Patterns of Care for Breast Radiotherapy in Italy: Breast IRRadiATA (Italian Repository of Radiotherapy dATA) Feasibility Study †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection Creation and Framework
2.2. Feasibility Study
3. Results
3.1. Results of Data Entry and Satisfaction Surveys
3.2. Results of Clinical Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
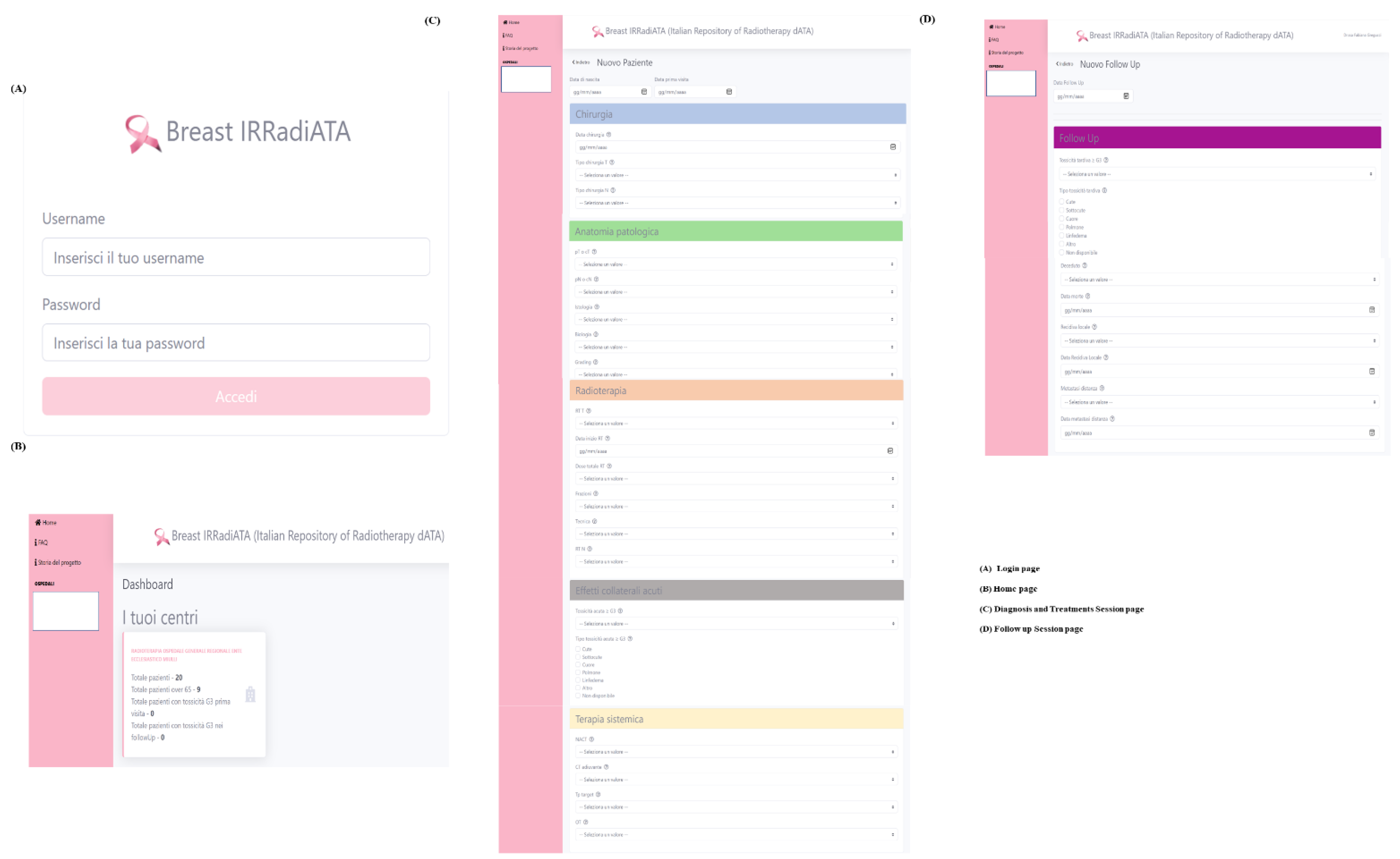
Appendix B

References
- World Health Organization. Women and Health: Today’s Evidence Tomorrow’s Agenda; World Health Organization: Geneva, Switzerland, 2009; Available online: https://apps.who.int/iris/handle/10665/44168 (accessed on 1 June 2021).
- I Numeri Del Cancro in Italia. Available online: https://www.aiom.it/wp-content/uploads/2021/10/2021_NumeriCancro_web.pdf (accessed on 1 June 2021).
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent advances in the treatment of breast cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Winer EPGnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.J.; Thürlimann, B. Panel members of the St. gallen international expert consensus on the primary therapy of early breast cancer 2017. De-escalating and escalating treatments for early-stage breast cancer: The St. gallen international expert consensus conference on the primary therapy of early breast cancer. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim SIl et Park, B.W. Characteristics and outcoms according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012, 21, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Corradini, S.; Krug, D.; Meattini, I.; Fastner, G.; Matuschek, C.; Cutuli, B. Challenges in radiotherapy. Breast Care 2019, 14, 152–158. [Google Scholar] [CrossRef]
- Veronesi, U.; Del Vecchio, M.; Luini, A.; Rasponi, A.; Zucali, R. The quadrantectomy, axillary dissection and radiotherapy (QU.A.RT) technique in early breast cancer. Int. Adv. Surg. Oncol. 1983, 6, 141–165. [Google Scholar]
- Meduri, B.; Gregucci, F.; D’Angelo, E.; Alitto, A.R.; Ciurlia, E.; Desideri, I.; Marino, L.; Borghetti, P.; Fiore, M.; Fiorentino, A. AIRO Giovani-Italian association of radiation oncology-young members. volume de-escalation in radiation therapy: State of the art and new perspectives. J. Cancer Res. Clin. Oncol. 2020, 146, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Bauer-Nilsen, K.; McNulty, R.H.; Vicini, F. Novel radiation therapy approaches for breast cancer treatment. Semin. Oncol. 2021, 47, 209–216. [Google Scholar] [CrossRef]
- Ciabattoni, A.; Gregucci, F.; De Rose, F.; Falivene, S.; Fozza, A.; Daidone, A.; Morra, A.; Smaniotto, D.; Barbara, R.; Lozza, L.; et al. AIRO breast cancer group best clinical practice 2022 update. Tumori J. 2022, 108, 1–144. [Google Scholar] [CrossRef]
- Fozza, A.; Giaj-Levra, N.; De Rose, F.; Ippolito, E.; Silipigni, S.; Meduri, B.; Fiorentino, A.; Gregucci, F.; Marino, L.; Di Grazia, A.; et al. Lymph nodal radiotherapy in breast cancer: What are the unresolved issues? Expert Rev. Anticancer Ther. 2021, 21, 827–840. [Google Scholar] [CrossRef]
- O’Hea, E.L.; Creamer, S.; Flahive, J.M.; Keating, B.A.; Crocker, C.R.; Williamson, S.R.; Edmiston, K.L.; Harralson, T.; Boudreaux, E.D. Survivorship care planning, quality of life, and confidence to transition to survivorship: A randomized controlled trial with women ending treatment for breast cancer. J. Psychosoc. Oncol. 2021, 40, 574–594. [Google Scholar] [CrossRef] [PubMed]
- Cancer.Net. The Importance of Follow-Up Care Approved by the Cancer.Net Editorial Board, April 2018. Available online: https://www.cancer.net/survivorship/follow-care-after-cancer-treatment/importance-follow-care (accessed on 1 June 2021).
- AIRO: I Centri di Radioterapia in Italia (Giugno 2020). Available online: https://www.radioterapiaitalia.it/ricerca-centri-radioterapia (accessed on 1 June 2021).
- Macfarlane, A.J.R. What is clinical governance? BJA Educ. 2019, 19, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Maissenhaelter, B.E.; Woolmore, A.L.; Schlag, P.M. Real-world evidence research based on big data: Motivation-challenges-success factors. Der Onkol. 2018, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Gregucci, F.; Fozza, A.; Falivene, S.; Smaniotto, D.; Morra, A.; Daidone, A.; Barbara, R.; Ciabattoni, A.; Italian Society of Radiotherapy and Clinical Oncology (AIRO) Breast Group. Present clinical practice of breast cancer radiotherapy in Italy: A nationwide survey by the Italian society of radiotherapy and clinical oncology (AIRO) breast group. Radiol. Med. 2020, 125, 674–682. [Google Scholar] [CrossRef]
- Enewold, L.; Parsons, H.; Zhao, L.; Bott, D.; Rivera, D.R.; Barrett, M.J.; Virnig, B.A.; Warren, J.L. Updated overview of the SEER-medicare data: Enhanced content and applications. J. Natl. Cancer Inst. Monogr. 2020, 55, 3–13. [Google Scholar]
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, A.; Køtlum, J.E.; Olafsdóttir, E.; Pukkala, E.; Storm, H.H. NORDCAN-a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Nathan, H. Strengths and limitations of registries in surgical oncology research. J. Gastrointest. Surg. 2021, 25, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Guidelines 2/2019 on the Processing of Personal Data under Article 6 (1) (b) GDPR in the Context of the Provision of Online Services to Data Subjects-Version Adopted after Public Consultation. Available online: https://edpb.europa.eu/our-work-tools/our-documents/guidelines/guidelines-22019-processing-personal-data-under-article-61b_en (accessed on 1 June 2020).
- Zakeri, K.; Coleman, C.; Vikram, B. Radiation oncology in the 21st century: Prospective randomized trials that changed practice or didn’t! Front. Oncol. 2018, 8, 130. [Google Scholar] [CrossRef]
- Real World Data E Real World Evidence: Considerazioni e Proposte Da Un Network Di Società Scientifiche. Available online: https://www.chrp.it/wp-content/uploads/documento-rwe-2017_-vfinale.pdf (accessed on 1 June 2021).
- Fung, C.Y.; Chen, E.; Vapiwala, N.; Pohar, S.; Trifiletti, D.; Truong, M.T.; Uschold, G.; Schuster, J.; Patel, A.; Jani, A.; et al. The American society for radiation oncology 2017 radiation oncologist workforce study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 547–556. [Google Scholar] [CrossRef]
- Clare, S.E.; Shaw, P.L. “Big Data” for breast cancer: Where to look and what you will find. NPJ Breast Cancer 2016, 2, 16031. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Available online: https://seer.cancer.gov/ (accessed on 1 June 2021).
- Hernandez-Boussard, T.; Bozkurt, S.; Ioannidis, J.P.A.; Shah, N.H. MINIMAR (MINimum Information for Medical AI Reporting): Developing reporting standards for artificial intelligence in health care. J. Am. Med. Inform. Assoc. 2020, 27, 2011–2015. [Google Scholar] [CrossRef]
- Goel, A.K.; Campbell, W.S.; Moldwin, R. Structured data capture for oncology. JCO Clin. Cancer Inform. 2021, 5, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, F.; Masiello, V.; Masciocchi, C.; Merluzzi, M.; Saldi, S.; Belli, P.; Boldrini, L.; Capocchiano, N.D.; Di Leone, A.; Magno, S.; et al. The assisi think tank meeting breast large database for standardized data collection in breast cancer-ATTM.BLADE. J Pers. Med. 2021, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The national cancer data base: A powerful initiative to improve cancer care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Forsea, A.M. Cancer registries in Europe-going forward is the only option. Ecancermedicalscience 2016, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, Z.; Ghazisaeedi, M.; Nahvijou, A.; Rostam Niakan Kalhori, S.; Davoodi, S.; Zendehdel, K. Systematic review of hospital based cancer registries (HBCRs): Necessary tool to improve quality of care in cancer patients. Asian Pac. J. Cancer Prev. 2017, 18, 2027–2033. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- Van Dam, P.A.; Tomatis, M.; Marotti, L.; Heil, J.; Wilson, R.; Rosselli Del Turco, M.; Mayr, C.; Costa, A.; Danei, M.; Denk, A.; et al. The effect of EUSOMA certification on quality of breast cancer care. Eur. J. Surg. Oncol. 2015, 41, 1423–1429. [Google Scholar] [CrossRef]
- Meattini, I.; Becherini, C.; Boersma, L.; Kaidar-Person, O.; Marta, G.N.; Montero, A.; Offersen, B.V.; Aznar, M.C.; Belka, C.; Brunt, A.M.; et al. European society for radiotherapy and oncology advisory committee in radiation oncology practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022, 23, e21–e31. [Google Scholar] [CrossRef]
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhuis, C.; Horiot, J.C.; et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015, 16, 47–56. [Google Scholar] [CrossRef]
- Cooper, B.T.; Formenti-Ujlaki, G.F.; Li, X.; Shin, S.M.; Fenton-Kerimian, M.; Guth, A.; Roses, D.F.; Hitchen, C.J.; Rosenstein, B.S.; Dewyngaert, J.K.; et al. Prospective randomized trial of prone accelerated intensity modulated breast radiation therapy with a daily versus weekly boost to the tumor bed. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gregucci, F.; Mazzola, R.; Figlia, V.; Ricchetti, F.; Sicignano, G.; Giajlevra, N.; Ruggieri, R.; Fersino, S.; Naccarato, S.; et al. Intensity-modulated radiotherapy and hypofractionated volumetric modulated arc therapy for elderly patients with breast cancer: Comparison of acute and late toxicities. Radiol. Med. 2019, 124, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Cante, D.; Sciacero, P.; Girelli, G.; La Porta, M.R.; Ricardi, U. Tumor bed boost integration during whole breast radiotherapy: A review of the current evidence. Breast Care 2015, 10, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ciabattoni, A.; Gregucci, F.; Fastner, G.; Cavuto, S.; Spera, A.; Drago, S.; Ziegler, I.; Mirri, M.A.; Consorti, R.; Sedlmayer, F. IOERT versus external beam electrons for boost radiotherapy in stage I/II breast cancer: 10-year results of a phase III randomized study. Breast Cancer Res. 2021, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Ciabattoni, A.; Gregucci, F.; Llange, K.; Alessandro, M.; Corazzi, F.; Ivaldi, G.B.; Zuccoli, P.; Stefanelli, A.; Cristaudo, A.; Fusco, V.; et al. Intra-operative electron radiation therapy (IOERT) anticipated boost in breast cancer treatment: An Italian multicenter experience. Cancers 2022, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Fadavi, P.; Nafissi, N.; Mahdavi, S.R.; Jafarnejadi, B.; Javadinia, S.A. Outcome of hypofractionated breast irradiation and intraoperative electron boost in early breast cancer: A randomized non-inferiority clinical trial. Cancer Rep. 2021, 4, e1376. [Google Scholar] [CrossRef] [PubMed]
- Homaei Shandiz, F.; Fanipakdel, A.; Forghani, M.N. Clinical efficacy and side effects of IORT as tumor bed boost during breast-conserving surgery in breast cancer patients following neoadjuvant chemotherapy. Indian J. Gynecol. Oncol. 2020, 18, 46. [Google Scholar] [CrossRef]
- Keramati, A.; Javadinia, S.A.; Gholamhosseinian, H. A review of intraoperative radiotherapy after neoadjuvant chemotherapy in patients with locally advanced breast cancer: From bench to bedside. Indian J. Gynecol. Oncol. 2020, 18, 110. [Google Scholar] [CrossRef]

| Folder | Item | Description | Options |
|---|---|---|---|
| SURGERY | SURGERY_DATA | Specify data of breast surgery | Day/Month/Year |
| T_SURGERY_TYPE | Specify type of breast surgery | Lumpectomy | |
| Mastectomy | |||
| Other | |||
| Not available | |||
| N_SURGERY_TYPE | Specify type of lymph node surgery | Sentinel Lymph node Biopsy | |
| Axillary Lymph node Dissection | |||
| Other | |||
| Not available | |||
| PATHOLOGICAL ANATOMY | cT or pT | Specify the local extent of the disease (clinical or pathological) (AJCC 2017 classification, Eighth edition) | Tx |
| Tis | |||
| T1 | |||
| T2 | |||
| T3 | |||
| T4 | |||
| Not available | |||
| cN or pN | Specify the lymph node involvement of the disease (clinical or pathological) (AJCC 2017 classification, Eighth edition) | Nx | |
| N0 | |||
| N1 | |||
| N2 | |||
| N3 | |||
| Not available | |||
| HISTOLOGY | Specify the histology of the disease | Ductal Carcinoma In Situ | |
| Invasive Ductal Carcinoma | |||
| Invasive Lobular Carcinoma | |||
| Other | |||
| Not available | |||
| BIOLOGY | Specify the biology of the disease (St. Gallen 2013 classification) | Luminal A | |
| Luminal B HER2 negative | |||
| Luminal B HER2 positive | |||
| HER2 positive | |||
| Triple Negative | |||
| Not applicable | |||
| Not available | |||
| GRADING | Specify the grading of the disease | G1 | |
| G2 | |||
| G3 | |||
| Not available | |||
| RADIOTHERAPY | RT_T | Specify whether or not the patient has undergone adjuvant radiotherapy on the breast or chest wall | Yes |
| Not | |||
| Not available | |||
| RT_DATA | Specify data of breast radiotherapy | Day/Month/Year | |
| RT_total_dose | Specify the total dose for whole breast irradiation excluding boost | 50 Gy | |
| 40.5 Gy | |||
| 42.75 Gy | |||
| 30 Gy | |||
| 26 Gy | |||
| Other | |||
| Not available | |||
| RT_fractions | Specify the total fractions for whole breast irradiation excluding boost | 25 | |
| 15 | |||
| 16 | |||
| 5 | |||
| Other | |||
| Not available | |||
| RT_technique | Specify the technique used for breast irradiation | 3D Conformal RadioTherapy | |
| Intensity Modulated RadioTherapy | |||
| Volumetric Modulated Arc Therapy | |||
| Deep Inspiration Breath Hold | |||
| IntraOperative RadioTherapy | |||
| Partial Breast Irradiation | |||
| Brachytherapy | |||
| Other | |||
| Not available | |||
| RT_LNF | Specify whether or not the patient has undergone adjuvant radiotherapy on the regional lymph node | Yes | |
| Not | |||
| Not available | |||
| ACUTE TOXICITY | A_tox > G3 | Specify whether the patient reported acute side effects of a grade equal to or greater than G3 according to the RTOG scale, regardless of the anatomical district, during or at the end of RT | Yes |
| Not | |||
| Not available | |||
| A_tox_type | If YES, specify the anatomical district of acute toxicity | Skin | |
| Soft tissue | |||
| Heart | |||
| Lung | |||
| Lymphedema | |||
| Other | |||
| Not available | |||
| SYSTEMIC THERAPY | NACT | Specify whether or not the patient has undergone neoadjuvant chemotherapy | Yes |
| Not | |||
| Not available | |||
| ADJUV_CT | Specify whether or not the patient has undergone adjuvant chemotherapy | Yes | |
| Not | |||
| Not available | |||
| TARGET_tp | Specify whether or not the patient has undergone target therapy | Yes | |
| Not | |||
| Not available | |||
| HT | Specify whether or not the patient has undergone hormone therapy | Yes | |
| Not | |||
| Not available | |||
| FOLLOW UP | FU_DATA | Specify data of last follow up | Day/Month/Year |
| L_tox ≥ G3 | Specify whether the patient reported late side effects of a grade equal to or greater than G3 according to the RTOG scale, regardless of the anatomical district, during or at the end of RT | Yes | |
| Not | |||
| Not available | |||
| L_tox_type | If YES, specify the anatomical district of late toxicity | Skin | |
| Soft tissue | |||
| Heart | |||
| Lung | |||
| Lymphedema | |||
| Other | |||
| Not available | |||
| Dead | Specify whether the patient is alive or dead | Alive | |
| Dead | |||
| Dead_DATA | If DEAD, specify data | Day/Month/year | |
| Loc_relapse | Specify if the patient has developed a local recurrence event (breast, chest wall, regional lymph nodes) | Yes | |
| Not | |||
| Not available | |||
| Loc_relapse _DATA | If YES, specify data | Day/Month/year | |
| Distant_mets | Specify if the patient has developed a distant metastases event | Yes | |
| Not | |||
| Not available | |||
| Distant_mets _DATA | If YES, specify data | Day/Month/year |
| Items | Questions | Answers | Responders |
|---|---|---|---|
| Patients per year | In your center, on average, how many breast cancer patients are treated each year? | 50–200 | 3 (18%) |
| 200–500 | 9 (53%) | ||
| >500 | 5 (29%) | ||
| Multidisciplinary Board | Are breast cancer patients from your center discussed within a Multidisciplinary Tumor Board? | Yes, ever | 8 (48%) |
| Yes, in most cases (80–60%) | 6 (35%) | ||
| Yes, in minority cases (50–20%) | 3 (17%) | ||
| Not, never | 0 | ||
| WBI technique | What is the most commonly used technique for whole breast irradiation to treat these patients? | 3DCRT | 13 (76%) |
| IMRT | 3 (18%) | ||
| VMAT | 1 (6%) | ||
| Boost administration | In your center, if indicated, is the boost delivered sequentially or concomitant? | Sequentially | 11 (64%) |
| Concomitant (SIB) | 3 (18%) | ||
| Both | 3 (18%) | ||
| PBI | Is partial breast irradiation performed in your center? | Yes | 11 (64%) |
| Not | 6 (36%) | ||
| PBI | If yes, with what technique? | EBRT | 9 (82%) |
| IORT | 1 (9%) | ||
| Brachytherapy | 1 (9%) | ||
| DIBH | In your center, in cases of left breast irradiation, is the Deep Inspiration Breath Hold technique used? | Yes, ever | 2 (12%) |
| Yes, in selected cases | 10 (59%) | ||
| Not, never | 5 (29%) | ||
| Follow-up | Is the radiotherapy follow-up of patients treated for breast cancer currently performed in your center? | Yes, ever | 9 (54%) |
| Yes, only in complex cases | 4 (23%) | ||
| Yes, only in cases of toxicity | 4 (23%) | ||
| Not, never | 0 | ||
| Follow-up | If yes, the follow-up is generally performed how often? | Every 3–4 months | 2 (12%) |
| Every 6–12 months | 10 (59%) | ||
| Variable interval on particular needs | 5 (29%) |
| Items | Questions | Answers | Responders |
|---|---|---|---|
| Time-consuming | On average, how long did it take to enter the data relating to the diagnosis and treatment of the individual patient? | 1–2 min | 0 (0%) |
| 2–5 min | 11 (64.7%) | ||
| 5–10 min | 6 (35.3%) | ||
| >10 min | 0 (0%) | ||
| Time-consuming | On average, how long did it take to enter the data relating to the follow-up of the individual patient? | 1–2 min | 7 (41.2%) |
| 2–5 min | 7 (41.2%) | ||
| 5–10 min | 3 (17.6%) | ||
| >10 min | 0 (0%) | ||
| Satisfaction | Report the degree of satisfaction with the operation of the project (ease of access to the site, intuitiveness, and simplicity in filling in the required fields, user-friendly) | Excellent | 14 (82.3%) |
| Good | 3 (17.7%) | ||
| Sufficient | 0 (0%) | ||
| Poor | 0 (0%) | ||
| Satisfaction | Report the degree of satisfaction regarding the relevance of the project (interest in the data collected, completeness of the data collected, relevance of the response options) | Excellent | 12 (70.6%) |
| Good | 5 (29.4%) | ||
| Sufficient | 0 (0%) | ||
| Poor | 0 (0%) | ||
| Satisfaction | Report the degree of satisfaction with the purpose of the project (usefulness in the clinical setting, use for all treated patients, diffusion at national level) | Excellent | 13 (76.5%) |
| Good | 4 (23.5%) | ||
| Sufficient | 0 (0%) | ||
| Poor | 0 (0%) | ||
| Satisfaction | Report the degree of general satisfaction with the project in its entirety | Excellent | 14 (82.3%) |
| Good | 3 (17.7%) | ||
| Sufficient | 0 (0%) | ||
| Poor | 0 (0%) | ||
| Improvement | Are there any aspects of the project that you would improve? If YES, please state which ones and how | Free answer | 6 (35.3%) * |
| Folder | Item | Options | Response |
|---|---|---|---|
| SURGERY | T_SURGERY_TYPE | Lumpectomy | 309 (92%) |
| Mastectomy | 25 (7%) | ||
| Other | 1 (1%) | ||
| Not available | 0 | ||
| N_SURGERY_TYPE | Sentinel Lymph node Biopsy | 239 (71%) | |
| Axillary Lymph node Dissection | 72 (21%) | ||
| Other | 11 (3%) | ||
| Not available | 13 (5%) | ||
| PATHOLOGICAL ANATOMY | cT or pT | Tx | 3 (0.9%) |
| Tis | 35 (10%) | ||
| T1 | 229 (68%) | ||
| T2 | 61 (18%) | ||
| T3 | 1 (0.2%) | ||
| T4 | 4 (1%) | ||
| Not available | 2 (1.9%) | ||
| cN or pN | Nx | 22 (6%) | |
| N0 | 248 (74%) | ||
| N1 | 41 (12%) | ||
| N2 | 15 (4%) | ||
| N3 | 6 (3%) | ||
| Not available | 3 (1%) | ||
| HISTOLOGY | Ductal Carcinoma In Situ | 35 (10%) | |
| Invasive Ductal Carcinoma | 245 (73%) | ||
| Invasive Lobular Carcinoma | 45 (13%) | ||
| Other | 7 (3%) | ||
| Not available | 3 (1%) | ||
| BIOLOGY | Luminal A | 180 (54%) | |
| Luminal B HER2 negative | 70 (21%) | ||
| Luminal B HER2 positive | 25 (7%) | ||
| Her2 positive | 9 (3%) | ||
| Triple Negative | 27 (8%) | ||
| Not applicable | 13 (4%) | ||
| Not available | 11 (3%) | ||
| GRADING | G1 | 61 (18%) | |
| G2 | 168 (50%) | ||
| G3 | 100 (30%) | ||
| Not available | 6 (2%) | ||
| RADIOTHERAPY | RT_T | Yes | 332 (99%) |
| Not | 1 (0.3%) | ||
| Not available | 2 (0.7%) | ||
| RT_total_dose | 50 Gy | 136 (40%) | |
| 40.5 Gy | 107 (32%) | ||
| 42.75 Gy | 19 (6%) | ||
| 30 Gy | 4 (1%) | ||
| 26 Gy | 10 (3%) | ||
| Other | 58 (17%) | ||
| Not available | 1 (1%) | ||
| RT_fractions | 25 | 122 (36%) | |
| 15 | 120 (36%) | ||
| 16 | 32 (10%) | ||
| 5 | 18 (5%) | ||
| Other | 40 (12%) | ||
| Not available | 3 (1%) | ||
| RT_technique | 3D Conformal RadioTherapy | 239 (71%) | |
| Intensity Modulated RadioTherapy | 19 (6%) | ||
| Volumetric Modulated Arc Therapy | 35 (10%) | ||
| Deep Inspiration Breath Hold | 4 (2%) | ||
| IntraOperative RadioTherapy | 0 | ||
| Partial Breast Irradiation | 14 (4%) | ||
| Brachytherapy | 0 | ||
| Other | 24 (7%) | ||
| Not available | 0 | ||
| RT_LNF | Yes | 36 (10%) | |
| Not | 298 (89%) | ||
| Not available | 1 (1%) | ||
| ACUTE TOXICITY | A_tox > G3 | Yes | 9 (3%) |
| Not | 312 (93%) | ||
| Not available | 14 (4%) | ||
| A_tox_type | Skin | 7 (78%) | |
| Soft tissue | 1 (11%) | ||
| Heart | 0 | ||
| Lung | 0 | ||
| Lymphedema | 1 (11%) | ||
| Other | 0 | ||
| Not available | 0 | ||
| SYSTEMIC THERAPY | NACT | Yes | 25 (8%) |
| Not | 295 (88%) | ||
| Not available | 15 (4%) | ||
| ADJUV_CT | Yes | 72 (22%) | |
| Not | 245 (73%) | ||
| Not available | 18 (5%) | ||
| TARGET_tp | Yes | 18 (5%) | |
| Not | 294 (88%) | ||
| Not available | 23 (7%) | ||
| HT | Yes | 268 (81%) | |
| Not | 52 (15%) | ||
| Not available | 15 (4%) | ||
| FOLLOW UP | L_tox ≥ G3 | Yes | 0 |
| Not | 327 (98%) | ||
| Not available | 8 (2%) | ||
| L_tox_type | Skin | 0 | |
| Soft tissue | 0 | ||
| Heart | 0 | ||
| Lung | 0 | ||
| Lymphedema | 0 | ||
| Other | 0 | ||
| Not available | 0 | ||
| Dead | Alive | 335 (100%) | |
| Dead | 0 | ||
| Loc_relapse | Yes | 0 | |
| Not | 335 (100%) | ||
| Not available | 0 | ||
| Distant_mets | Yes | 0 | |
| Not | 335 (100%) | ||
| Not available | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciabattoni, A.; Gregucci, F.; D’Ermo, G.; Dolfi, A.; Cucciarelli, F.; Palumbo, I.; Borghesi, S.; Gava, A.; Cesaro, G.M.; Baldissera, A.; et al. Patterns of Care for Breast Radiotherapy in Italy: Breast IRRadiATA (Italian Repository of Radiotherapy dATA) Feasibility Study. Cancers 2022, 14, 3927. https://doi.org/10.3390/cancers14163927
Ciabattoni A, Gregucci F, D’Ermo G, Dolfi A, Cucciarelli F, Palumbo I, Borghesi S, Gava A, Cesaro GM, Baldissera A, et al. Patterns of Care for Breast Radiotherapy in Italy: Breast IRRadiATA (Italian Repository of Radiotherapy dATA) Feasibility Study. Cancers. 2022; 14(16):3927. https://doi.org/10.3390/cancers14163927
Chicago/Turabian StyleCiabattoni, Antonella, Fabiana Gregucci, Giuseppe D’Ermo, Alessandro Dolfi, Francesca Cucciarelli, Isabella Palumbo, Simona Borghesi, Alessandro Gava, Giovanna Maria Cesaro, Antonella Baldissera, and et al. 2022. "Patterns of Care for Breast Radiotherapy in Italy: Breast IRRadiATA (Italian Repository of Radiotherapy dATA) Feasibility Study" Cancers 14, no. 16: 3927. https://doi.org/10.3390/cancers14163927
APA StyleCiabattoni, A., Gregucci, F., D’Ermo, G., Dolfi, A., Cucciarelli, F., Palumbo, I., Borghesi, S., Gava, A., Cesaro, G. M., Baldissera, A., Giammarino, D., Daidone, A., Maurizi, F., Mignogna, M., Mazzuoli, L., Ravo, V., Falivene, S., Pedretti, S., Ippolito, E., ... Formenti, S. C. (2022). Patterns of Care for Breast Radiotherapy in Italy: Breast IRRadiATA (Italian Repository of Radiotherapy dATA) Feasibility Study. Cancers, 14(16), 3927. https://doi.org/10.3390/cancers14163927









