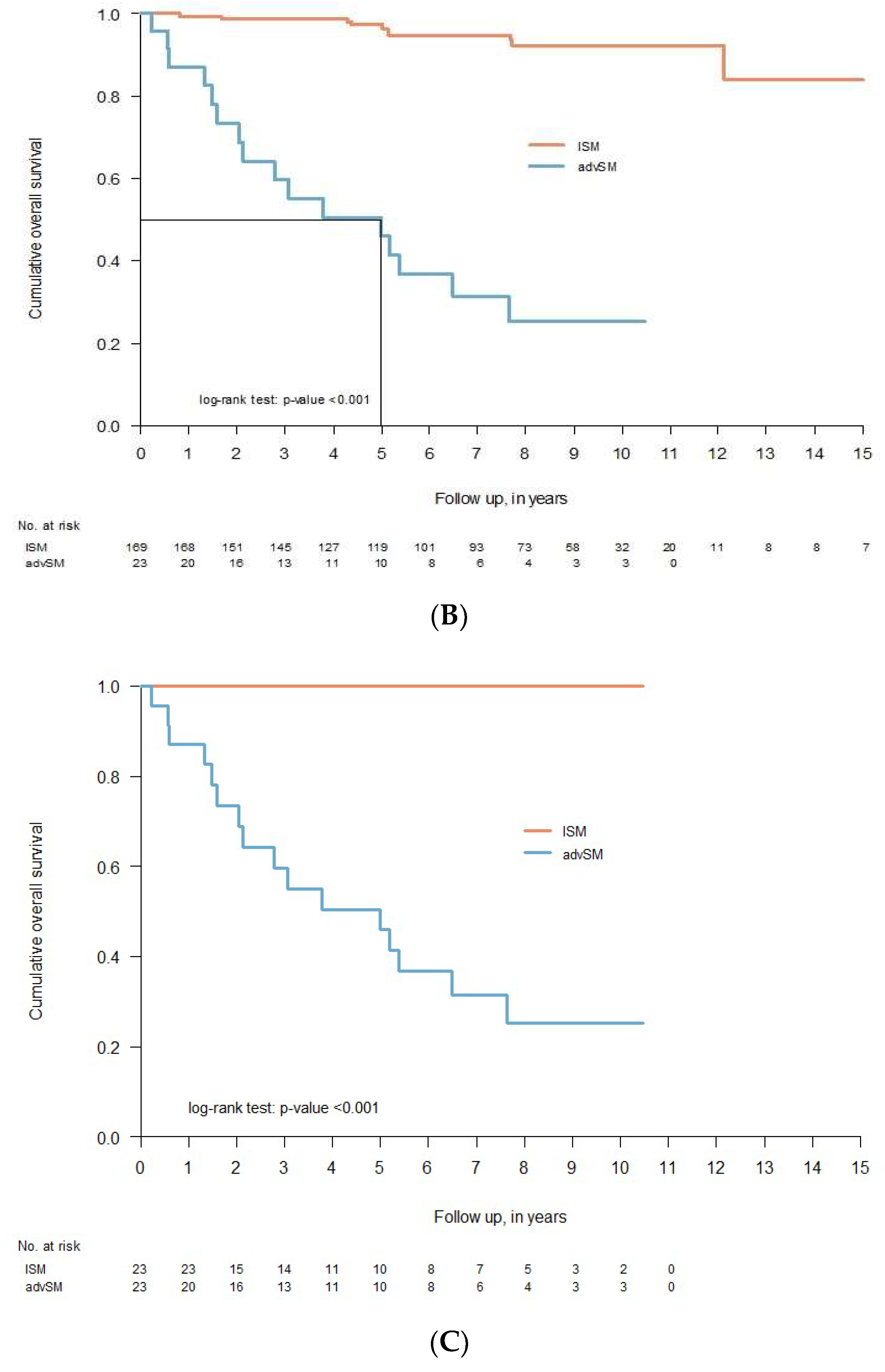
3.6. Clinical Course and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gülen, T.; Hägglund, H.; Dahlén, B.; Nilsson, G. Mastocytosis: The puzzling clinical spectrum and challenging diagnostic aspects of an enigmatic disease. J. Intern. Med. 2016, 279, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood J. Am. Soc. Hematol. 2017, 129, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Trizuljak, J.; Sperr, W.R.; Nekvindova, L.; Elberink, H.O.; Gleixner, K.V.; Gorska, A.; Lange, M.; Hartmann, K.; Illerhaus, A.; Bonifacio, M.; et al. Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification. Allergy 2020, 75, 1923–1934. [Google Scholar] [CrossRef]
- Zanotti, R.; Bonifacio, M.; Lucchini, G.; Sperr, W.R.; Scaffidi, L.; van Anrooij, B.; Elberink, H.N.O.; Rossignol, J.; Hermine, O.; Gorska, A.; et al. Refined diagnostic criteria for bone marrow mastocytosis: A proposal of the European competence network on mastocytosis. Leukemia 2022, 36, 516–524. [Google Scholar] [CrossRef]
- Horny, H.P.; Sotlar, K.; Sperr, W.R.; Valent, P. Systemic mastocytosis with associated clonal haematological non-mast cell lineage diseases: A histopathological challenge. J. Clin. Pathol. 2004, 57, 604–608. [Google Scholar] [CrossRef]
- Hagen, W.; Schwarzmeier, J.; Walchshofer, S.; Zojer, N.; Chott, A.; Sillaber, C.; Ackermann, J.; Simonitsch, I.; Bühring, H.J.; Drach, J.; et al. A case of bone marrow mastocytosis associated with multiple myeloma. Ann. Hematol. 1998, 76, 167–174. [Google Scholar] [CrossRef]
- Gulen, T.; Sander, B.; Nilsson, G.; Palmblad, J.; Sotlar, K.; Horny, H.P.; Hägglund, H. Systemic mastocytosis: Progressive evolution of an occult disease into fatal mast cell leukemia: Unique findings on an unusual hematological neoplasm. Med. Oncol. 2012, 29, 3540–3546. [Google Scholar] [CrossRef]
- van Doormaal, J.J.; Arends, S.; Brunekreeft, K.L.; van der Wal, V.B.; Sietsma, J.; van Voorst Vader, P.C.; Oude Elberink, J.N.G.; Kluin-Nelemans, J.C.; van der Veer, E.; de Monchy, J.G.R. Prevalence of Indolent Systemic Mastocytosis in a Dutch Region. J. Allergy Clin. Immunol. 2013, 131, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.S.; Skovbo, S.; Vestergaard, H.; Kristensen, T.; Møller, M.; Bindslev-Jensen, C.; Fryzek, J.P.; Broesby-Olsen, S. Epidemiology of Systemic Mastocytosis in Denmark. Br. J. Haematol. 2014, 166, 521–528. [Google Scholar] [CrossRef]
- Zanotti, R.; Bonifacio, M.; Isolan, C.; Tanasi, I.; Crosera, L.; Olivieri, F.; Orsolini, G.; Schena, D.; Bonadonna, P. A Multidisciplinary Diagnostic Approach Reveals a Higher Prevalence of Indolent Systemic Mastocytosis: 15-Years’ Experience of the GISM Network. Cancers 2021, 13, 6380. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Gülen, T.; Hägglund, H.; Dahlén, B.; Nilsson, G. High prevalence of anaphylaxis in patients with systemic—A single-centre experience. Clin. Exp. Allergy 2014, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, N.; Desoutter, J.; Chandesris, O.; Merlusca, L.; Henry, I.; Georgin-Lavialle, S.; Barete, S.; Hirsch, I.; Bouredji, D.; Royer, B.; et al. Bone complications of mastocytosis: A link between clinical and biological characteristics. Am. J. Med. 2013, 126, 75.e1–75.e7. [Google Scholar] [CrossRef]
- Jennings, S.; Russell, N.; Jennings, B.; Slee, V.; Sterling, L.; Castells, M.; Valent, P.; Akin, C. The Mastocytosis Society survey on mast cell disorders: Patient experiences and perceptions. J. Allergy Clin. Immunol. Pract. 2014, 2, 70–76. [Google Scholar] [CrossRef]
- Gülen, T.; Hägglund, H.; Sander, B.; Dahlén, B.; Nilsson, G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy 2014, 44, 1179–1187. [Google Scholar] [CrossRef]
- Gülen, T.; Ljung, C.; Nilsson, G.; Akin, C. Risk factor analysis to predict anaphylactic reactions in patients with systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 2017, 5, 1248–1255. [Google Scholar] [CrossRef]
- Jarkvist, J.; Brockow, K.; Gulen, T. Low frequency of IgE-mediated food-hypersensitivity in mastocytosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3093–3101. [Google Scholar] [CrossRef]
- Jarkvist, J.; Salehi, C.; Akin, C.; Gülen, T. Venom immunotherapy in patients with clonal mast cell disorders: IgG4 correlates with protection. Allergy 2020, 75, 169–177. [Google Scholar] [CrossRef]
- Gülen, T.; Hägglund, H.; Dahlén, S.E.; Sander, B.; Dahlén, B.; Nilsson, G. Flushing, fatigue, and recurrent anaphylaxis: A delayed diagnosis of mastocytosis. Lancet 2014, 383, 1608. [Google Scholar] [CrossRef]
- Teodosio, C.; Mayado, A.; Sanchez-Munoz, L.; Morgado, J.M.; Jara-Acevedo, M.; Alvarez-Twose, I.; Garci’ A-Montero, A.S.C.; Matito, A.; Caldas, C.; Escribano, L.; et al. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J. Leukoc. Biol. 2015, 97, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.; Vestergaard, H.; Bindslev-Jensen, C.; Møller, M.B.; Broesby-Olsen, S.; Mastocytosis Centre, Odense University Hospital (MastOUH). KIT D816V Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am. J. Hematol. 2014, 89, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Austin, P.C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat. Med. 2008, 27, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.A.W.; Rietveld, M.J.A.; van Laar, J.A.M.; Dalm, V.A.S.H.; Verburg, M.; Pasmans, S.G.M.A.; van Wijk, R.G.; van Hagen, P.M.; van Daele, P.L.A. Systemic mastocytosis: A cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur. J. Intern. Med. 2016, 30, 25–30. [Google Scholar] [CrossRef]
- Sperr, W.R.; Kundi, M.; Alvarez-Twose, I.; van Anrooij, B.; Oude Elberink, J.N.G.; Gorska, A.; Niedoszytko, M.; Gleixner, K.V.; Hadzijusufovic, E.; Zanotti, R.; et al. International Prognostic Scoring System for Mastocytosis (IPSM): A Retrospective Cohort Study. Lancet Haematol. 2019, 6, e638–e649. [Google Scholar] [CrossRef]
- Lim, K.H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield, J.H.; McClure, R.F.; Li, C.; Pardanani, A. Systemic Mastocytosis in 342 Consecutive Adults: Survival Studies and Prognostic Factors. Blood J. Am. Soc. Hematol. 2009, 113, 5727–5736. [Google Scholar] [CrossRef]
- Kluin-Nelemans, H.C.; Jawhar, M.; Reiter, A.; van Anrooij, B.; Gotlib, J.; Hartmann, K.; Illerhaus, A.; Elberink, H.N.O.; Gorska, A.; Niedoszytko, M.; et al. Cytogenetic and Molecular Aberrations and Worse Outcome for Male Patients in Systemic Mastocytosis. Theranostics 2021, 11, 292–303. [Google Scholar] [CrossRef]
- Schwaab, J.; Cabral do O Hartmann, N.; Naumann, N.; Jawhar, M.; Weiss, C.; Metzgeroth, G.; Schmid, A.; Lübke, J.; Reiter, L.; Fabarius, A.; et al. Importance of adequate diagnostic workup for correct diagnosis of advanced systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3121–3127. [Google Scholar] [CrossRef]
- Barete, S.; Assous, N.; de Gennes, C.; Grandpeix, C.; Feger, F.; Palmerini, F.; Dubreuil, P.; Arock, M.; Roux, C.; Launay, J.M.; et al. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann. Rheum. Dis. 2010, 69, 1838–1841. [Google Scholar] [CrossRef]
- Svedbom, A.; Hernlund, E.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, H.; Sander, B.; Gülen, T.; Lindelöf, B.; Nilsson, G. Increased risk of malignant melanoma in patients with systemic mastocytosis? Acta Derm. Venereol. 2014, 94, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Broesby-Olsen, S.; Farkas, D.K.; Vestergaard, H.; Hermann, A.P.; Møller, M.B.; Mortz, C.G.; Kristensen, T.K.; Bindslev-Jensen, C.; Sørensen, H.T.; Frederiksen, H. Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients with systemic mastocytosis: A nationwide population-based study. Am. J. Hematol. 2016, 91, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, G.; Gleixner, K.V.; Dinu, G.E.; Kundi, M.; Greiner, G.; Wimazal, F.; Hadzijusufovic, E.; Mitterbauer, G.; Mannhalter, C.; Valent, P.; et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy 2014, 69, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, M.; Schwaab, J.; Hausmann, D.; Clemens, J.; Naumann, N.; Henzler, T.; Horny, H.P.; Sotlar, K.; Schoenberg, S.O.; Cross, N.C.P.; et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia 2016, 30, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Reichard, K.K.; Zblewski, D.; Abdelrahman, R.A.; Wassie, E.A.; Morice, I.I.; Brooks, C.; Grogg, K.L.; Hanson, C.A.; Tefferi, A.; et al. CD123 immunostaining patterns in systemic mastocytosis: Differential expression in disease subgroups and potential prognostic value. Leukemia 2016, 30, 914–918. [Google Scholar] [CrossRef]
- Jawhar, M.; Schwaab, J.; Schnittger, S.; Meggendorfer, M.; Pfirrmann, M.; Sotlar, K.; Horny, H.P.; Metzgeroth, G.; Kluger, S.; Naumann, N.; et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia 2016, 30, 136–143. [Google Scholar] [CrossRef]
- Greiner, G.; Gurbisz, M.; Ratzinger, F.; Witzeneder, N.; Class, S.V.; Eisenwort, G.; Simonitsch-Klupp, I.; Esterbauer, H.; Mayerhofer, M.; Müllauer, L.; et al. Molecular quantification of tissue disease burden is a new biomarker and independent predictor of survival in mastocytosis. Haematologica 2020, 105, 366–374. [Google Scholar] [CrossRef]
- Pardanani, A.; Lasho, T.L.; Reichard, K.K.; Hanson, C.A.; Tefferi, A. World Health Organization class independent risk categorization in mastocytosis. Blood Cancer J. 2019, 9, 29. [Google Scholar] [CrossRef]
- Escribano, L.; Alvarez-Twose, I.; Sánchez-Muñoz, L.; Garcia-Montero, A.; Núñez, R.; Almeida, J.; Jara-Acevedo, M.; Teodósio, C.; García-Cosío, M.; Bellas, C.; et al. Prognosis in adult indolent systemic mastocytosis: A long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J. Allergy Clin. Immunol. 2009, 124, 514–521. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, L.; Alvarez-Twose, I.; García-Montero, A.C.; Teodosio, C.; Jara-Acevedo, M.; Pedreira, C.E.; Matito, A.; Morgado, J.M.T.; Sánchez, M.L.; Mollejo, M.; et al. Evaluation of the WHO criteria for the classification of patients with mastocytosis. Mod. Pathol. 2011, 24, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, J.I.; Álvarez-Twose, I.; Jara-Acevedo, M.; Henriques, A.; Viñas, E.; Prieto, C.; Sánchez-Muñoz, L.; Caldas, C.; Mayado, A.; Matito, A.; et al. Frequency and prognostic impact of KIT and other genetic variants in indolent systemic mastocytosis. Blood J. Am. Soc. Hematol. 2019, 134, 456–468. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | All Subjects | ISM | SSM | ASM | SM-AHN | p-Value # | ¤ Adj-p-Value |
|---|---|---|---|---|---|---|---|
| Number of subjects, n (%) | 195 | 169 (86.6) | 3 (1.6) | 8 (4.1) | 15 (7.7) | n/a | n/a |
| Age, median (range) | 57.0 (20–84) | 53.0 (20–84) | 49 (48–60) | 71 (53–84) | 70.0 (49–83) | <0.001 | <0.001 |
| Male, n (%) | 87 (44.6) | 73 (43.2) | 2 (66.7) | 4 (50.0) | 8 (53.3) | 0.731 | 1.000 |
| Skin engagement, n (%) | 132 (67.7) | 122 (72.1) | 3 (100) | 3 (37.5) | 4 (26.6) | <0.001 | <0.001 |
| Major criterion, n (%) | 112 (57.4) | 89 (52.7) | 3 (100) | 8 (100) | 12 (80.0) | 0.019 | 0.285 |
| Spindle-shaped MCs, n (%) | 180 (92.3) | 155 (91.7) | 3 (100) | 8 (100) | 14 (93.3) | 0.928 | 1.000 |
| KIT D816V *, positive, n (%) | 175 (96.7) * 14 n/a | 150 (96.8) * 14 n/a | 3 (100) | 8 (100) | 14 (93.3) | 0.419 | 1.000 |
| CD25/CD2 positive, n (%) | 192 (98.5) | 166 (98.2) | 3 (100) | 8 (100) | 15 (100) | 0.127 | 1.000 |
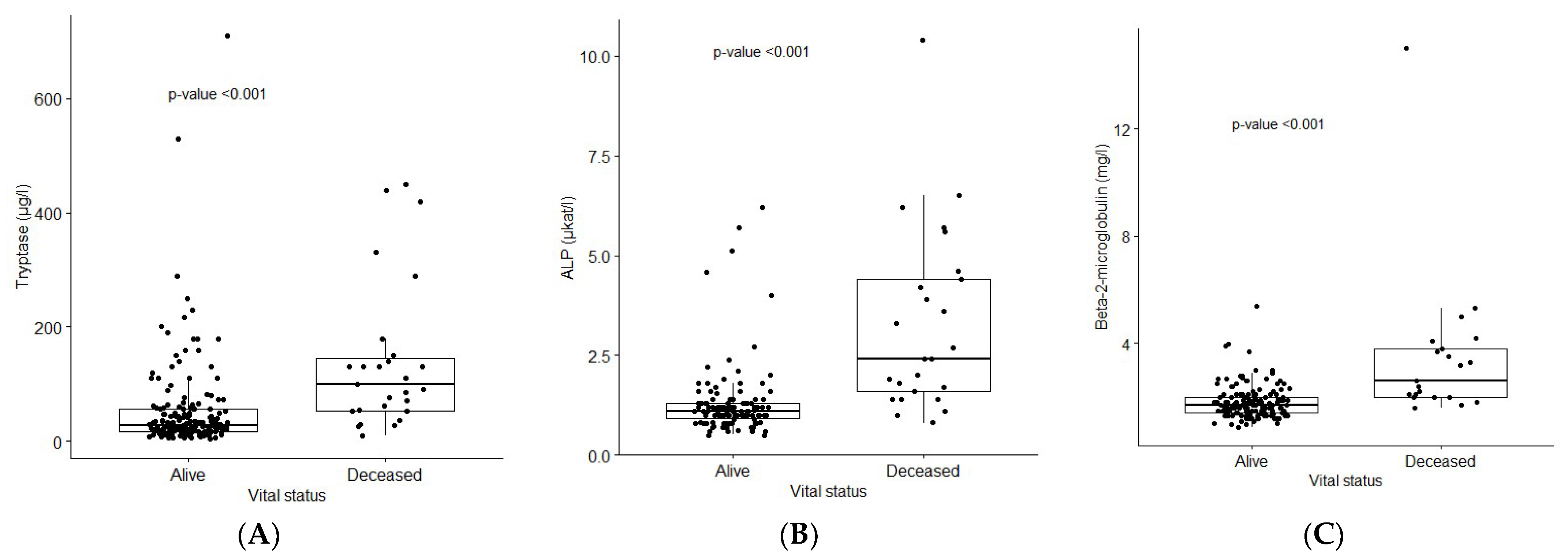
| Tryptase µg/L, median (range) | 32 (4.3–710) | 27.5 (4.3–530] | 450 (290–710) | 140 (62–290) | 130 (10–440) | <0.001 | <0.001 |
| ALP µkat/L, median (range) | 1.2 (0.5–10.4) | 1.1 (0.5–5.7) | 4.0 (1.1–5.7) | 4.1 (1.2–10.4) | 2.3 (0.8–6.0) | <0.001 | <0.001 |
| ALP mg/L, ≥1.9 (%) | 25 (18.0) | 7 (6.1) | 2 (66.7) | 7 (88.0) | 9 (66.7) | <0.001 | <0.001 |
| β2-Microglobulin mg/L, median (range) | 1.8 (0.9–15.0) | 1.7 (0.9–5.4) | 2.4 (2.3–2.5) | 3.1 (2.0–4.2) | 3.5 (2.0–15.0) | <0.001 | <0.001 |
| β2-Microglobulin mg/L, ≥2.2, n (%) | 37 (19.0) | 20 (11.8) | 1 (33.3) | 5 (55.6) | 11 (71.4) | <0.001 | <0.001 |
| Eosinophils > 0.5, median (range), n = 19 | 1.4 (0.5–14.8) | 0.7 (0.5–1.5) | 1.4 (1.4–1.4) | 3.1 (0.6–5.3) | 2.2 (1.2–14.8) | 0.028 | 0.420 |
| Monocytes > 0.8, median (range), n = 15 | 1.3 (0.9–5.5) | 1.0 (0.9–1.7) | None | 1.1 (1.1–3.2) | 1.7 (1.30–5.5) | 0.037 | 0.555 |
| Osteoporosis **, n (%) | 36 (21.9) 31 n/a | 32 (22.2) 25 n/a | None | 2 (28.5) 1 n/a | 2 (20) 5 n/a | 0.977 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungerstedt, J.; Ljung, C.; Klimkowska, M.; Gülen, T. Clinical Outcomes of Adults with Systemic Mastocytosis: A 15-Year Multidisciplinary Experience. Cancers 2022, 14, 3942. https://doi.org/10.3390/cancers14163942
Ungerstedt J, Ljung C, Klimkowska M, Gülen T. Clinical Outcomes of Adults with Systemic Mastocytosis: A 15-Year Multidisciplinary Experience. Cancers. 2022; 14(16):3942. https://doi.org/10.3390/cancers14163942
Chicago/Turabian StyleUngerstedt, Johanna, Christopher Ljung, Monika Klimkowska, and Theo Gülen. 2022. "Clinical Outcomes of Adults with Systemic Mastocytosis: A 15-Year Multidisciplinary Experience" Cancers 14, no. 16: 3942. https://doi.org/10.3390/cancers14163942
APA StyleUngerstedt, J., Ljung, C., Klimkowska, M., & Gülen, T. (2022). Clinical Outcomes of Adults with Systemic Mastocytosis: A 15-Year Multidisciplinary Experience. Cancers, 14(16), 3942. https://doi.org/10.3390/cancers14163942







