Theranostics Using Indocyanine Green Lactosomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Molecular Design of Indocyanine Green-Loaded Lactosomes
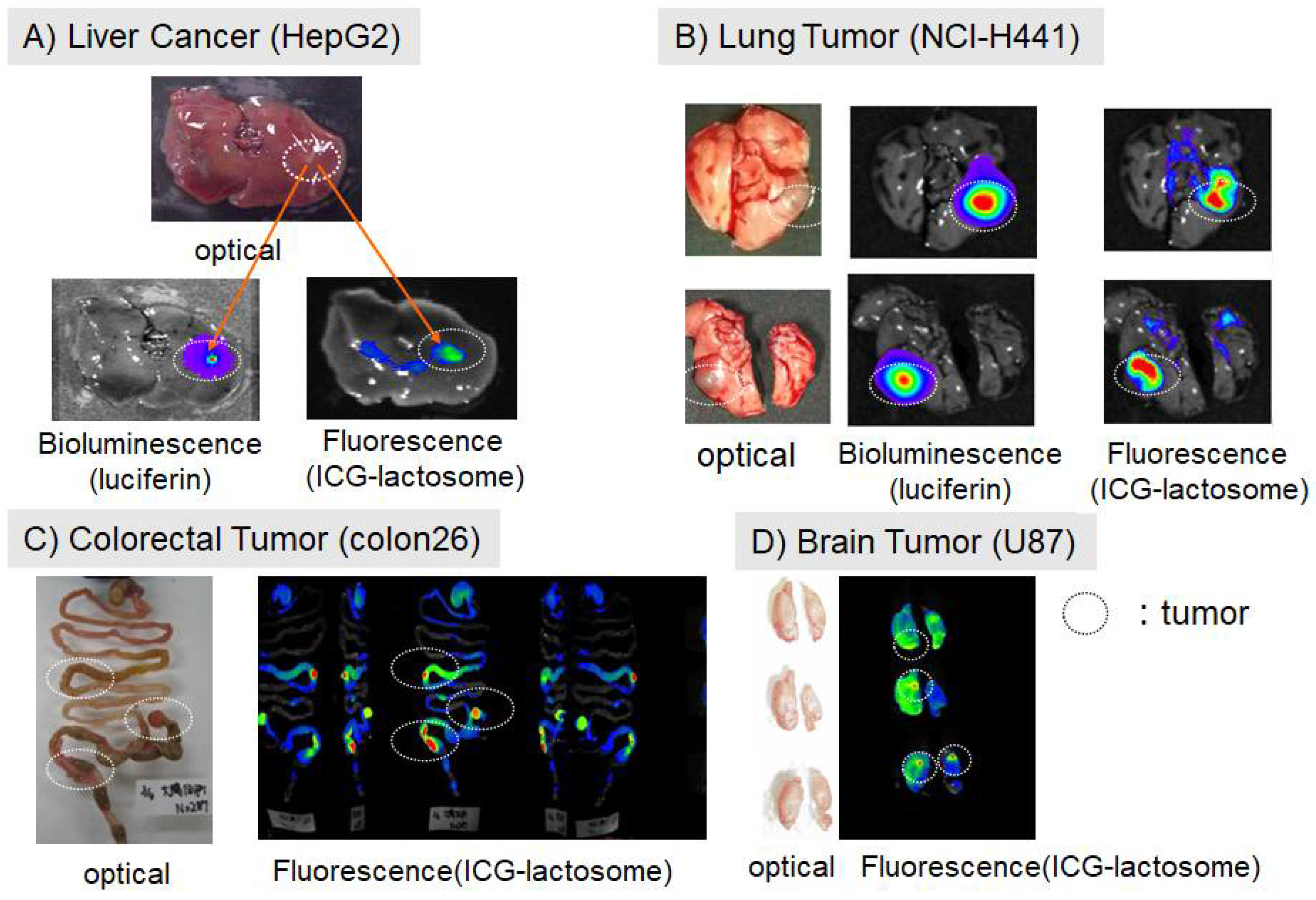
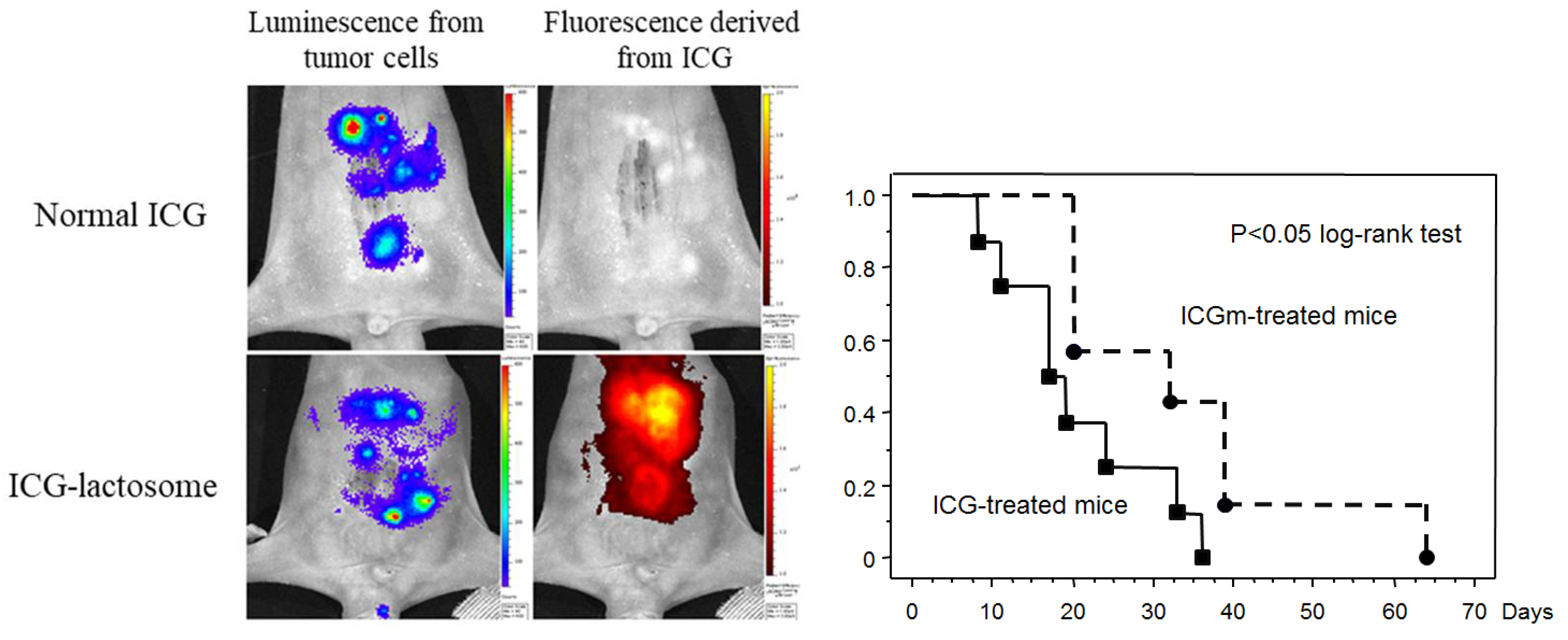
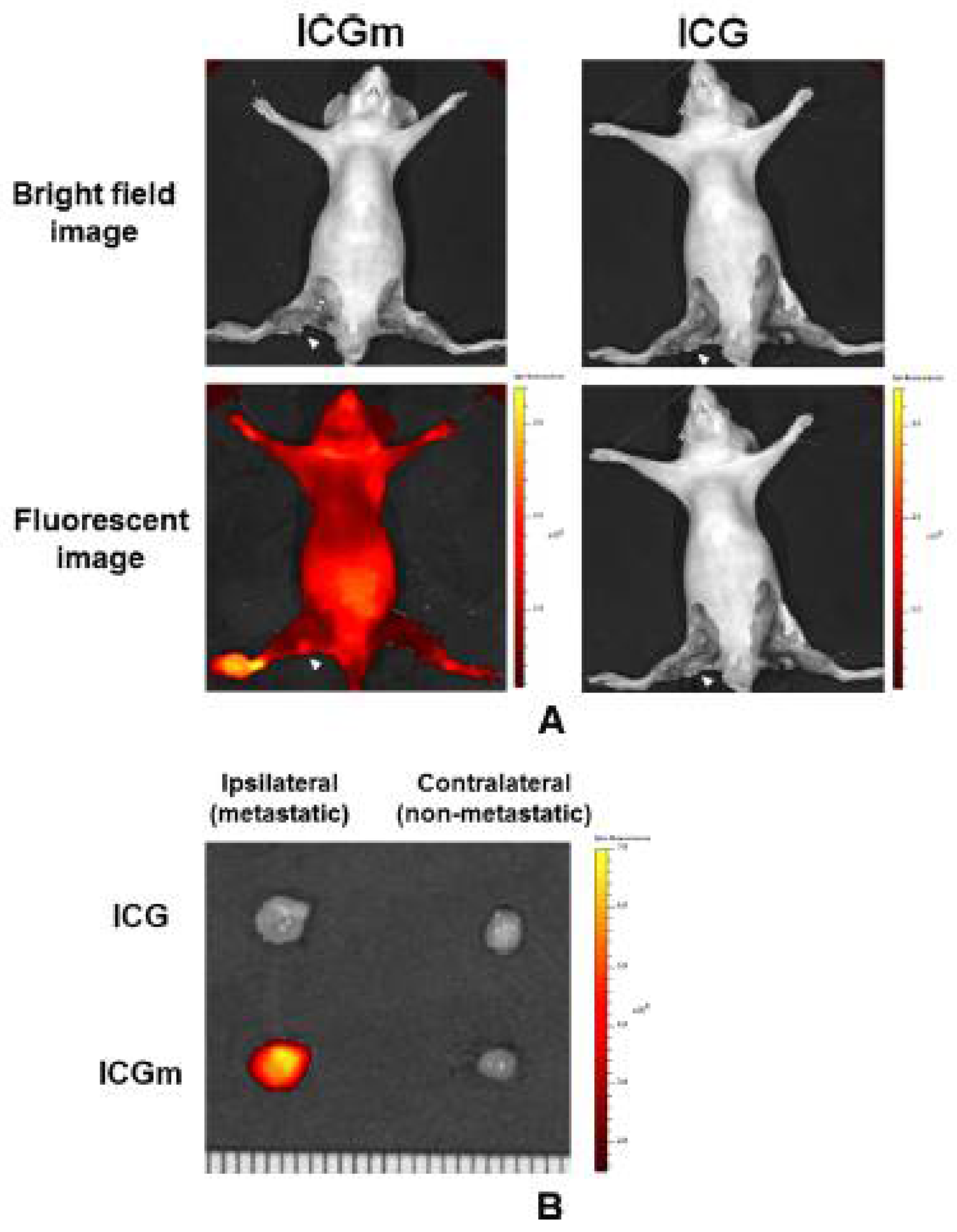
2.1. Accumulation of Lactosomes in Cancer Tissues
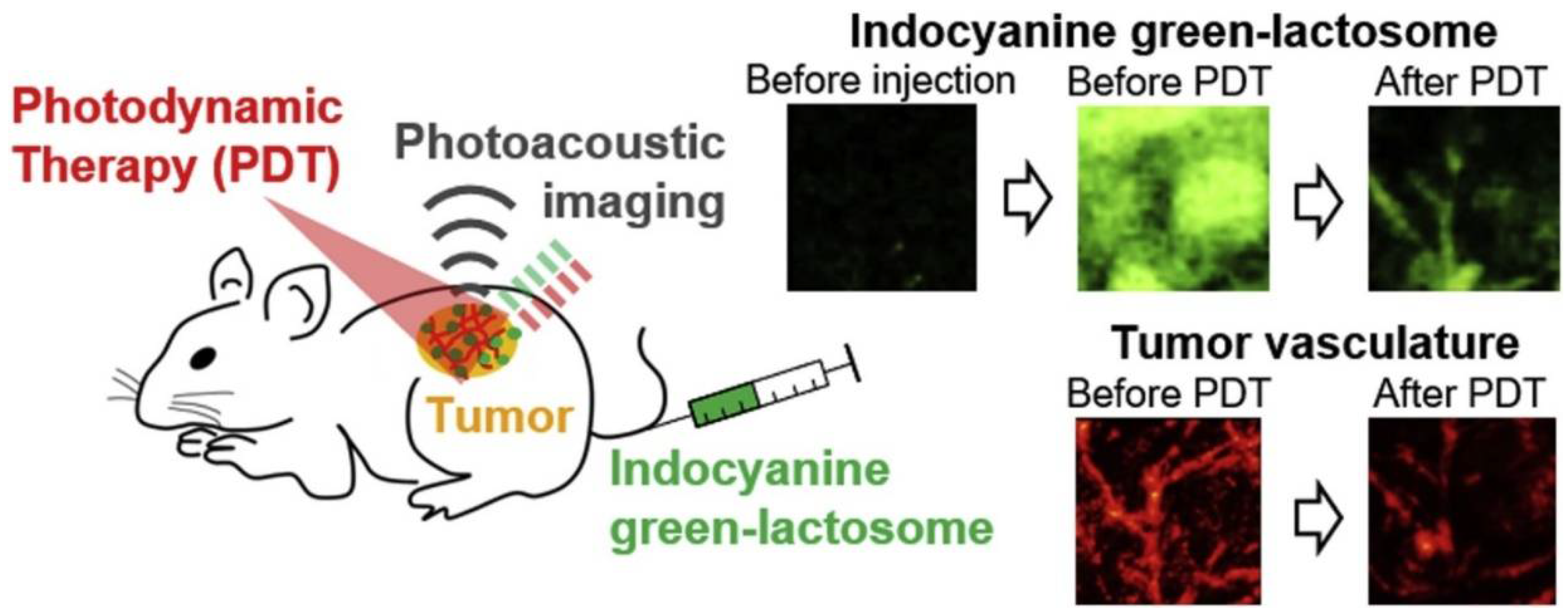
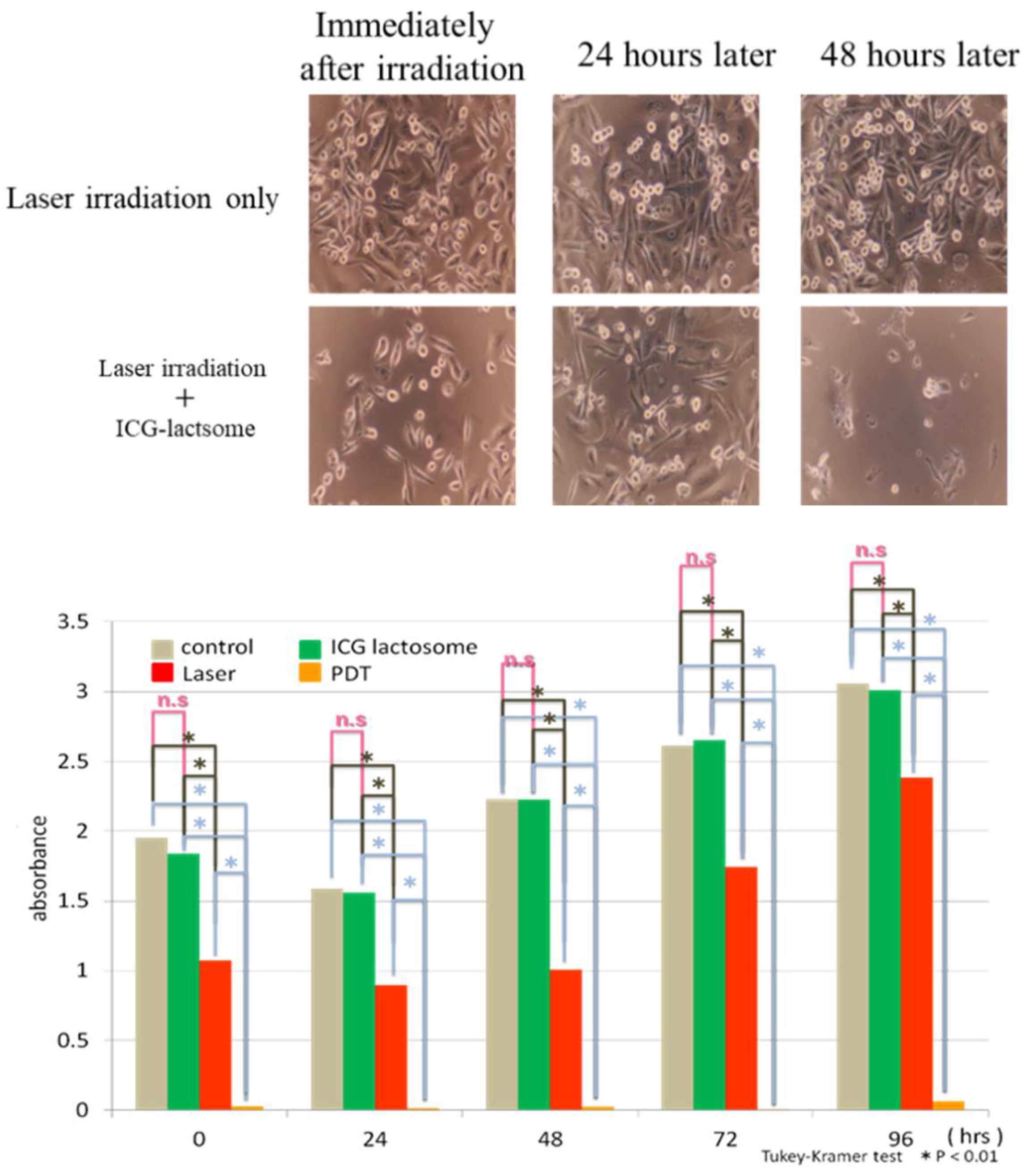
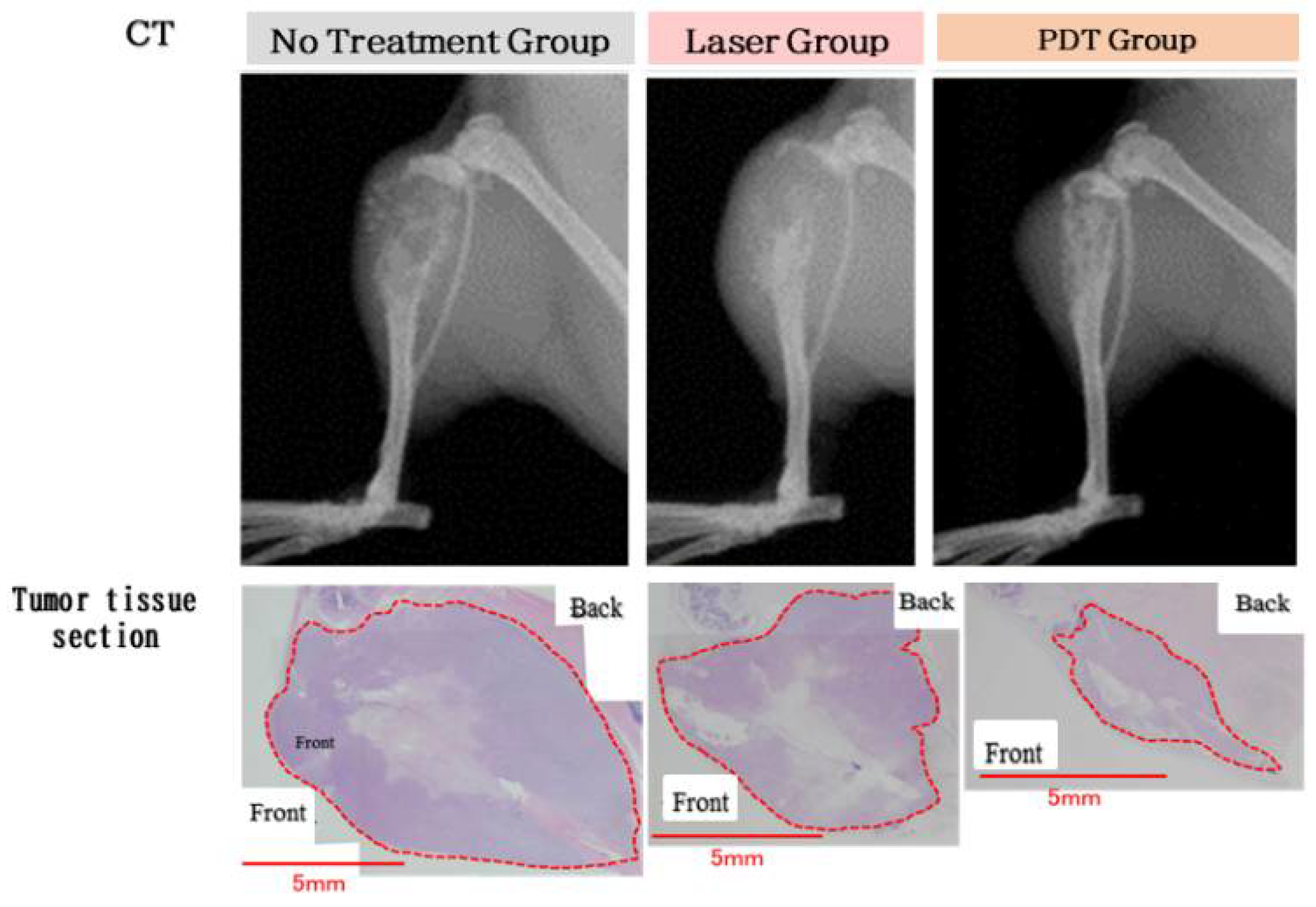
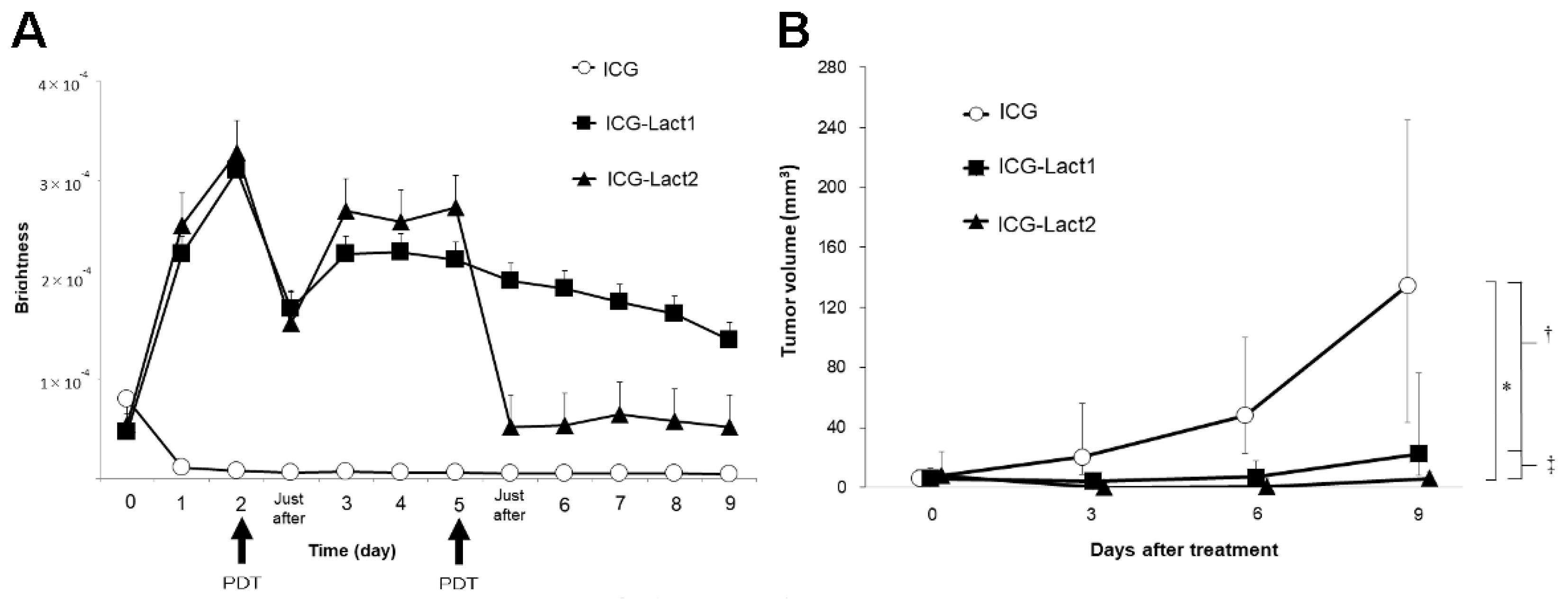
2.2. Theranostics Application with ICG-Lactosomes
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Lukyanov, A.N.; Hartner, W.C.; Torchilin, V.P. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J. Control. Release 2004, 94, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Kizaka-Kondoh, S.; Yamahara, R.; Hara, I.; Kanzaki, T.; Ozeki, E.; Hiraoka, M.; Kimura, S. Near-infrared fluorescence tumor imaging using nanocarrier composed of poly(L-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide. Biomaterials 2009, 30, 5156–5160. [Google Scholar] [CrossRef]
- Makino, A.; Hara, E.; Hara, I.; Yamahara, R.; Kurihara, K.; Yamanoto, F.; Kimura, S. Control of in vivo blood clearance time of polymeric micelle by stereochemistry of amphiphilic polydepsipeptides. J. Control. Release 2012, 161, 821–825. [Google Scholar] [CrossRef]
- Hara, E.; Makino, A.; Kurihara, K.; Sugai, M.; Shimizu, A.; Hara, I.; Ozeki, E.; Kimura, S. Evasion from accelerated blood clearance of nanocarrier named as “Lactosome” induced by excessive administration of Lactosome. Biochim. Biophys. Acta 2013, 1830, 4046–4052. [Google Scholar] [CrossRef]
- Hara, E.; Ueda, M.; Makino, A.; Hara, I.; Ozeki, E.; Kimura, S. Factors influencing in vivo disposition of polymeric micelles on multiple administrations. ACS Med. Chem. Lett. 2014, 5, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Makino, A.; Hara, E.; Hara, I.; Ozeki, E.; Kimura, S. Size control of core-shell-type polymeric micelle with a nanometer precision. Langmuir 2014, 30, 669–674. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Morimoto, Y.; Takahata, R.; Nomura, S.; Yoshida, K.; Horiguchi, H.; Hiraki, S.; Ono, S.; Miyazaki, H.; Saito, D.; et al. Photodynamic therapy using nanoparticle loaded with indocyanine green for experimental peritoneal dissemination of gastric cancer. Cancer Sci. 2014, 105, 1626–1630. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Weisiger, R.; Gollan, J.; Ockner, R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science 1981, 211, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Forker, E.L.; Luxon, B.A. Albumin-mediated transport of rose bengal by perfused rat liver. Kinetics of the reaction at the cell surface. J. Clin. Investig. 1983, 72, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Tsunoi, Y.; Araki, K.; Ozeki, E.; Hara, I.; Shiotani, A.; Terakawa, M.; Sato, S. Photoacoustic diagnosis of pharmacokinetics and vascular shutdown effects in photodynamic treatment with indocyanine green-lactosome for a subcutaneous tumor in mice. Photodiagn. Photodyn. Ther. 2019, 26, 436–441. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, P.; Birke, A.; Huesmann, D.; Weber, B.; Fischer, K.; Reske-Kunz, A.; Bros, M.; Barz, M. Introducing PeptoPlexes: Polylysine-block-polysarcosine based polyplexes for transfection of HEK 293T cells. Macromol. Biosci. 2014, 14, 1380–1395. [Google Scholar] [CrossRef]
- Birke, A.; Huesmann, D.; Kelsch, A.; Weilbächer, M.; Xie, J.; Bros, M.; Bopp, T.; Becker, C.; Landfester, K.; Barz, M. Polypeptoid-block-polypeptide copolymers: Synthesis, characterization, and application of amphiphilic block Copolypept(o)ides in drug formulations and miniemulsion techniques. Biomacromolecule 2014, 15, 548–557. [Google Scholar] [CrossRef]
- Ozeki, E.; Hara, I. Theranostics using a LACTOSOME® nanocarrier composed of the amphiphilic polydepsipeptides, poly(L-lactic acid) and poly(sarcosine). Shimadzu Hyoron 2016, 72, 89–99. (In Japanese) [Google Scholar]
- Zhou, Y.; Li, G.; Zhu, L.; Li, C.; Cornelius, L.A.; Wang, L.V. Handheld photoacoustic probe to detect both melanoma depth and volume at high speed in vivo. J. Biophotonics 2015, 8, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Tsukanishi, T.; Funayama, T.; Ozeki, E.; Hara, I.; Abe, T.; Onishi, S.; Yamazaki, M.; Sakane, M. Indocyanine green-lactosome and near-infrared light-based intraoperative imaging and photodynamic therapy for metastatic bone tumors. J. Photopolym. Sci. Technol. 2014, 27, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Funayama, T.; Tsukanishi, T.; Hara, I.; Ozeki, E.; Sakane, M. Tumor-selective near-infrared photodynamic therapy with novel indocyanine green-loaded nanocarrier delays paralysis in rats with spinal metastasis. Photodiagnosis Photodyn. Ther. 2013, 10, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Morimoto, Y.; Takahata, R.; Nomura, S.; Yoshida, K.; Hiraki, S.; Horiguchi, H.; Miyazaki, H.; Ono, S.; Saito, D.; et al. Theranostic photosensitive nanoparticles for lymph node metastasis of gastric cancer. Ann. Surg. Oncol. 2015, 22, S923–S928. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Kaibori, M.; Hishikawa, H.; Nakatake, R.; Okumura, T.; Ozeki, E.; Hara, I.; Morimoto, Y.; Yoshii, K.; Kon, M. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosome has antineoplastic effects for hepatocellular carcinoma. PLoS ONE 2017, 12, e0183527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hishikawa, H.; Kaibori, M.; Tsuda, T.; Matsui, K.; Okumura, T.; Ozeki, E.; Yoshii, K. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosomes has antineoplastic effects for gallbladder cancer. Oncotarget 2019, 10, 5622–5631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, S.; Arake, M.; Morimoto, Y.; Tsujimoto, H.; Miyazaki, H.; Saitoh, D.; Shinomiya, N.; Hase, K.; Yamamoto, J.; Ueno, H. Thermal Sensor Circuit Using Thermography for Temperature-Controlled Laser Hyperthermia. J. Sens. 2017, 2017, 3738046. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Morimoto, Y.; Tsujimoto, H.; Arake, M.; Harada, M.; Saitoh, D.; Hara, I.; Ozeki, E.; Satoh, A.; Takayama, E.; et al. Highly reliable, targeted photothermal cancer therapy combined with thermal dosimetry using a near-infrared absorbent. Sci. Rep. 2020, 10, 9765. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Namikawa, T.; Iwabu, J.; Munekage, M.; Uemura, S.; Maeda, H.; Kitagawa, H.; Nakayama, T.; Inoue, K.; Sato, T.; Kobayashi, M.; et al. Evolution of photodynamic medicine based on fluorescence image-guided diagnosis using indocyanine green and 5-aminolevulinic acid. Surg. Today 2020, 50, 821–831. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Esposito, C.; Coppola, V.; Del Conte, F.; Cerulo, M.; Esposito, G.; Farina, A.; Crocetto, F.; Castagnetti, M.; Settimi, A.; Escolino, M. Near-Infrared fluorescence imaging using indocyanine green (ICG): Emerging applications in pediatric urology. J. Pediatr. Urol. 2020, 16, 700–707. [Google Scholar] [CrossRef]
- Esposito, C.; Settimi, A.; Del Conte, F.; Cerulo, M.; Coppola, V.; Farina, A.; Crocetto, F.; Ricciardi, E.; Esposito, G.; Escolino, M. Image-Guided Pediatric Surgery Using Indocyanine Green (ICG) Fluorescence in Laparoscopic and Robotic Surgery. Front. Pediatr. 2020, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y. 35 years of discussions with Prof. Maeda on the EPR effect and future directions. J. Control. Release 2022, 348, 966–969. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaibori, M.; Matsui, K.; Hayashi, M. Theranostics Using Indocyanine Green Lactosomes. Cancers 2022, 14, 3840. https://doi.org/10.3390/cancers14153840
Kaibori M, Matsui K, Hayashi M. Theranostics Using Indocyanine Green Lactosomes. Cancers. 2022; 14(15):3840. https://doi.org/10.3390/cancers14153840
Chicago/Turabian StyleKaibori, Masaki, Kosuke Matsui, and Mikio Hayashi. 2022. "Theranostics Using Indocyanine Green Lactosomes" Cancers 14, no. 15: 3840. https://doi.org/10.3390/cancers14153840
APA StyleKaibori, M., Matsui, K., & Hayashi, M. (2022). Theranostics Using Indocyanine Green Lactosomes. Cancers, 14(15), 3840. https://doi.org/10.3390/cancers14153840






