Different Prognostic Values of Tumour and Nodal Response to Neoadjuvant Chemotherapy Depending on Subtypes of Inflammatory Breast Cancer, a 317 Patient-Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Statistical Analysis
2.4. Outcomes
3. Results
3.1. Population
3.2. Responses to Treatment
3.3. Follow-Up
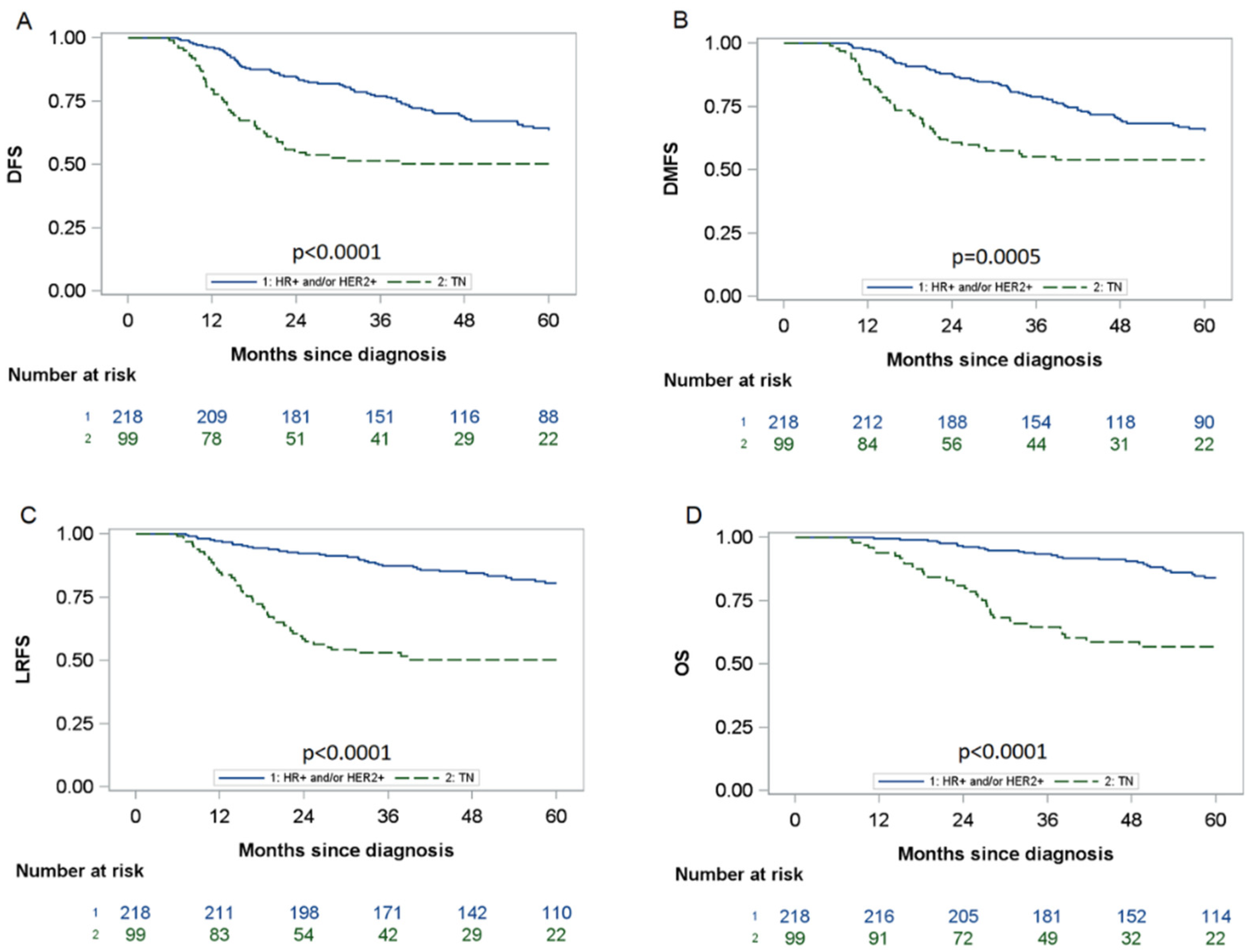
3.4. Impact of Pathological Responses on DFS by Subtype
3.5. Impact of Tumour and Node Responses on DFS According to Sataloff’s Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hance, K.W.; Anderson, W.F.; Devesa, S.S.; Young, H.A.; Levine, P.H. Trends in Inflammatory Breast Carcinoma Incidence and Survival: The Surveillance, Epidemiology, and End Results Program at the National Cancer Institute. JNCI J. Natl. Cancer Inst. 2005, 97, 966–975. [Google Scholar] [CrossRef]
- Ueno, N.T.; Espinosa Fernandez, J.R.; Cristofanilli, M.; Overmoyer, B.; Rea, D.; Berdichevski, F.; El-Shinawi, M.; Bellon, J.; Le-Petross, H.T.; Lucci, A.; et al. International Consensus on the Clinical Management of Inflammatory Breast Cancer from the Morgan Welch Inflammatory Breast Cancer Research Program 10th Anniversary Conference. J. Cancer 2018, 9, 1437–1447. [Google Scholar] [CrossRef]
- Dawood, S.; Merajver, S.D.; Viens, P.; Vermeulen, P.B.; Swain, S.M.; Buchholz, T.A.; Dirix, L.Y.; Levine, P.H.; Lucci, A.; Krishnamurthy, S.; et al. International Expert Panel on Inflammatory Breast Cancer: Consensus Statement for Standardized Diagnosis and Treatment. Ann. Oncol. 2011, 22, 515–523. [Google Scholar] [CrossRef]
- Ueno, N.T.; Buzdar, A.U.; Singletary, S.E.; Ames, F.C.; McNeese, M.D.; Holmes, F.A.; Theriault, R.L.; Strom, E.A.; Wasaff, B.J.; Asmar, L.; et al. Combined-Modality Treatment of Inflammatory Breast Carcinoma: Twenty Years of Experience at M. D. Anderson Cancer Center. Cancer Chemother. Pharmacol. 1997, 40, 321–329. [Google Scholar] [CrossRef]
- Baldini, E.; Gardin, G.; Evangelista, G.; Prochilo, T.; Collecchi, P.; Lionetto, R. Long-Term Results of Combined-Modality Therapy for Inflammatory Breast Carcinoma. Clin. Breast Cancer 2004, 5, 358–363. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Buzdar, A.U.; Sneige, N.; Smith, T.; Wasaff, B.; Ibrahim, N.; Booser, D.; Rivera, E.; Murray, J.L.; Valero, V.; et al. Paclitaxel in the Multimodality Treatment for Inflammatory Breast Carcinoma. Cancer 2001, 92, 1775–1782. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Gonzalez-Angulo, A.M.; Buzdar, A.U.; Kau, S.-W.; Frye, D.K.; Hortobagyi, G.N. Paclitaxel Improves the Prognosis in Estrogen Receptor—Negative Inflammatory Breast Cancer: The M. D. Anderson Cancer Center Experience. Clin. Breast Cancer 2004, 4, 415–419. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Moliterni, A.; Vazquez, F.; Byakhov, M.J.; Lichinitser, M.; et al. Neoadjuvant and Adjuvant Trastuzumab in Patients with HER2-Positive Locally Advanced Breast Cancer (NOAH): Follow-up of a Randomised Controlled Superiority Trial with a Parallel HER2-Negative Cohort. Lancet Oncol. 2014, 15, 640–647. [Google Scholar] [CrossRef]
- Grova, M.M.; Strassle, P.D.; Navajas, E.E.; Gallagher, K.K.; Ollila, D.W.; Downs-Canner, S.M.; Spanheimer, P.M. The Prognostic Value of Axillary Staging Following Neoadjuvant Chemotherapy in Inflammatory Breast Cancer. Ann. Surg. Oncol. 2021, 28, 2182–2190. [Google Scholar] [CrossRef]
- Rosso, K.J.; Tadros, A.B.; Weiss, A.; Warneke, C.L.; DeSnyder, S.; Kuerer, H.; Ueno, N.T.; Stecklein, S.R.; Woodward, W.A.; Lucci, A. Improved Locoregional Control in a Contemporary Cohort of Nonmetastatic Inflammatory Breast Cancer Patients Undergoing Surgery. Ann. Surg. Oncol. 2017, 24, 2981–2988. [Google Scholar] [CrossRef]
- Bertucci, F.; Tarpin, C.; Charafe-Jauffret, E.; Bardou, V.-J.; Braud, A.-C.; Tallet, A.; Gravis, G.; Viret, F.; Gonçalves, A.; Houvenaeghel, G.; et al. Multivariate Analysis of Survival in Inflammatory Breast Cancer: Impact of Intensity of Chemotherapy in Multimodality Treatment. Bone Marrow Transpl. 2004, 33, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Warren, L.E.G.; Guo, H.; Regan, M.M.; Nakhlis, F.; Yeh, E.D.; Jacene, H.A.; Hirshfield-Bartek, J.; Overmoyer, B.A.; Bellon, J.R. Inflammatory Breast Cancer: Patterns of Failure and the Case for Aggressive Locoregional Management. Ann. Surg. Oncol. 2015, 22, 2483–2491. [Google Scholar] [CrossRef]
- Van Uden, D.J.P.; van Maaren, M.C.; Bult, P.; Strobbe, L.J.A.; van der Hoeven, J.J.M.; Blanken-Peeters, C.F.J.M.; Siesling, S.; de Wilt, J.H.W. Pathologic Complete Response and Overall Survival in Breast Cancer Subtypes in Stage III Inflammatory Breast Cancer. Breast Cancer Res. Treat. 2019, 176, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Jindal, C.; Fitzgerald, T.L.; Efird, J.T. Pathologic Complete Response (PCR) and Survival of Women with Inflammatory Breast Cancer (IBC): An Analysis Based on Biologic Subtypes and Demographic Characteristics. Int. J. Environ. Res. Public Health 2019, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Sataloff, D.M.; Mason, B.A.; Prestipino, A.J.; Seinige, U.L.; Lieber, C.P.; Baloch, Z. Pathologic Response to Induction Chemotherapy in Locally Advanced Carcinoma of the Breast: A Determinant of Outcome. J. Am. Coll. Surg. 1995, 180, 297–306. [Google Scholar]
- Pierga, J.-Y.; Petit, T.; Delozier, T.; Ferrero, J.-M.; Campone, M.; Gligorov, J.; Lerebours, F.; Roché, H.; Bachelot, T.; Charafe-Jauffret, E.; et al. Neoadjuvant Bevacizumab, Trastuzumab, and Chemotherapy for Primary Inflammatory HER2-Positive Breast Cancer (BEVERLY-2): An Open-Label, Single-Arm Phase 2 Study. Lancet Oncol. 2012, 13, 375–384. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Abrial, C.; Raoelfils, I.; Cayre, A.; Mouret-Reynier, M.-A.; Leheurteur, M.; Durando, X.; Achard, J.-L.; Gimbergues, P.; Chollet, P. Comparison of the Prognostic Significance of Chevallier and Sataloff’s Pathologic Classifications after Neoadjuvant Chemotherapy of Operable Breast Cancer. Hum. Pathol. 2008, 39, 1221–1228. [Google Scholar] [CrossRef]
- Rueth, N.M.; Lin, H.Y.; Bedrosian, I.; Shaitelman, S.F.; Ueno, N.T.; Shen, Y.; Babiera, G. Underuse of Trimodality Treatment Affects Survival for Patients With Inflammatory Breast Cancer: An Analysis of Treatment and Survival Trends From the National Cancer Database. J. Clin. Oncol. 2014, 32, 2018–2024. [Google Scholar] [CrossRef]
- Bossuyt, V.; Provenzano, E.; Symmans, W.F.; Boughey, J.C.; Coles, C.; Curigliano, G.; Dixon, J.M.; Esserman, L.J.; Fastner, G.; Kuehn, T.; et al. Recommendations for Standardized Pathological Characterization of Residual Disease for Neoadjuvant Clinical Trials of Breast Cancer by the BIG-NABCG Collaboration. Ann. Oncol. 2015, 26, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP Inhibitor Veliparib plus Carboplatin or Carboplatin Alone to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer (BrighTNess): A Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Long-Term Efficacy and Safety of Addition of Carboplatin with or without Veliparib to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: 4-Year Follow-up Data from BrighTNess, a Randomized Phase III Trial. Ann. Oncol. 2022, 34, 384–394. [Google Scholar] [CrossRef]
- Bertucci, A.; Bertucci, F.; Zemmour, C.; Lerebours, F.; Pierga, J.-Y.; Levy, C.; Dalenc, F.; Grenier, J.; Petit, T.; Berline, M.; et al. PELICAN-IPC 2015-016/Oncodistinct-003: A Prospective, Multicenter, Open-Label, Randomized, Non-Comparative, Phase II Study of Pembrolizumab in Combination With Neo Adjuvant EC-Paclitaxel Regimen in HER2-Negative Inflammatory Breast Cancer. Front. Oncol. 2020, 10, 575978. [Google Scholar] [CrossRef]
- Loap, P.; Loirat, D.; Berger, F.; Ricci, F.; Vincent-Salomon, A.; Ezzili, C.; Mosseri, V.; Fourquet, A.; Ezzalfani, M.; Kirova, Y. Combination of Olaparib and Radiation Therapy for Triple Negative Breast Cancer: Preliminary Results of the RADIOPARP Phase 1 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 436–440. [Google Scholar] [CrossRef]

| All N = 317 | HR+ and/or HER2+ N = 218 | HR- HER2- N = 99 | p-Value | |
|---|---|---|---|---|
| Age, years | 53.0 (45.2–61.4) | 53.0 (46.1–61.4) | 51.8 (42–61.8) | 0.37 |
| Age, years (category) | 0.52 | |||
| <40 | 45 (14.3%) | 27 (12.4%) | 18 (18.4%) | |
| 40–49 | 85 (27.0%) | 58 (26.7%) | 27 (27.6%) | |
| 50–59 | 90 (28.6%) | 65 (30.0%) | 25 (25.5%) | |
| ≥60 | 95 (30.2%) | 67 (30.9%) | 28 (28.6%) | |
| NA | 2 (0.6%) | 1 (0.5%) | 1 (1.0%) | |
| Body Mass Index, kg/m2 | 0.040 | |||
| <25 | 103 (32.6%) | 61 (28.1%) | 42 (42.4%) | |
| ≥25 <30 | 105 (33.2%) | 76 (35.0%) | 29 (29.3%) | |
| ≥30 | 108 (34.2%) | 80 (36.9%) | 28 (28.3%) | |
| NA | 1 (0.3%) | 1 (0.5%) | 0 | |
| Histology | 0.15 | |||
| Ductal | 280 (88.9%) | 188 (87%) | 92 (92.9%) | |
| Lobular | 18 (5.7%) | 16 (7.4%) | 2 (2.0%) | |
| Other | 17 (5.4%) | 12 (5.6%) | 5 (5.1%) | |
| NA | 2 (0.6%) | 2 (0.9%) | 0 | |
| Grade SBR | <0.0001 | |||
| 1–2 | 137 (44.1%) | 115 (53.5%) | 22 (22.9%) | |
| 3 | 174 (56.0%) | 100 (46.5%) | 74 (77.1%) | |
| NA | 6 (1.9%) | 3 (1.4%) | 3 (3.0%) | |
| cN | 0.047 | |||
| N0 | 51 (16.2%) | 41 (19.0%) | 10 (10.1%) | |
| N+ | 264 (83.8%) | 175 (81.0%) | 89 (89.9%) | |
| NA | 2 (0.6%) | 2 (0.9%) | 0 | |
| WHO performance status | 0.91 | |||
| 0 | 263 (83.5%) | 180 (83%) | 83 (84.7%) | |
| 1 | 49 (15.6%) | 35 (16.1%) | 14 (14.3%) | |
| 2–3 | 3 (1.0%) | 2 (0.9%) | 1 (1.0%) | |
| NA | 2 (0.6%) | 1 (0.5%) | 1 (1.0%) | |
| Ki67 | 0.0002 | |||
| <10% | 3 (1.8%) | 3 (2.5%) | 0 | |
| 10–30% | 62 (36.3%) | 55 (45.5%) | 7 (14.0%) | |
| >30% | 106 (62.0%) | 63 (52.1%) | 43 (86.0%) | |
| NA | 146 (46.1%) | 97 (44.5%) | 49 (49.5%) | |
| Sataloff T | 0.064 | |||
| TA | 116 (37.2%) | 74 (34.3%) | 42 (43.8%) | |
| TB | 100 (32.1%) | 79 (36.6%) | 21 (21.9%) | |
| TC | 74/(23.7%) | 50 (23.2%) | 24 (25.0%) | |
| TD | 22 (7.1%) | 13 (6.0%) | 9 (9.4%) | |
| Not available | 5 (1.6%) | 2 (0.9%) | 3 (3.0%) | |
| Sataloff N | 0.096 | |||
| NA | 97 (31.2%) | 65 (30.0%) | 32 (34.0%) | |
| NB | 44 (14.2%) | 26 (12.0%) | 18 (19.2%) | |
| NC | 116 (37.3%) | 90 (41.5%) | 26 (27.7%) | |
| ND | 54 (17.4%) | 36 (16.6%) | 18 (19.2%) | |
| Not available | 6 (1.9%) | 1 (0.5%) | 5 (5.1%) | |
| ypN | NC | |||
| N0 | 141 (44.5%) | 90 (41.3%) | 51 (51.5%) | |
| N1 | 71 (22.4%) | 51 (23.4%) | 20 (20.2%) | |
| N2 | 75 (23.7%) | 56 (25.7%) | 19 (19.2%) | |
| N3 | 25 (7.9%) | 18 (8.3%) | 7 (7.1%) | |
| Nx | 5 (1.6%) | 3 (1.4%) | 2 (2.0%) | |
| ypT | NC | |||
| ypT0 | 72 (22.7%) | 42 (19.3%) | 30 (30.3%) | |
| ypTis | 19 (6.0%) | 13 (6.0%) | 6 (6.1%) | |
| ypT1 | 78 (24.6%) | 59 (27.1%) | 19 (19.2%) | |
| ypT2 | 69 (21.8%) | 47 (21.6%) | 22 (22.2%) | |
| ypT3 | 42 (13.2%) | 30 (13.8%) | 12 (12.1%) | |
| ypT4 | 27 (8.5%) | 19 (8.7%) | 8 (8.1%) | |
| ypTx | 10 (3.2%) | 8 (3.7%) | 2 (2.0%) | |
| Pathological complete response according to Sataloff | 95 (30.4%) | 60 (27.8%) | 35 (36.5%) | 0.12 |
| Not available | 5 (1.6%) | 2 (0.9%) | 3 (3.0%) | |
| Pathological complete response according to ypTNM | 84 (26.5%) | 51 (23.4%) | 33 (33.3%) | 0.063 |
| Not available | 0 | 0 | 0 |
| All N = 317 | HR+ and/or HER2+ N = 218 | TN N = 99 | p-Value | |
|---|---|---|---|---|
| Neoadjuvant chemotherapy protocol | 317 (100%) | 218 (100%) | 99 (100%) | NC |
| Number of cycles | 8 (7–8) | 8 (7–8) | 8 (7–12) | 0.014 |
| (F)EC-T | 233 (73.5%) | 169 (77.5%) | 64 (64.7%) | 0.016 |
| AC-T | 34 (10.7%) | 24 (11%) | 10 (10.1%) | 0.81 |
| Taxanes received | 316 (99.7%) | 217 (99.5%) | 99 (100%) | NC |
| Anthracyclines received | 284 (89.6%) | 193 (88.5%) | 91 (91.9%) | 0.36 |
| Platinium salts received | 3 (1%) | 0 | 3 (3%) | NC |
| Trastuzumab alone | 77 (24.3%) | 75 (34.4%) | 2 (2%) | <0.0001 |
| Trastuzumab + Pertuzumab | 14 (4.4%) | 14 (6.4%) | 0 | |
| Adjuvant systemic treatment | 219 (69.1%) | 204 (93.6%) | 15 (15.2%) | <0.0001 |
| Hormonotherapy | 168 (53%) | 164 (75.2%) | 4 (4%) | <0.0001 |
| Trastuzumab | 87 (27.4%) | 85 (39%) | 2 (2%) | <0.001 |
| Adjuvant chemotherapy | 10 (3.2%) | 1 (0.5%) | 9 (9.1%) | <0.0001 |
| TDM-1 | 3 (1%) | 3 (1.4%) | 0 | NC |
| Capecitabine | 8 (2.5%) | 1 (0.5%) | 7 (7.1%) | 0.0015 |
| Surgery | 317 (100%) | 218 (100%) | 99 (100%) | NC |
| Mastectomy + SLND | 3 (0.9%) | 1 (0.5%) | 2 (2.0%) | NC |
| Mastectomy + ALND | 310 (97.8%) | 214 (98.2%) | 96 (97.0%) | |
| Tumourectomy + SLND | 0 | 0 | 0 | |
| Tumourectomy + ALND | 4 (1.3%) | 3 (1.4%) | 1 (1.0%) | |
| Radiotherapy | 309 (97.5%) | 215 (98.6%) | 94 (95%) | 0.11 |
| Before surgery | 18 (5.7%) | 9 (4.1%) | 9 (9.1%) | 0.0028 |
| After surgery | 291 (91.8%) | 206 (94.5%) | 85 (85.9%) | |
| Dose (Gy) | 50 (50–50) | 50 (50–50) | 50 (49–50) | 0.0099 |
| Fractions | 25 (24–25) | 25 (25–25) | 25 (23–25) | 0.039 |
| Overall treatment time (days) | 37 (37–40) | 37 (35–41) | 37 (35–40) | 0.66 |
| Target area | ||||
| B or CW alone a | 11 (3.5%) | 7 (3.2%) | 4 (4.1%) | 0.74 |
| B/CW + Level 2-3-4 a | 294 (93.9%) | 206 (95.4%) | 88 (90.7%) | 0.11 |
| Internal mammary node b | 211 (69.4%) | 148 (69.8%) | 63 (68.5%) | 0.82 |
| Level 1 b | 72 (23.7%) | 52 (24.5%) | 20 (21.7%) | 0.60 |
| HR+ and/or HER2+, N = 218 | TN, N = 99 | ||||
|---|---|---|---|---|---|
| HR and 95% CI | p-Value | HR and 95% CI | p-Value | ||
| Age, years (category) | <40 | 1 | 1 | ||
| 40–49 | 0.76 [0.36; 1.60] | 0.46 | 0.62 [0.27; 1.41] | 0.26 | |
| 50–59 | 0.98 [0.48; 2.00] | 0.95 | 0.65 [0.29; 1.48] | 0.30 | |
| ≥60 | 0.66 [0.31; 1.39] | 0.27 | 0.60 [0.27; 1.34] | 0.21 | |
| BMI, kg/m2 | <25 | 1 | 1 | ||
| ≥25 <30 | 1.43 [0.77; 2.66] | 0.26 | 1.35 [0.69; 2.62] | 0.38 | |
| ≥30 | 1.33 [0.72; 2.45] | 0.37 | 0.97 [0.48; 1.97] | 0.94 | |
| Histology | Ductal | 1 | 1 | ||
| Lobular | 0.76 [0.31; 1.90] | 0.56 | 1.33 [0.18; 9.68] | 0.78 | |
| Other | 0.47 [0.12; 1.92] | 0.29 | 1.96 [0.61; 6.32] | 0.26 | |
| SBR | 1–2 | 1 | 1 | ||
| 3 | 0.95 [0.60; 1.51] | 0.82 | 0.85 [0.43; 1.68] | 0.64 | |
| cN | N0 | 1 | 1 | ||
| N+ | 0.97 [0.55; 1.71] | 0.90 | 1.51 [0.54; 4.21] | 0.43 | |
| WHO performance status | 0 | 1 | 1 | ||
| 1–3 | 1.10 [0.60; 2.01] | 0.75 | 1.74 [0.86; 3.50] | 0.12 | |
| Preoperative radiotherapy | No | 1 | 1 | ||
| Yes | 1.84 [0.67; 5.05] | 0.24 | 3.46 [1.66; 7.21] | 0.0009 | |
| Sataloff T | TA | 1 | 1 | ||
| TB | 1.12 [0.61; 2.05] | 0.73 | 2.06 [0.86; 4.94] | 0.11 | |
| TC | 2.05 [1.12; 3.77] | 0.020 | 5.66 [2.64; 12.16] | <0.0001 | |
| TD | 2.79 [1.17; 6.64] | 0.020 | 1.92 [0.60; 6.14] | 0.27 | |
| Sataloff T | TA-TB | 1 | 1 | ||
| TC-TD | 2.06 [1.29; 3.30] | 0.002 | 3.17 [1.75; 5.74] | <0.0001 | |
| Sataloff N | NA | 1 | 1 | ||
| NB | 1.02 [0.42; 2.49] | 0.96 | 1.10 [0.26; 4.58] | 0.90 | |
| NC | 1.44 [0.79; 2.62] | 0.24 | 7.10 [2.65; 19.01] | <0.0001 | |
| ND | 2.29 [1.15; 4.53] | 0.018 | 9.59 [3.44; 26.70] | <0.0001 | |
| Sataloff N | NA-NB | 1 | 1 | ||
| NC-ND | 1.64 [0.99; 2.69] | 0.052 | 7.69 [3.53; 16.75] | <0.0001 | |
| HR+ and/or HER2+, N = 218 | HR and 95% CI | p-Value | |
|---|---|---|---|
| Sataloff T | TA-TB | 1 | |
| TC-TD | 1.85 [1.10; 3.11] | 0.020 | |
| Sataloff N | NA-NB | 1 | |
| NC-ND | 1.3 [0.75; 2.24] | 0.35 | |
| TN, N = 99 | HR and 95% CI | p-value | |
| Sataloff T | TA-TB | 1 | |
| TC-TD | 1.33 [0.69; 2.54] | 0.39 | |
| Sataloff N | NA-NB | 1 | |
| NC-ND | 6.06 [2.59; 14.2] | <0.0001 | |
| Preoperative radiotherapy | No | 1 | |
| Yes | 1.82 [0.85; 3.89] | 0.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogé, M.; Salleron, J.; Kirova, Y.; Guigo, M.; Cailleteau, A.; Levy, C.; Leheurteur, M.; Nebbache, R.; Rivin Del Campo, E.; Lazarescu, I.; et al. Different Prognostic Values of Tumour and Nodal Response to Neoadjuvant Chemotherapy Depending on Subtypes of Inflammatory Breast Cancer, a 317 Patient-Study. Cancers 2022, 14, 3928. https://doi.org/10.3390/cancers14163928
Rogé M, Salleron J, Kirova Y, Guigo M, Cailleteau A, Levy C, Leheurteur M, Nebbache R, Rivin Del Campo E, Lazarescu I, et al. Different Prognostic Values of Tumour and Nodal Response to Neoadjuvant Chemotherapy Depending on Subtypes of Inflammatory Breast Cancer, a 317 Patient-Study. Cancers. 2022; 14(16):3928. https://doi.org/10.3390/cancers14163928
Chicago/Turabian StyleRogé, Maximilien, Julia Salleron, Youlia Kirova, Marin Guigo, Axel Cailleteau, Christelle Levy, Marianne Leheurteur, Rafik Nebbache, Eleonor Rivin Del Campo, Ioana Lazarescu, and et al. 2022. "Different Prognostic Values of Tumour and Nodal Response to Neoadjuvant Chemotherapy Depending on Subtypes of Inflammatory Breast Cancer, a 317 Patient-Study" Cancers 14, no. 16: 3928. https://doi.org/10.3390/cancers14163928
APA StyleRogé, M., Salleron, J., Kirova, Y., Guigo, M., Cailleteau, A., Levy, C., Leheurteur, M., Nebbache, R., Rivin Del Campo, E., Lazarescu, I., Servagi, S., Aumont, M., Thariat, J., & Thureau, S. (2022). Different Prognostic Values of Tumour and Nodal Response to Neoadjuvant Chemotherapy Depending on Subtypes of Inflammatory Breast Cancer, a 317 Patient-Study. Cancers, 14(16), 3928. https://doi.org/10.3390/cancers14163928








