The Hypercoagulable Profile of Patients with Bone Tumors: A Pilot Observational Study Using Rotational Thromboelastometry
Abstract
:Simple Summary
Abstract
1. Introduction
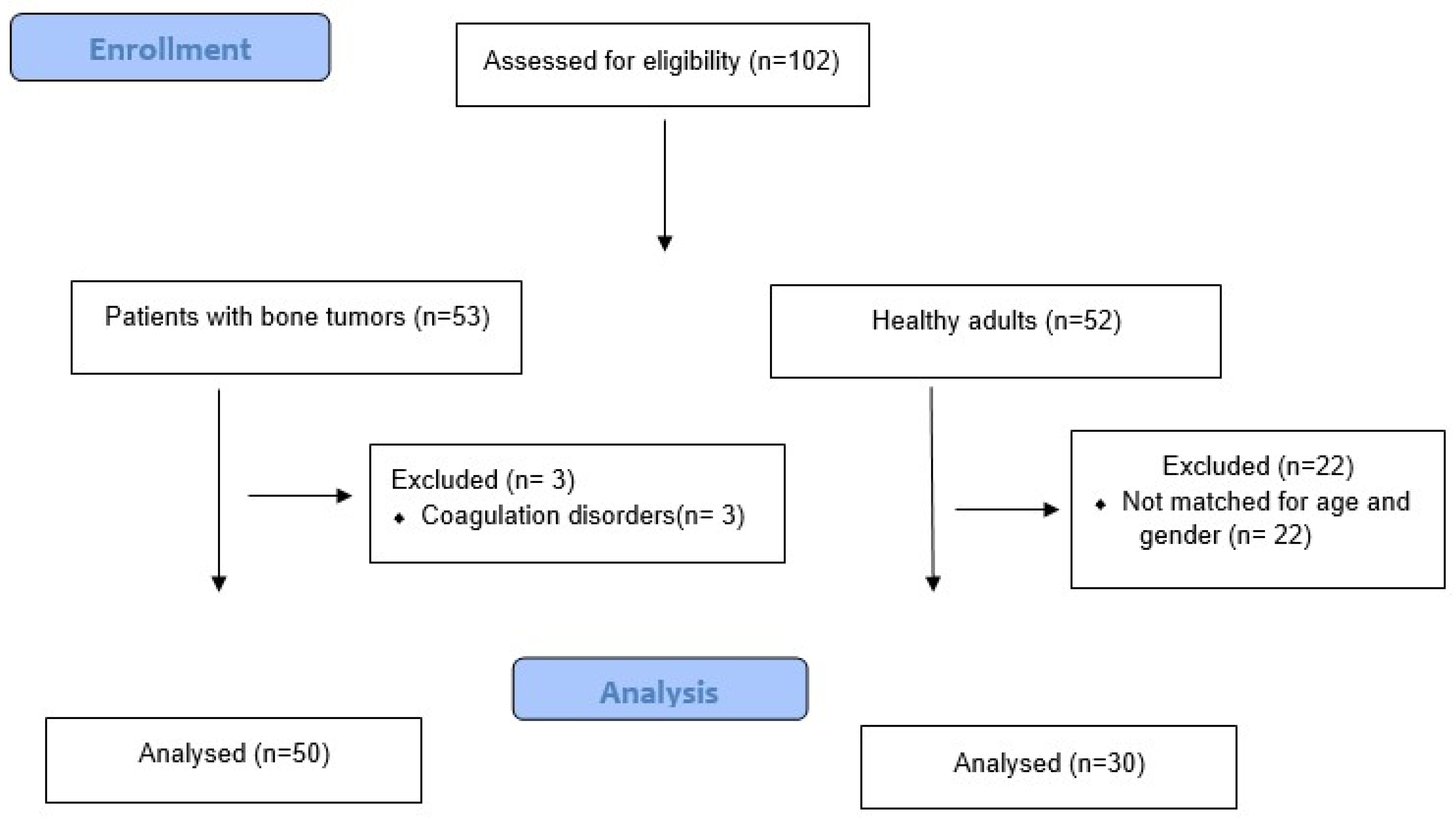
2. Methods
2.1. Sample Collection and ROTEM Analysis
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delegates, I.-V.O. Recommendations from the ICM-VTE: Oncology. J. Bone Joint Surg. Am. 2022, 104 (Suppl. 1), 232–237. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Montagnana, M.; Targher, G.; Manzato, F.; Lippi, G. Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J. Thromb. Thrombolysis 2007, 24, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 110, 2339–2346. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Cancer-associated thrombosis. Blood Cells Mol. Dis. 2006, 36, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Moore, E.E.; Moore, H.; Thomas, S.; Lune, S.V.; Zimmer, D.; Dynako, J.; Hake, D.; Crowell, Z.; McCauley, R.; et al. Use of Viscoelastography in Malignancy-Associated Coagulopathy and Thrombosis: A Review. Semin. Thromb. Hemost. 2019, 45, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef]
- Odent, T.; de Courtivron, B.; Gruel, Y. Thrombotic risk in children undergoing orthopedic surgery. Orthop. Traumatol. Surg. Res. 2020, 106 (Suppl. 1), S109–S114. [Google Scholar] [CrossRef]
- Imberti, D.; Bianchi, C.; Zambon, A.; Parodi, A.; Merlino, L.; Gallerani, M.; Corrao, G. Venous thromboembolism after major orthopaedic surgery: A population-based cohort study. Intern. Emerg. Med. 2012, 7, 243–249. [Google Scholar] [CrossRef]
- Morii, T.; Mochizuki, K.; Tajima, T.; Aoyagi, T.; Satomi, K. Venous thromboembolism in the management of patients with musculoskeletal tumor. J. Orthop. Sci. 2010, 15, 810–815. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Matsumine, A.; Niimi, R.; Nakamura, T.; Matsubara, T.; Asanuma, K.; Hasegawa, M.; Sudo, A. Deep-vein thrombosis after resection of musculoskeletal tumours of the lower limb. Bone Joint J. 2013, 95, 1280–1284. [Google Scholar] [CrossRef]
- Volod, O.; Bunch, C.M.; Zackariya, N.; Moore, E.E.; Moore, H.B.; Kwaan, H.C.; Neal, M.D.; Al-Fadhl, M.D.; Patel, S.S.; Wiarda, G. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J. Clin. Med. 2022, 11, 860. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Vaiopoulos, A.G.; Piovani, D.; Nikolopoulos, G.K.; Kokoris, S.I.; et al. The Prognostic Performance of Rotational Thromboelastometry for Excessive Bleeding and Increased Transfusion Requirements in Hip Fracture Surgeries. Thromb. Haemost. 2021, 122, 895–904. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Piovani, D.; Kriebardis, A.G.; Gialeraki, A.; Nikolopoulos, G.K.; et al. Rotational Thromboelastometry Findings Are Associated with Symptomatic Venous Thromboembolic Complications after Hip Fracture Surgery. Clin. Orthop. Relat. Res. 2021, 479, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Savvidou, O.D.; Kontogeorgakos, V.A.; Piovani, D.; Kriebardis, A.G.; et al. Higher coagulation activity in hip fracture patients: A case-control study using rotational thromboelastometry. Int. J. Lab. Hematol. 2021, 43, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G. Rotational Thromboelastometry Predicts Transfusion Requirements in Total Joint Arthroplasties. Semin. Thromb. Hemost. 2022. Ahead of print. [Google Scholar]
- Walsh, M.; Fries, D.; Moore, E.; Moore, H.; Thomas, S.; Kwaan, H.C.; Marsee, M.K.; Grisoli, A.; McCauley, R.; Vande Lune, S.; et al. Whole Blood for Civilian Urban Trauma Resuscitation: Historical, Present, and Future Considerations. Semin. Thromb. Hemost. 2020, 46, 221–234. [Google Scholar] [CrossRef]
- Hvas, C.L.; Larsen, J.B.; Adelborg, K.; Christensen, S.; Hvas, A.M. Dynamic Hemostasis and Fibrinolysis Assays in Intensive Care COVID-19 Patients and Association with Thrombosis and Bleeding-A Systematic Review and a Cohort Study. Semin. Thromb. Hemost. 2022, 48, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, A.; Sokou, R.; Tsantes, A.G.; Parastatidou, S.; Bonovas, S.; Kouskouni, E.; Gounaris, A.K.; Tsantes, A.E.; Iacovidou, N. Thromboelastometry Variables in Neonates with Perinatal Hypoxia. Semin. Thromb. Hemost. 2020, 46, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sokou, R.; Tsantes, A.G.; Konstantinidi, A.; Ioakeimidis, G.; Lampridou, M.; Parastatidou, S.; Theodoraki, M.; Piovani, D.; Iliodromiti, Z.; Boutsikou, T.; et al. Rotational Thromboelastometry in Neonates Admitted to a Neonatal Intensive Care Unit: A Large Cross-sectional Study. Semin. Thromb. Hemost. 2021, 47, 875–884. [Google Scholar] [PubMed]
- Souliotis, K.; Golna, C.; Nikolaidi, S.; Dreden, P.V.; Vatheia, G.; Gerotziafas, G.T. Public Awareness on Cancer-Associated Thrombosis among the Greek Population: First Findings from the ROADMAP-CAT Awareness Study. TH Open 2022, 6, e89–e95. [Google Scholar] [CrossRef]
- Muntz, J. Thromboprophylaxis in orthopedic surgery: How long is long enough? Am. J. Orthop. 2009, 38, 394–401. [Google Scholar] [PubMed]
- Walsh, M.; Kwaan, H.; McCauley, R.; Marsee, M.; Speybroeck, J.; Thomas, S.; Hatch, J.; Vande Lune, S.; Grisoli, A.; Wadsworth, S.; et al. Viscoelastic testing in oncology patients (including for the diagnosis of fibrinolysis): Review of existing evidence, technology comparison, and clinical utility. Transfusion 2020, 60 (Suppl. 6), S86–S100. [Google Scholar] [CrossRef] [PubMed]
- Toukh, M.; Siemens, D.R.; Black, A.; Robb, S.; Leveridge, M.; Graham, C.H.; Othman, M. Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thromb. Res. 2014, 133, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wehrum, M.J.; Hines, J.F.; Hayes, E.B.; Kost, E.R.; Hall, K.L.; Paidas, M.J. Comparative assessment of hypercoagulability in women with and without gynecologic malignancies using the thromboelastograph coagulation analyzer. Blood Coagul. Fibrinolysis 2010, 21, 140–143. [Google Scholar] [CrossRef]
- Akay, O.M.; Ustuner, Z.; Canturk, Z.; Mutlu, F.S.; Gulbas, Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med. Oncol. 2009, 26, 358–364. [Google Scholar] [CrossRef]
- Davies, N.A.; Harrison, N.K.; Sabra, A.; Lawrence, M.J.; Noble, S.; Davidson, S.J.; Evans, V.J.; Morris, R.H.; Hawkins, K.; Williams, P.R.; et al. Application of ROTEM to assess hypercoagulability in patients with lung cancer. Thromb. Res. 2015, 135, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Qin, Q.; Que, X.; Chen, Y.; Wei, Y.; Chen, H.; Li, Q.; Meng, C.; Liang, Z. Utility of Thromboelastography to Identify Hypercoagulability in Lung Cancer Related Ischemic Stroke Patients. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620975502. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Goumenos, S.; Piovani, D.; Nikolopoulos, G.K.; Gialeraki, A.; Bonovas, S.; Papagelopoulos, P.J.; Kontogeorgakos, V.A.; et al. The Safety and Efficacy of Tranexamic Acid in Oncology Patients Undergoing Endoprosthetic Reconstruction and a ROTEM-Based Evaluation of Their Hemostatic Profile: A Pilot Study. Cancers 2021, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.E.; Frantzeskaki, F.; Tsantes, A.G.; Rapti, E.; Rizos, M.; Kokoris, S.I.; Paramythiotou, E.; Katsadiotis, G.; Karali, V.; Flevari, A.; et al. The haemostatic profile in critically ill COVID-19 patients receiving therapeutic anticoagulant therapy: An observational study. Medicine 2020, 99, e23365. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Goumenos, S.; Piovani, D.; Tsante, K.A.; Mavrogenis, A.F.; Vaiopoulos, A.G.; Koulouvaris, P.; Nikolopoulos, G.K.; et al. The Procoagulant Effect of COVID-19 on the Thrombotic Risk of Patients with Hip Fractures Due to Enhanced Clot Strength and Fibrinolysis Shutdown. J. Clin. Med. 2021, 10, 3397. [Google Scholar] [CrossRef]
- Tsantes, A.E.; Tsantes, A.G.; Kokoris, S.I.; Bonovas, S.; Frantzeskaki, F.; Tsangaris, I.; Kopterides, P. COVID-19 Infection-Related Coagulopathy and Viscoelastic Methods: A Paradigm for Their Clinical Utility in Critical Illness. Diagnostics 2020, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Paniccia, A.; Lawson, P.J.; Torphy, R.J.; Nydam, T.L.; Moore, E.E.; McCarter, M.D.; Schulick, R.D.; Edil, B.H. Utility of Viscoelastic Assays Beyond Coagulation: Can Preoperative Thrombelastography Indices Predict Tumor Histology, Nodal Disease, and Resectability in Patients Undergoing Pancreatectomy? J. Am. Coll Surg. 2018, 227, 55–62. [Google Scholar] [CrossRef]
- Hincker, A.; Feit, J.; Sladen, R.; Wagener, G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit. Care 2014, 18, 549. [Google Scholar] [CrossRef]

| Patients with Tumors (n = 50) | Control Group (n = 30) | p-Value | |
|---|---|---|---|
| Age (years) | 47.9± 22.9, 53 (22–66) | 54.8 ± 4.3, 55 (53–59) | 0.62 |
| Gender (males %) | 24 (48.0) | 16 (51.6) | 0.86 |
| BMI (kgr/m2) | 20.7 ± 2.1, 21.0 (19.0–22.0) | 21.8 ± 2.3, 22 (20–23) | 0.10 |
| Smoking | 9 (18.0) | 4 (13.3) | 0.54 |
| Tumor Primary Metastatic | 38 (76.0) 12 (24.0) | - | |
| Location Proximal femur Distal femur Proximal tibia | 15 (30.0) 32 (64.0) 3 (6.0) | - |
| Variables | Patients with Tumors (n = 50) | Control Group (n = 30) | p-Value |
|---|---|---|---|
| PLTs (count × 103/mL) | 260.5 ± 101.3, 259.0 (182.0–324.0) | 233.8 ± 20.8, 231.0 (225.0–245.0) | 0.17 |
| aPTT (s) | 30.9 ± 3.8, 30.6 (28.5–30.6) | 31.7 ± 2.8, 31.6 (30.0–34.0) | 0.14 |
| PT (s) | 12.2 ± 1.6, 11.8 (11.2–12.8) | 12.1 ± 1.8, 12.0 (10.4–13.2) | 0.78 |
| Variables | Patients with Tumors (n = 50) | Control Group (n = 30) | p-Value |
|---|---|---|---|
| EXTEM CT (s) | 61.2 ± 6.2, 61 (58.0–65.0) | 64.9 ± 7.3, 65.0 (59.0–70.0) | 0.034 |
| EXTEM CFT (s) | 53.1 ± 19.6, 51.5 (47.0–55.0) | 88.9 ± 5.7, 90.0 (86.0–93.0) | <0.001 |
| EXTEM A10 (mm) | 62.9 ± 8.3, 64.0 (59.0–67.0) | 52.0 ± 4.2, 51.0 (49.0–54.0) | <0.001 |
| EXTEM MCF (mm) | 69.5 ± 8.0, 70.0 (63.0–75.0) | 58.9 ± 3.5, 59.0 (57.0–61.0) | <0.001 |
| EXTEM Alpha angle (°) | 80.4 ± 6.5, 80.0 (77.0–83.5) | 70.8 ± 2.7, 70.5 (69.0–73.0) | <0.001 |
| EXTEM LI60 (%) | 92.5 ± 2.5, 93.0 (91.0–94.0) | 90.6 ± 4.2, 91.0 (88.0–93.5) | 0.022 |
| INTEM CT (s) | 179.6 ± 10.2, 178.0 (175.0 −182.0) | 185.1 ± 5.2, 186.0 (182.0–189.0) | <0.001 |
| INTEM CFT (s) | 64.8 ± 15.1, 64.0 (61.0–68.0) | 72.7 ± 4.8, 73.0 (70.0–74.0) | <0.001 |
| INTEM A10 (mm) | 66.7 ± 8.9, 67.0 (62.5–71.0) | 55.6 ± 4.1, 57.0 (51.0–58.0) | <0.001 |
| INTEM MCF (mm) | 70.9 ± 7.9, 72.0 (67.0–75.0) | 59.0 ± 4.0, 59.0 (58.0–60.0) | <0.001 |
| INTEM Alpha angle (°) | 82.0 ± 5.8, 82.0 (80.0–84.0) | 76.0 ± 4.1, 76.0 (74.0–78.0) | <0.001 |
| INTEM LI60 (%) | 93.6 ± 3.2, 94 (92.0–96.0) | 88.0 ± 3.5, 88.0 (85.0–91.0) | <0.001 |
| Variables | Bone Tumor | ||
|---|---|---|---|
| Coefficient | 95% CI | p-Value | |
| EXTEM CT (s) | −3.62 | −6.95 to −0.28 | 0.034 |
| EXTEM CFT (s) | −34.71 | −41.67 to −27.75 | <0.001 |
| EXTEM A10 (mm) | +10.71 | +7.31 to +14.11 | <0.001 |
| EXTEM MCF (mm) | +10.46 | +7.27 to +13.64 | <0.001 |
| EXTEM alpha angle (°) | +10.11 | +7.64 to +12.57 | <0.001 |
| EXTEM LI60 (%) | +1.92 | +0.28 to +3.56 | 0.022 |
| INTEM CT (s) | −5.43 | −9.67 to −1.19 | 0.013 |
| INTEM CFT (s) | −6.50 | −11.49 to −1.52 | 0.011 |
| INTEM A10 (mm) | +11.32 | +7.69 to +14.94 | <0.001 |
| INTEM MCF (mm) | +12.20 | +8.92 to +15.47 | <0.001 |
| INTEM alpha angle (°) | +6.53 | +3.88 to +9.18 | <0.001 |
| INTEM LI60 (%) | +5.42 | +3.71 to +7.12 | <0.001 |
| Variables | Preoperatively (n = 50) | Postoperatively (n = 50) | p-Value |
|---|---|---|---|
| EXTEM CT (s) | 61.2 ± 6.2, 61 (58.0–65.0) | 56.3 ± 8.9, 56 (51.0–62.0) | 0.003 |
| EXTEM CFT (s) | 53.1 ± 19.6, 51.5 (47.0–55.0) | 48.9 ± 9.7, 47.0 (45.0–50.0) | 0.002 |
| EXTEM A10 (mm) | 62.9 ± 8.3, 64.0 (59.0–67.0) | 67.4 ± 8.0, 68.0 (62.0–75.0) | 0.007 |
| EXTEM MCF (mm) | 69.5 ± 8.0, 70.0 (63.0–75.0) | 74.0 ± 5.5, 75.0 (71.0–78.0) | 0.003 |
| EXTEM LI60 (%) | 92.5 ± 2.5, 93.0 (91.0–94.0) | 92.8 ± 2.3, 93.0 (91.0–95.0) | 0.33 |
| INTEM CT (s) | 179.6 ± 10.2, 178 (175 −182) | 175.8 ± 11.7, 175 (170.0–181.5) | 0.013 |
| INTEM CFT (s) | 64.8 ± 15.9, 64.0 (61.0–68.0) | 60.7 ± 12.1, 60.0 (54.5–67.0) | 0.018 |
| INTEM A10 (mm) | 66.7 ± 8.9, 67.0 (62.5–71.0) | 71.0 ± 6.7, 71.5 (67.0–76.0) | 0.009 |
| INTEM MCF (mm) | 70.9 ± 7.9, 72 (67.0–75.0) | 75.7 ± 6.6, 77 (73.0–79.0) | 0.005 |
| INTEM LI60 (%) | 93.6 ± 3.2, 94 (92.0–96.0) | 94.3 ± 3.0, 94.5 (93.0–97.0) | 0.08 |
| Variables | Metastastic (n = 12) | Primary (n = 18) | p-Value |
|---|---|---|---|
| EXTEM CT (s) | 62.0 ± 3.1, 61.5 (59.5–65.0) | 60.9 ± 7.1, 61.0 (56.0–65.0) | 0.54 |
| EXTEM CFT (s) | 46.2 ± 11.1, 48.5 (36.0–54.5) | 57.4 ± 19.5, 52.0 (48.0–58.0) | 0.15 |
| EXTEM A10 (mm) | 72.3 ± 4.6, 73.5 (68.0–77.0) | 59.2 ± 6.3, 62.0 (52.0–64.0) | <0.001 |
| EXTEM MCF (mm) | 79.1 ± 3.8, 79.5 (76.0–81.0) | 65.8 ± 5.9, 68.0 (60.0–71.0) | <0.001 |
| EXTEM LI60 (%) | 92.9 ± 2.1, 92.0 (91.0–95.0) | 92.4 ± 2.6, 93.0 (91.0–94.0) | 0.81 |
| INTEM CT (s) | 176.6 ± 6.6, 177 (170 −183) | 180.8 ± 11.1, 178 (176.0–182.0) | 0.34 |
| INTEM CFT (s) | 63.0 ± 5.7, 62.0 (62.0–65.0) | 67.6 ± 14.1, 64.0 (61.0–68.0) | 0.30 |
| INTEM A10 (mm) | 70.7 ± 8.9, 72.0 (66.5–73.0) | 65.2 ± 8.6, 67.0 (62.0–69.0) | 0.040 |
| INTEM MCF (mm) | 79.2 ± 4.8, 80.0 (75.0–82.0) | 67.8 ± 6.4, 71 (61.0–72.0) | <0.001 |
| INTEM LI60 (%) | 94.2 ± 3.0, 95 (93.0–97.0) | 93.0 ± 3.1, 94.0 (92.0–95.0) | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsantes, A.G.; Loukopoulou, I.; Papadopoulos, D.V.; Trikoupis, I.G.; Roustemis, A.G.; Goumenos, S.; Sokou, R.; Tsante, K.A.; Kriebardis, A.G.; Koulouvaris, P.; et al. The Hypercoagulable Profile of Patients with Bone Tumors: A Pilot Observational Study Using Rotational Thromboelastometry. Cancers 2022, 14, 3930. https://doi.org/10.3390/cancers14163930
Tsantes AG, Loukopoulou I, Papadopoulos DV, Trikoupis IG, Roustemis AG, Goumenos S, Sokou R, Tsante KA, Kriebardis AG, Koulouvaris P, et al. The Hypercoagulable Profile of Patients with Bone Tumors: A Pilot Observational Study Using Rotational Thromboelastometry. Cancers. 2022; 14(16):3930. https://doi.org/10.3390/cancers14163930
Chicago/Turabian StyleTsantes, Andreas G., Ilectra Loukopoulou, Dimitrios V. Papadopoulos, Ioannis G. Trikoupis, Anastasios G. Roustemis, Stavros Goumenos, Rozeta Sokou, Konstantina A. Tsante, Anastasios G. Kriebardis, Panagiotis Koulouvaris, and et al. 2022. "The Hypercoagulable Profile of Patients with Bone Tumors: A Pilot Observational Study Using Rotational Thromboelastometry" Cancers 14, no. 16: 3930. https://doi.org/10.3390/cancers14163930
APA StyleTsantes, A. G., Loukopoulou, I., Papadopoulos, D. V., Trikoupis, I. G., Roustemis, A. G., Goumenos, S., Sokou, R., Tsante, K. A., Kriebardis, A. G., Koulouvaris, P., Houhoula, D., Piovani, D., Papagelopoulos, P. J., Bonovas, S., & Tsantes, A. E. (2022). The Hypercoagulable Profile of Patients with Bone Tumors: A Pilot Observational Study Using Rotational Thromboelastometry. Cancers, 14(16), 3930. https://doi.org/10.3390/cancers14163930










