An Update on Prevalence, Assessment, and Risk Factors for Sleep Disturbances in Patients with Advanced Cancer—Implications for Health Care Providers and Clinical Research
Abstract
Simple Summary
Abstract
1. Introduction
2. Sleep Disorders and Sleep Disturbances
- (1)
- Difficulty initiating sleep (greater than 30 min to sleep onset) and/or difficulty maintaining sleep (greater than 30 min nocturnal waking time);
- (2)
- Sleep difficulty at least 3 nights per week;
- (3)
- Sleep difficulty that causes significant impairment of daytime functioning.
3. Sleep Assessment
4. Prevalence of Poor Sleep Quality
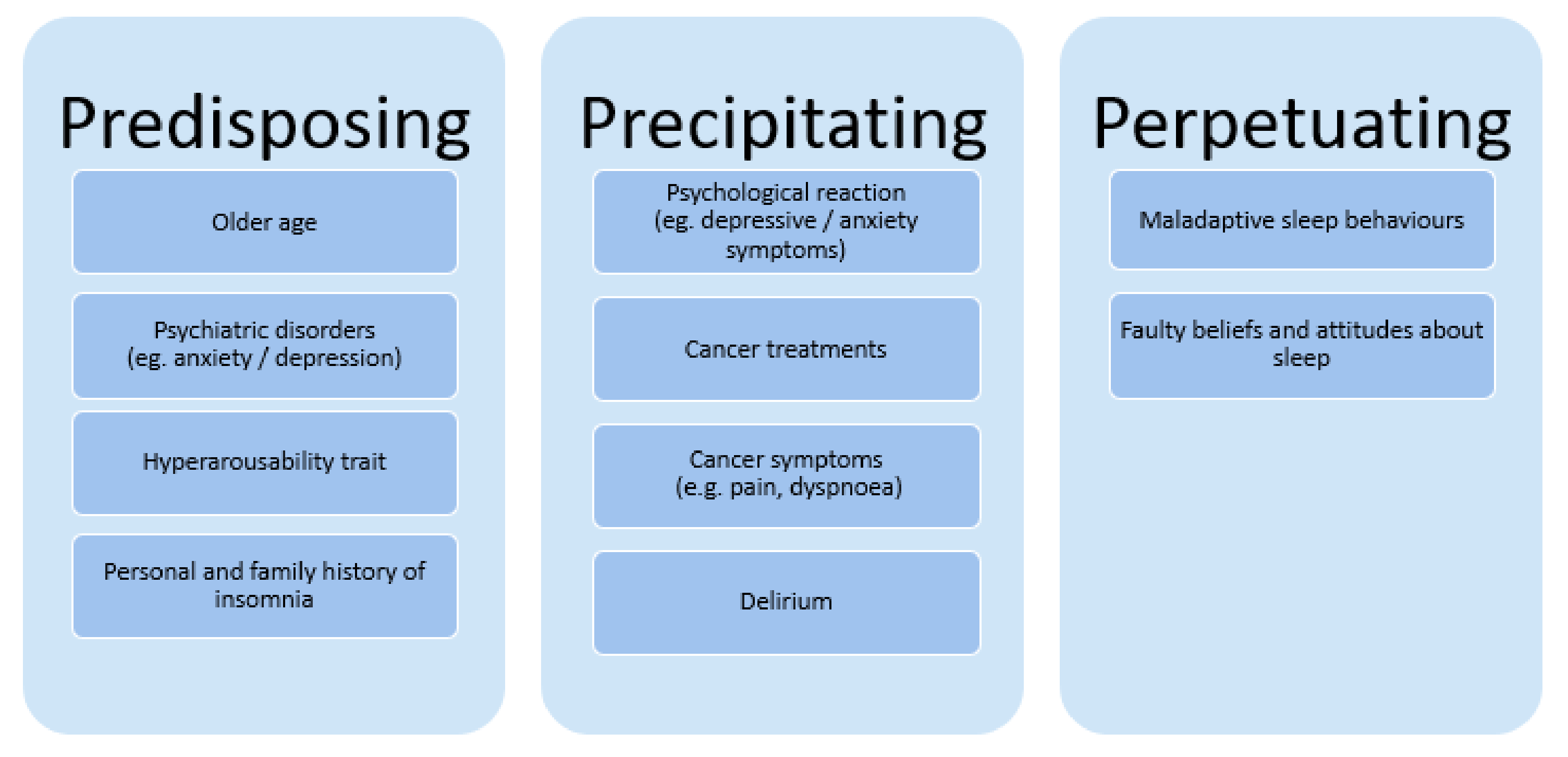
5. Predisposing Factors for Insomnia in Advanced Cancer and Consequences for Other Symptoms
6. Implications for Clinical Practice and Future Research
6.1. Prevalence of Poor Sleep Quality
6.2. Cancer-Related Factors for Insomnia in Advanced Cancer
6.3. Sleep Assessment
6.4. Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, K.; Aapro, M.; Kaasa, S.; Ripamonti, C.I.; Scotte, F.; Strasser, F.; Young, A.; Bruera, E.; Herrstedt, J.; Keefe, D.; et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann. Oncol. 2018, 29, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, S.C.; Wesker, W.; Kruitwagen, C.; de Haes, H.C.; Voest, E.E.; de Graeff, A. Symptom prevalence in patients with incurable cancer: A systematic review. J. Pain Symptom Manag. 2007, 34, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S.; Zhao, F.; Chang, V.T.; Sloan, J.A.; O’Mara, A.M.; Gilman, P.B.; Weiss, M.; Mendoza, T.R.; Lee, J.W.; Fisch, M.J. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer 2013, 119, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Klepstad, P.; Fladvad, T.; Skorpen, F.; Bjordal, K.; Caraceni, A.; Dale, O.; Davies, A.; Kloke, M.; Lundstrom, S.; Maltoni, M.; et al. Influence from genetic variability on opioid use for cancer pain: A European genetic association study of 2294 cancer pain patients. Pain 2011, 152, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Pathiaki, M.; Gennatas, K.; Smyrniotis, V.; Vassiliou, I. The relationship of subjective sleep quality, pain, and quality of life in advanced cancer patients. Sleep 2007, 30, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.P.; Bliwise, D.L.; Ribeiro, M.; Jain, S.R.; Vena, C.I.; Kohles-Baker, M.K.; Rogatko, A.; Xu, Z.; Harris, W.B. Sleep/Wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J. Clin. Oncol. 2008, 26, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Good, P.; Pinkerton, R.; Bowler, S.; Craig, J.; Hardy, J. Impact of Opioid Therapy on Sleep and Respiratory Patterns in Adults With Advanced Cancer Receiving Palliative Care. J. Pain Symptom Manag. 2018, 55, 962–967. [Google Scholar] [CrossRef]
- National Cancer Institute. Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/advanced-cancer (accessed on 24 June 2022).
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders. In Diagnostic and Coding Manual, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Savard, J.; Morin, C.M. Insomnia in the context of cancer: A review of a neglected problem. J. Clin. Oncol. 2001, 19, 895–908. [Google Scholar] [CrossRef]
- Grandner, M.A. Addressing sleep disturbances: An opportunity to prevent cardiometabolic disease? Int. Rev. Psychiatry 2014, 26, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Anothaisintawee, T.; Reutrakul, S.; Van Cauter, E.; Thakkinstian, A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med. Rev. 2016, 30, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Kvale, E.A.; Shuster, J.L. Sleep disturbance in supportive care of cancer: A review. J. Palliat. Med. 2006, 9, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yin, Z.; Fang, B. Measurements and status of sleep quality in patients with cancers. Support. Care Cancer 2018, 26, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.T.; Huang, C.; Gogenur, I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: A systematic review. Sleep Med. Rev. 2015, 20, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Wielgus, K.K.; Young-McCaughan, S.; Fischer, P.; Farr, L.; Lee, K.A. Methodological challenges when using actigraphy in research. J. Pain Symptom Manag. 2008, 36, 191–199. [Google Scholar] [CrossRef]
- Langford, D.J.; Lee, K.; Miaskowski, C. Sleep disturbance interventions in oncology patients and family caregivers: A comprehensive review and meta-analysis. Sleep Med. Rev. 2012, 16, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.L.; Schwartz, A.L.; Towsley, G.; Dudley, W.; Barsevick, A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J. Pain Symptom Manag. 2004, 27, 140–148. [Google Scholar] [CrossRef]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Balachandran, D.; Pedraza Cardozo, S.L.; Berg, E.A.; Chisholm, G.B.; Reddy, A.; DeLa Cruz, V.; Williams, J.L.; Bruera, E. Patient-reported sleep disturbance in advanced cancer: Frequency, predictors and screening performance of the Edmonton Symptom Assessment System sleep item. BMJ Support. Palliat. Care 2017, 7, 274–280. [Google Scholar] [CrossRef]
- Watanabe, S.M.; Nekolaichuk, C.; Beaumont, C.; Johnson, L.; Myers, J.; Strasser, F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J. Pain Symptom Manag. 2011, 41, 456–468. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Barla, S.R.; Arthur, J.; Chisholm, G.B.; Bruera, E. Frequency and characteristics of drowsiness, somnolence, or daytime sleepiness in patients with advanced cancer. Palliat. Support. Care 2019, 17, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Akman, T.; Yavuzsen, T.; Sevgen, Z.; Ellidokuz, H.; Yilmaz, A.U. Evaluation of sleep disorders in cancer patients based on Pittsburgh Sleep Quality Index. Eur. J. Cancer Care 2015, 24, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Lin, C.Y.; Cheng, A.S.K.; Nejati, B.; Imani, V.; Ulander, M.; Browall, M.; Griffiths, M.D.; Broström, A.; Pakpour, A.H. A thorough psychometric comparison between Athens Insomnia Scale and Insomnia Severity Index among patients with advanced cancer. J. Sleep Res. 2020, 29, e12891. [Google Scholar] [CrossRef]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef]
- Jakobsen, G.; Engstrom, M.; Thronaes, M.; Lohre, E.T.; Kaasa, S.; Fayers, P.; Hjermstad, M.J.; Klepstad, P. Sleep quality in hospitalized patients with advanced cancer: An observational study using self-reports of sleep and actigraphy. Support. Care Cancer 2020, 28, 2015–2023. [Google Scholar] [CrossRef]
- Palesh, O.; Haitz, K.; Levi, F.; Bjarnason, G.A.; Deguzman, C.; Alizeh, I.; Ulusakarya, A.; Packer, M.M.; Innominato, P.F. Relationship between subjective and actigraphy-measured sleep in 237 patients with metastatic colorectal cancer. Qual. Life Res. 2017, 26, 2783–2791. [Google Scholar] [CrossRef]
- Bernatchez, M.S.; Savard, J.; Savard, M.H.; Aubin, M.; Ivers, H. Sleep-wake difficulties in community-dwelling cancer patients receiving palliative care: Subjective and objective assessment. Palliat. Support. Care 2018, 16, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.L.; Chang, W.P.; Lin, C.C. Rest/activity rhythm is related to the coexistence of pain and sleep disturbance among advanced cancer patients with pain. Support. Care Cancer 2014, 22, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Boyne, K.; Sherry, D.D.; Gallagher, P.R.; Olsen, M.; Brooks, L.J. Accuracy of computer algorithms and the human eye in scoring actigraphy. Sleep Breath. Schlaf Atm. 2013, 17, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Martin, J.L.; Blackwell, T.; Buenaver, L.; Liu, L.; Meltzer, L.J.; Sadeh, A.; Spira, A.P.; Taylor, D.J. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav. Sleep Med. 2015, 13 (Suppl. 1), S4–S38. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef]
- Milanti, A.; Chan, D.N.S.; Li, C.; So, W.K.W. Actigraphy-measured rest-activity circadian rhythm disruption in patients with advanced cancer: A scoping review. Support. Care Cancer 2021, 29, 7145–7169. [Google Scholar] [CrossRef] [PubMed]
- Luyster, F.S.; Strollo, P.J., Jr.; Zee, P.C.; Walsh, J.K. Sleep: A health imperative. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef]
- Borbely, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Innominato, P.F.; Roche, V.P.; Palesh, O.G.; Ulusakarya, A.; Spiegel, D.; Levi, F.A. The circadian timing system in clinical oncology. Ann. Med. 2014, 46, 191–207. [Google Scholar] [CrossRef]
- Innominato, P.F.; Komarzynski, S.; Palesh, O.G.; Dallmann, R.; Bjarnason, G.A.; Giacchetti, S.; Ulusakarya, A.; Bouchahda, M.; Haydar, M.; Ballesta, A.; et al. Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med. 2018, 7, 4396–4405. [Google Scholar] [CrossRef] [PubMed]
- Al Maqbali, M.; Al Sinani, M.; Alsayed, A.; Gleason, A.M. Prevalence of Sleep Disturbance in Patients With Cancer: A Systematic Review and Meta-Analysis. Clin. Nurs. Res. 2022, 31, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J.; Lang, B.J. Sleep and cancer: Recent developments. Curr. Oncol. Rep. 2008, 10, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.; Oliver, T.K.; Keller-Olaman, S.; Davidson, J.R.; Garland, S.; Samuels, C.; Savard, J.; Harris, C.; Aubin, M.; Olson, K.; et al. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Ann. Oncol. 2014, 25, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Divani, A.; Heidari, M.E.; Ghavampour, N.; Parouhan, A.; Ahmadi, S.; Narimani Charan, O.; Shahsavari, H. Effect of cancer treatment on sleep quality in cancer patients: A systematic review and meta-analysis of Pittsburgh Sleep Quality Index. Support. Care Cancer 2022, 30, 4687–4697. [Google Scholar] [CrossRef] [PubMed]
- Nzwalo, I.; Aboim, M.A.; Joaquim, N.; Marreiros, A.; Nzwalo, H. Systematic Review of the Prevalence, Predictors, and Treatment of Insomnia in Palliative Care. Am. J. Hosp. Palliat. Care 2020, 37, 957–969. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Partinen, M. Insomnia and global sleep dissatisfaction in Finland. J. Sleep Res. 2002, 11, 339–346. [Google Scholar] [CrossRef]
- Pallesen, S.; Sivertsen, B.; Nordhus, I.H.; Bjorvatn, B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 2014, 15, 173–179. [Google Scholar] [CrossRef]
- Jakobsen, G.; Engstrom, M.; Fayers, P.; Hjermstad, M.J.; Kaasa, S.; Kloke, M.; Sabatowski, R.; Klepstad, P. Sleep quality with WHO Step III opioid use for cancer pain. BMJ Support. Palliat. Care 2019, 9, 307–315. [Google Scholar] [CrossRef]
- Collins, K.P.; Geller, D.A.; Antoni, M.; Donnell, D.M.; Tsung, A.; Marsh, J.W.; Burke, L.; Penedo, F.; Terhorst, L.; Kamarck, T.W.; et al. Sleep duration is associated with survival in advanced cancer patients. Sleep Med. 2017, 32, 208–212. [Google Scholar] [CrossRef]
- George, G.C.; Iwuanyanwu, E.C.; Anderson, K.O.; Yusuf, A.; Zinner, R.G.; Piha-Paul, S.A.; Tsimberidou, A.M.; Naing, A.; Fu, S.; Janku, F.; et al. Sleep quality and its association with fatigue, symptom burden, and mood in patients with advanced cancer in a clinic for early-phase oncology clinical trials. Cancer 2016, 122, 3401–3409. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Aielli, F.; Adile, C.; Ferrera, P.; Valle, A.; Cartoni, C.; Pizzuto, M.; Caruselli, A.; Parsi, R.; Cortegiani, A.; et al. Sleep Disturbances in Patients with Advanced Cancer in Different Palliative Care Settings. J. Pain Symptom Manag. 2015, 50, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, M.; Tamura, A.; Nagai, H.; Matsushima, E. Assessment of sleep disturbance in lung cancer patients: Relationship between sleep disturbance and pain, fatigue, quality of life, and psychological distress. Palliat. Support. Care 2015, 13, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Khoshknabi, D.; Walsh, D.; Lagman, R.; Platt, A. Insomnia in patients with advanced cancer. Am. J. Hosp. Palliat. Care 2014, 31, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Chisholm, G.; Palla, S.L.; Holmes, H.; Reuben, J.M.; Bruera, E. Self-reported sleep disturbance in patients with advanced cancer: Frequency, intensity, and factors associated with response to outpatient supportive care consultation—A preliminary report. Palliat. Support. Care 2013, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Guay, M.; Yennurajalingam, S.; Parsons, H.; Palmer, J.L.; Bruera, E. Association between self-reported sleep disturbance and other symptoms in patients with advanced cancer. J. Pain Symptom Manag. 2011, 41, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Valle, A.; Cartoni, C.; Pizzuto, M. Insomnia in patients with advanced lung cancer admitted to palliative care services. Int. J. Clin. Pract. 2021, 75, e14521. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Pathiaki, M.; Patiraki, E.; Galanos, A.; Vlahos, L. Sleep quality in advanced cancer patients. J. Psychosom. Res. 2007, 62, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Graci, G. Pathogenesis and management of cancer-related insomnia. J. Support. Oncol. 2005, 3, 349–359. [Google Scholar] [PubMed]
- Fleming, L.; Davidson, J.R. Sleep and Medical Disorders. In The Oxford Handbook of Sleep and Sleep Disorders; Morin, C.M., Espie, C., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 505–519. [Google Scholar]
- Matthews, E.E.; Wang, S.Y. Cancer-Related Sleep Wake Disturbances. Semin. Oncol. Nurs. 2022, 38, 151253. [Google Scholar] [CrossRef] [PubMed]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, W.J.; Klerman, E.B. Circadian Neurobiology and the Physiologic Regulation of Sleep and Wakefulness. Neurol. Clin. 2019, 37, 475–486. [Google Scholar] [CrossRef]
- Young, J.S.; Bourgeois, J.A.; Hilty, D.M.; Hardin, K.A. Sleep in hospitalized medical patients, part 1: Factors affecting sleep. J. Hosp. Med. 2008, 3, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Frisbee-Hume, S.; Palmer, J.L.; Delgado-Guay, M.O.; Bull, J.; Phan, A.T.; Tannir, N.M.; Litton, J.K.; Reddy, A.; Hui, D.; et al. Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J. Clin. Oncol. 2013, 31, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Liu, L.; Marler, M.R.; Parker, B.A.; Jones, V.; Sadler, G.R.; Dimsdale, J.; Cohen-Zion, M.; Fiorentino, L. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support. Care Cancer 2006, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rissling, M.; Natarajan, L.; Fiorentino, L.; Mills, P.J.; Dimsdale, J.E.; Sadler, G.R.; Parker, B.A.; Ancoli-Israel, S. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep 2012, 35, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Laugsand, E.A.; Kaasa, S.; de Conno, F.; Hanks, G.; Klepstad, P. Intensity and treatment of symptoms in 3,030 palliative care patients: A cross-sectional survey of the EAPC Research Network. J. Opioid Manag. 2009, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Meuser, T.; Pietruck, C.; Radbruch, L.; Stute, P.; Lehmann, K.A.; Grond, S. Symptoms during cancer pain treatment following WHO-guidelines: A longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001, 93, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Gennatas, C.; Galanos, A.; Vlahos, L. How is sleep quality affected by the psychological and symptom distress of advanced cancer patients? Palliat. Med. 2009, 23, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Renom-Guiteras, A.; Planas, J.; Farriols, C.; Mojal, S.; Miralles, R.; Silvent, M.A.; Ruiz-Ripoll, A.I. Insomnia among patients with advanced disease during admission in a Palliative Care Unit: A prospective observational study on its frequency and association with psychological, physical and environmental factors. BMC Palliat. Care 2014, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Akechi, T.; Okuyama, T.; Akizuki, N.; Shimizu, K.; Inagaki, M.; Fujimori, M.; Shima, Y.; Furukawa, T.A.; Uchitomi, Y. Associated and predictive factors of sleep disturbance in advanced cancer patients. Psychooncology 2007, 16, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.G.; Collie, K.; Batiuchok, D.; Tilston, J.; Koopman, C.; Perlis, M.L.; Butler, L.D.; Carlson, R.; Spiegel, D. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol. Psychol. 2007, 75, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lou, V.W.; Chen, E.J.; Jian, H.; Zhou, Z.; Zhu, J.; Li, G.; He, Y. Respiratory Symptoms, Sleep, and Quality of Life in Patients With Advanced Lung Cancer. J. Pain Symptom Manag. 2017, 53, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Strøm, L.; Danielsen, J.T.; Amidi, A.; Cardenas Egusquiza, A.L.; Wu, L.M.; Zachariae, R. Sleep During Oncological Treatment—A Systematic Review and Meta-Analysis of Associations With Treatment Response, Time to Progression and Survival. Front. Neurosci. 2022, 16, 199. [Google Scholar] [CrossRef] [PubMed]
- Tembo, A.C.; Parker, V. Factors that impact on sleep in intensive care patients. Intensive Crit. Care Nurs. 2009, 25, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.; Matsumura, C.; Tahara, Y.; Sako, M.; Kurosawa, H.; Nomura, T.; Eguchi, Y.; Ohba, K.; Yano, Y. Symptom clusters and their influence on prognosis using EORTC QLQ-C15-PAL scores in terminally ill patients with cancer. Support. Care Cancer 2022, 30, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Madero, R.; Alonso, A.; Martínez-Marín, V.; Vilches, Y.; Martínez, B.; Feliu, M.; Díaz, L.; Espinosa, E.; Feliu, J. Symptom clusters in advanced cancer. J. Pain Symptom Manag. 2011, 42, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, M.S.; Savard, J.; Aubin, M.; Ivers, H. Correlates of disrupted sleep-wake variables in patients with advanced cancer. BMJ Support. Palliat. Care 2018. Epub ahead of print. [Google Scholar] [CrossRef]
- Khater, W.; Masha’al, D.; Al-Sayaheen, A. Sleep assessment and interventions for patients living with cancer from the patients’ and nurses’ perspective. Int. J. Palliat. Nurs. 2019, 25, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Siefert, M.L.; Hong, F.; Valcarce, B.; Berry, D.L. Patient and clinician communication of self-reported insomnia during ambulatory cancer care clinic visits. Cancer Nurs. 2014, 37, E51–E59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nesbitt, L.; Goode, D. Nurses perceptions of sleep in the intensive care unit environment: A literature review. Intensive Crit. Care Nurs. 2014, 30, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Keane, K.; Hutton Johnson, S.; Dykes, P.C. How do clinicians assess, communicate about, and manage patient sleep in the hospital? J. Nurs. Adm. 2013, 43, 342–347. [Google Scholar] [CrossRef]
- Gellerstedt, L.; Medin, J.; Kumlin, M.; Rydell Karlsson, M. Nurses’ experiences of hospitalised patients’ sleep in Sweden: A qualitative study. J. Clin. Nurs. 2015, 24, 3664–3673. [Google Scholar] [CrossRef] [PubMed]
- Gellerstedt, L.; Medin, J.; Kumlin, M.; Rydell Karlsson, M. Sleep as a topic in nursing education programs? A mixed method study of syllabuses and nursing students’ perceptions. Nurse Educ. Today 2019, 79, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ritmala-Castren, M.; Virtanen, I.; Vahlberg, T.; Leivo, S.; Kaukonen, K.M.; Leino-Kilpi, H. Evaluation of patients’ sleep by nurses in an ICU. J. Clin. Nurs. 2016, 25, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Gellerstedt, L.; Medin, J.; Kumlin, M.; Rydell Karlsson, M. Nursing care and management of patients’ sleep during hospitalisation: A cross-sectional study. J. Clin. Nurs. 2019, 28, 3400–3407. [Google Scholar] [CrossRef] [PubMed]
- Induru, R.R.; Walsh, D. Cancer-related insomnia. Am. J. Hosp. Palliat. Care 2014, 31, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.E.; Shelby, R.A.; Mitchell, S.A.; Fawzy, M.R.; Hardy, N.C.; Husain, A.M.; Keefe, F.J.; Krystal, A.D.; Porter, L.S.; Reeve, B.B.; et al. Sleep-wake functioning along the cancer continuum: Focus group results from the Patient-Reported Outcomes Measurement Information System (PROMIS(®)). Psychooncology 2010, 19, 1086–1093. [Google Scholar] [CrossRef]
- Davidson, J.R.; Feldman-Stewart, D.; Brennenstuhl, S.; Ram, S. How to provide insomnia interventions to people with cancer: Insights from patients. Psychooncology 2007, 16, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, R.M. Impact of mood disturbance, sleep disturbance, fatigue and pain among patients receiving cancer therapy. Eur. J. Cancer Care 2013, 22, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Gullvåg, M.; Gjeilo, K.H.; Fålun, N.; Norekvål, T.M.; Mo, R.; Broström, A. Sleepless nights and sleepy days: A qualitative study exploring the experiences of patients with chronic heart failure and newly verified sleep-disordered breathing. Scand. J. Caring Sci. 2019, 33, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Lundeby, T.; Hjermstad, M.J.; Aass, N.; Kaasa, S. Integration of palliative care in oncology-the intersection of cultures and perspectives of oncology and palliative care. Ecancermedicalscience 2022, 16, 1376. [Google Scholar] [CrossRef] [PubMed]
- Grutsch, J.F.; Wood, P.A.; Du-Quiton, J.; Reynolds, J.L.; Lis, C.G.; Levin, R.D.; Ann Daehler, M.; Gupta, D.; Quiton, D.F.; Hrushesky, W.J. Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. J. Circadian. Rhythm. 2011, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.; Jarrin, D.C.; Leanza, Y.; Vallières, A.; Morin, C.M. Qualitative studies of insomnia: Current state of knowledge in the field. Sleep Med. Rev. 2017, 31, 58–69. [Google Scholar] [CrossRef]
- Absolon, N.A.; Balneaves, L.; Truant, T.L.; Cashman, R.L.; Wong, M.; Hamm, J.; Witmans, M. A Self-Administered Sleep Intervention for Patients With Cancer Experiencing Insomnia. Clin. J. Oncol. Nurs. 2016, 20, 289–297. [Google Scholar] [CrossRef]
- Hugel, H.; Ellershaw, J.E.; Cook, L.; Skinner, J.; Irvine, C. The prevalence, key causes and management of insomnia in palliative care patients. J. Pain Symptome Manag. 2004, 27, 316–321. [Google Scholar] [CrossRef]
- Garland, S.N.; Johnson, J.A.; Savard, J.; Gehrman, P.; Perlis, M.; Carlson, L.; Campbell, T. Sleeping well with cancer: A systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr. Dis. Treat. 2014, 10, 1113–1124. [Google Scholar] [CrossRef]
- Elliott, J.E.; McBride, A.A.; Balba, N.M.; Thomas, S.V.; Pattinson, C.L.; Morasco, B.J.; Wilkerson, A.; Gill, J.M.; Lim, M.M. Feasibility and preliminary efficacy for morning bright light therapy to improve sleep and plasma biomarkers in US Veterans with TBI. A prospective, open-label, single-arm trial. PLoS ONE 2022, 17, e0262955. [Google Scholar] [CrossRef]
- Palliative Care Network of Wisconsin. Fast Facts #105. Insomnia: Drug Therapies. Available online: https://www.mypcnow.org/fast-fact/insomnia-drug-therapies/ (accessed on 8 August 2022).
| Author, Country (Year) | N | Prevalence of Poor Sleep 1 | Questionnaire |
|---|---|---|---|
| Mercadante, Italy (2021) [59] | 182 | 50% | Athens Insomnia Scale |
| Jakobsen, Denmark, Germany, Lithuania, Norway, Switzerland (2018) [51] | 604 | 78% | PSQI |
| Collins, USA (2017) [52] | 292 | 59% | PSQI |
| Yennurajalingam USA (2017) [21] | 180 | 62% | PSQI |
| George, USA (2016) [53] | 256 | 64% | PSQI |
| Akman, Turkey (2015) [25] | 314 | 40% | PSQI |
| Nishiura, Tokyo (2015) [55] | 50 | 56% | Athens Insomnia Scale |
| Mercadante, Italy (2015) [54] | 820 | 61% | Athens Insomnia Scale |
| Davis, USA (2014) [56] | 715 | 14% | Insomnia Severity Index |
| Yennurajalingam, USA (2013) [57] | 442 | 75% | Sleep item on a 10-point scale |
| Delgado-Guay, USA (2011) [58] | 101 | 85% | PSQI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobsen, G.; Gjeilo, K.H.; Hjermstad, M.J.; Klepstad, P. An Update on Prevalence, Assessment, and Risk Factors for Sleep Disturbances in Patients with Advanced Cancer—Implications for Health Care Providers and Clinical Research. Cancers 2022, 14, 3933. https://doi.org/10.3390/cancers14163933
Jakobsen G, Gjeilo KH, Hjermstad MJ, Klepstad P. An Update on Prevalence, Assessment, and Risk Factors for Sleep Disturbances in Patients with Advanced Cancer—Implications for Health Care Providers and Clinical Research. Cancers. 2022; 14(16):3933. https://doi.org/10.3390/cancers14163933
Chicago/Turabian StyleJakobsen, Gunnhild, Kari Hanne Gjeilo, Marianne Jensen Hjermstad, and Pål Klepstad. 2022. "An Update on Prevalence, Assessment, and Risk Factors for Sleep Disturbances in Patients with Advanced Cancer—Implications for Health Care Providers and Clinical Research" Cancers 14, no. 16: 3933. https://doi.org/10.3390/cancers14163933
APA StyleJakobsen, G., Gjeilo, K. H., Hjermstad, M. J., & Klepstad, P. (2022). An Update on Prevalence, Assessment, and Risk Factors for Sleep Disturbances in Patients with Advanced Cancer—Implications for Health Care Providers and Clinical Research. Cancers, 14(16), 3933. https://doi.org/10.3390/cancers14163933







