Human Leucocyte Antigens as Prognostic Markers in Head and Neck Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Population
2.2. DNA Isolation and HLA Typing
2.3. Statistics
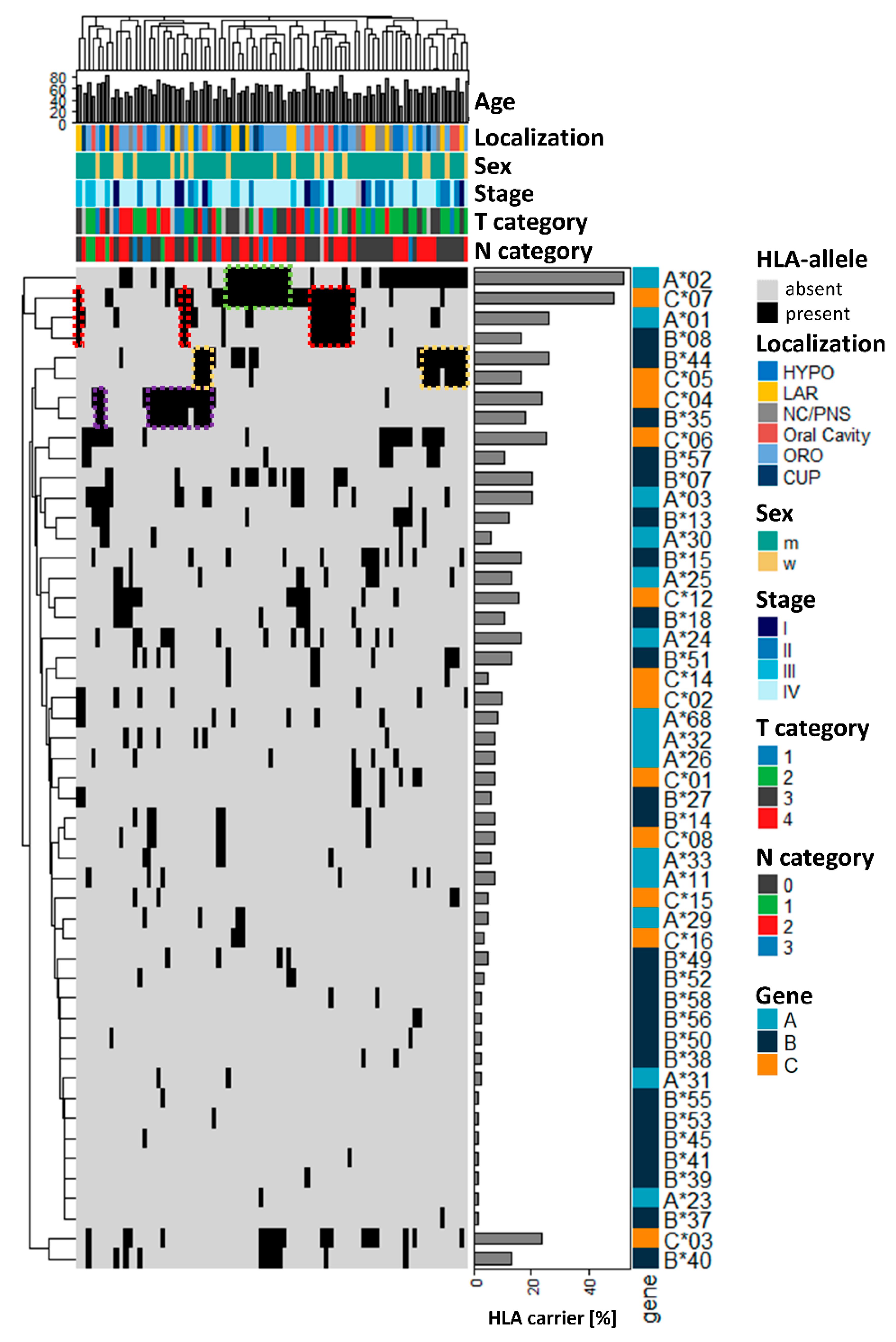
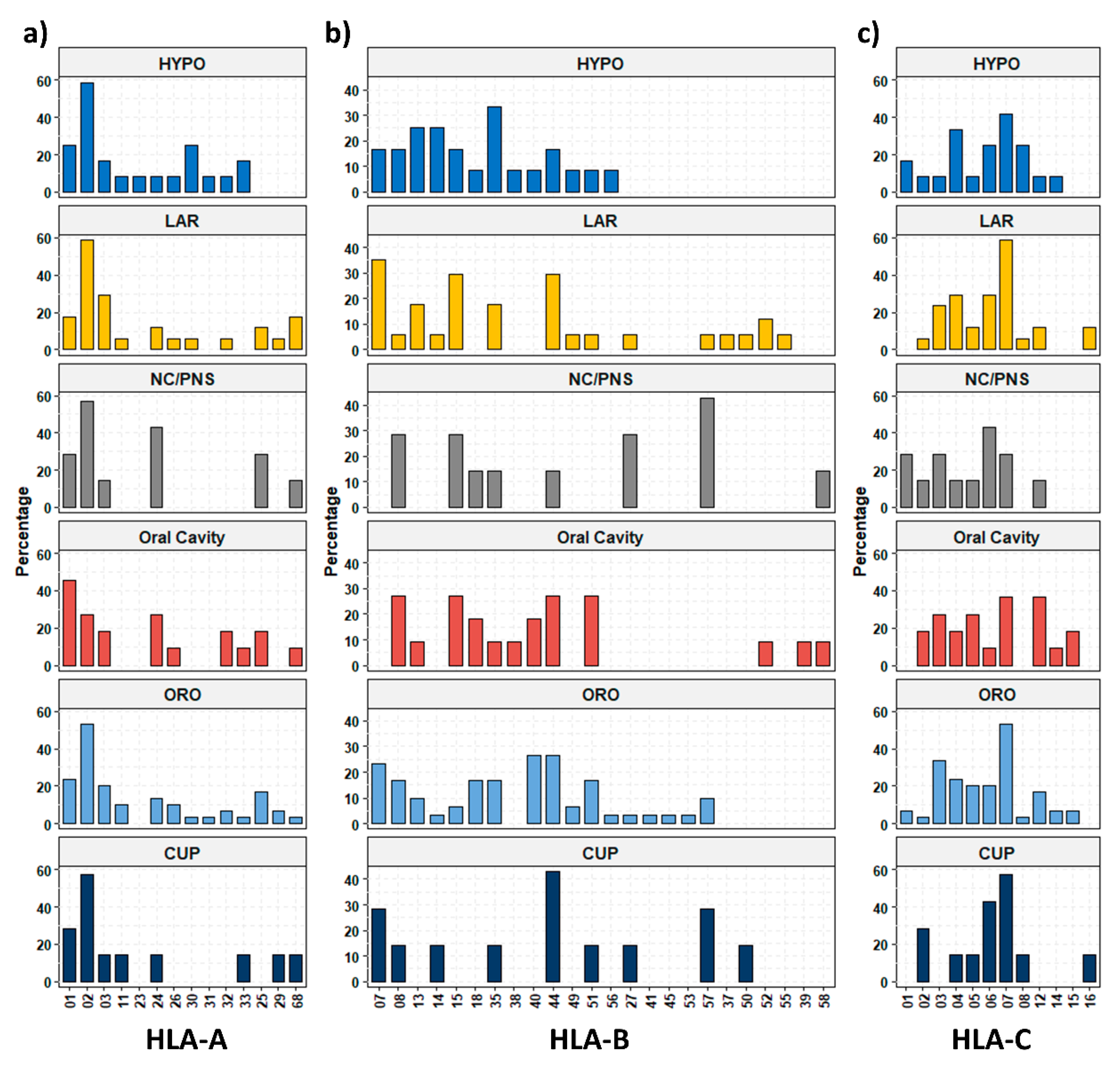
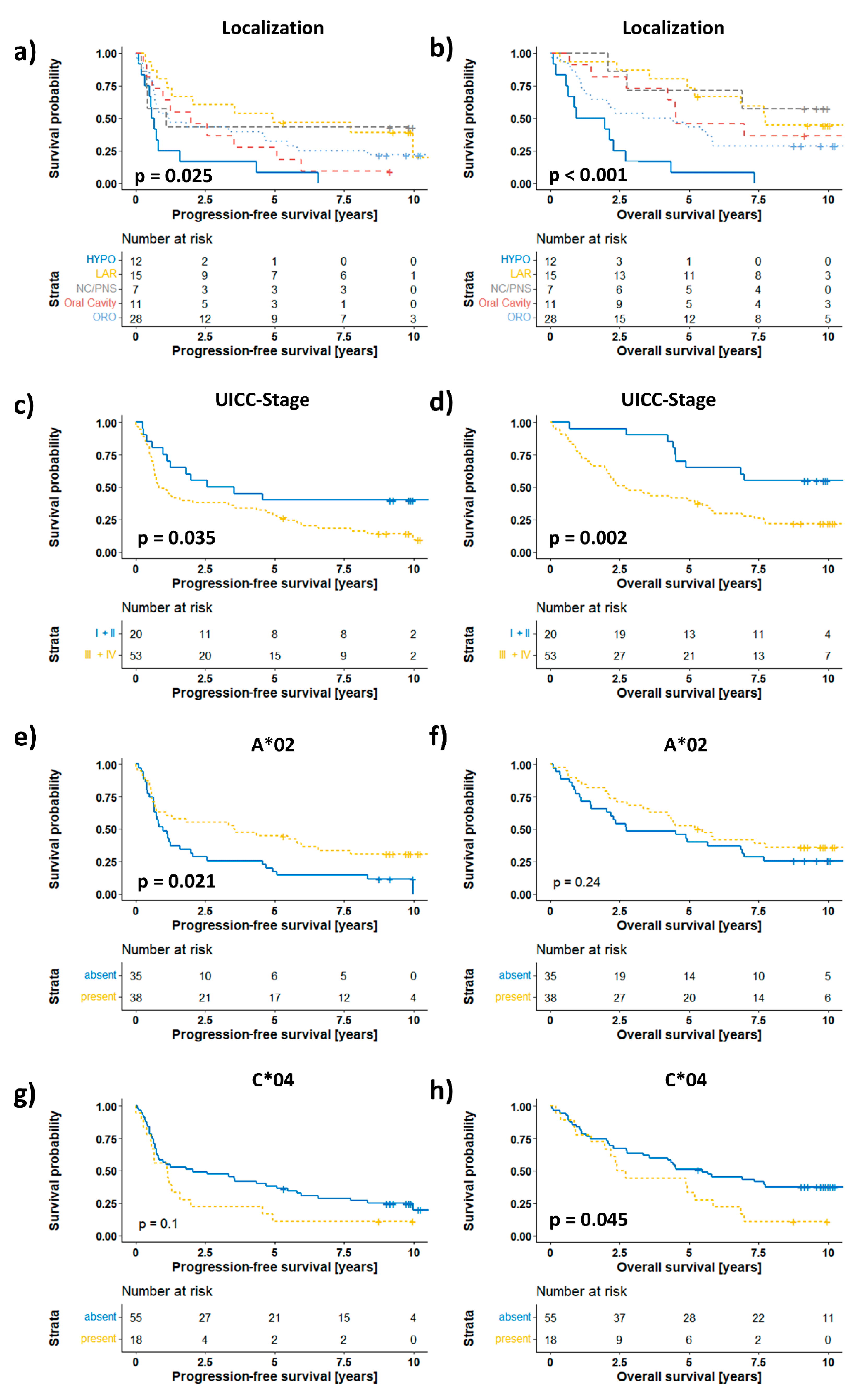
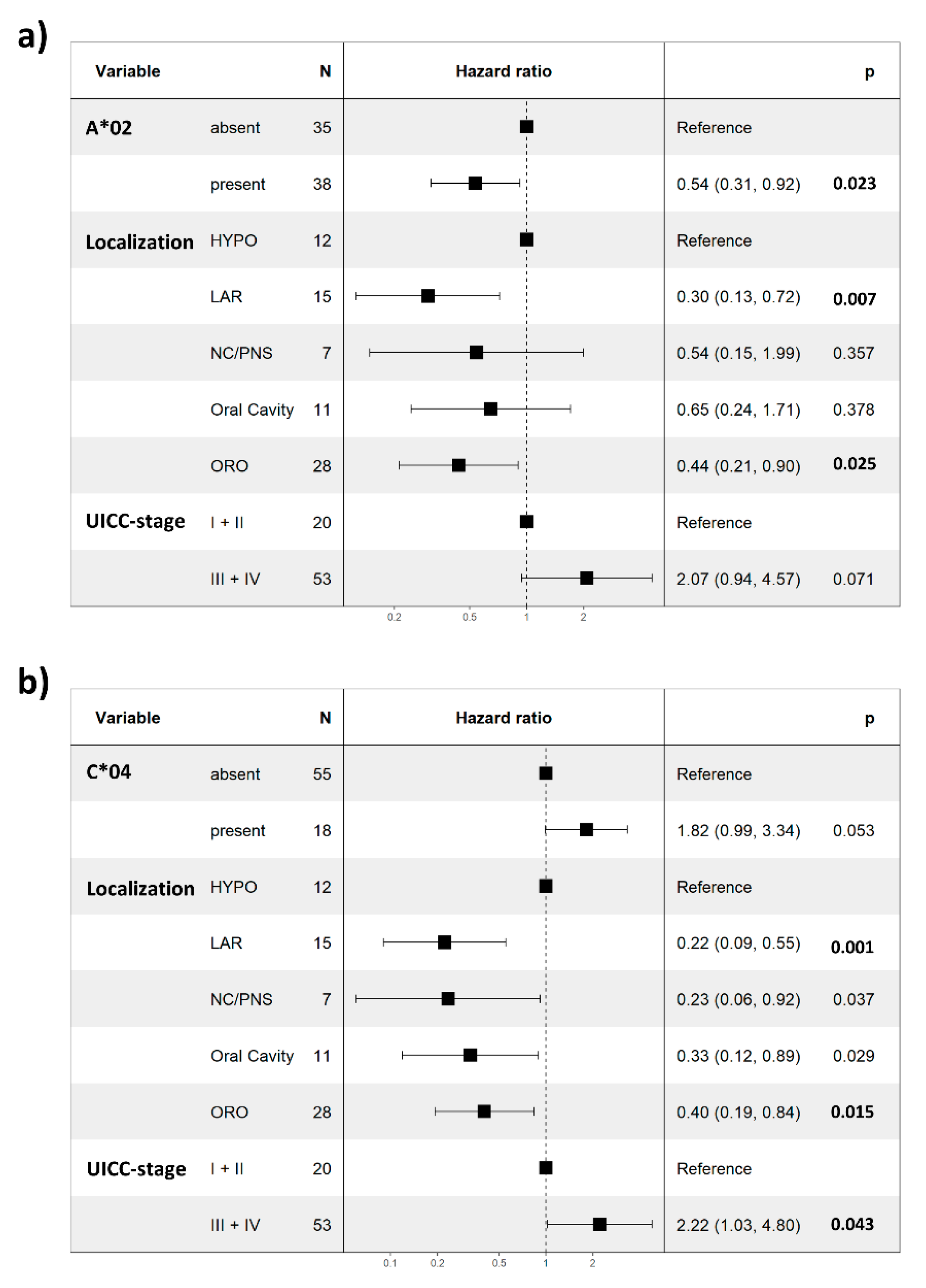
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.; He, M.; Li, L. HLA class I restricted epitopes prediction of common tumor antigens in white and East Asian populations: Implication on antigen selection for cancer vaccine design. PLoS ONE 2020, 15, e0229327. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sabbatino, F.; Liguori, L.; Polcaro, G.; Salvato, I.; Caramori, G.; Salzano, F.A.; Casolaro, V.; Stellato, C.; Col, J.D.; Pepe, S. Role of Human Leukocyte Antigen System as A Predictive Biomarker for Checkpoint-Based Immunotherapy in Cancer Patients. Int. J. Mol. Sci. 2020, 21, 7295. [Google Scholar] [CrossRef]
- La Gruta, N.L.; Gras, S.; Daley, S.R.; Thomas, P.G.; Rossjohn, J. Understanding the drivers of MHC restriction of T cell receptors. Nat. Rev. Immunol. 2018, 18, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Shen, K.Y.; Liu, B.S.; Chen, I.H.; Sher, Y.P.; Tseng, G.C.; Liu, S.J.; Sung, W.C. Immunological evaluation of a novel HLA-A2 restricted phosphopeptide of tumor associated Antigen, TRAP1, on cancer therapy. Vaccine X 2019, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Orenbuch, R.; Filip, I.; Rabadan, R. HLA Typing from RNA Sequencing and Applications to Cancer. Methods Mol. Biol. 2020, 2120, 71–92. [Google Scholar] [CrossRef]
- Liu, X.S.; Mardis, E.R. Applications of Immunogenomics to Cancer. Cell 2017, 168, 600–612. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Alvaro-Benito, M.; Stolzenberg, S.; Noe, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Shiina, T.; Hosomichi, K.; Inoko, H.; Kulski, J.K. The HLA genomic loci map: Expression, interaction, diversity and disease. J. Hum. Genet. 2009, 54, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, G.; Herchenhahn, C.; Boehm, A.; Mozet, C.; Hofer, M.; Fischer, M.; Kolb, M.; Dietz, A. HLA traits linked to development of head and neck squamous cell carcinoma affect the progression-free survival of patients. Oral Oncol. 2017, 69, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Ernst, A.; Orth, M.; Herrmann, M.; Belka, C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front. Oncol. 2012, 2, 116. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Kim, S.S.; Pang, J.; Gold, K.A.; Gutkind, J.S.; Califano, J.A.; Mell, L.K.; Cohen, E.E.W.; Sharabi, A.B. Immune Modulation of Head and Neck Squamous Cell Carcinoma and the Tumor Microenvironment by Conventional Therapeutics. Clin. Cancer Res. 2019, 25, 4211–4223. [Google Scholar] [CrossRef]
- Naranbhai, V.; Viard, M.; Dean, M.; Groha, S.; Braun, D.A.; Labaki, C.; Shukla, S.A.; Yuki, Y.; Shah, P.; Chin, K.; et al. HLA-A*03 and response to immune checkpoint blockade in cancer: An epidemiological biomarker study. Lancet Oncol. 2022, 23, 172–184. [Google Scholar] [CrossRef]
- Tisch, M.; Kyrberg, H.; Weidauer, H.; Mytilineos, J.; Conradt, C.; Opelz, G.; Maier, H. Human leukocyte antigens and prognosis in patients with head and neck cancer: Results of a prospective follow-up study. Laryngoscope 2002, 112, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.2-13. Available online: https://CRAN.R-project.org/package=survival (accessed on 1 June 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Gonzalez-Galarza, F.F.; McCabe, A.; Santos, E.; Jones, J.; Takeshita, L.; Ortega-Rivera, N.D.; Cid-Pavon, G.M.D.; Ramsbottom, K.; Ghattaoraya, G.; Alfirevic, A.; et al. Allele frequency net database (AFND) 2020 update: Gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2020, 48, D783–D788. [Google Scholar] [CrossRef]
- Li, X.; Zhou, C.; Chen, K.; Huang, B.; Liu, Q.; Ye, H. Benchmarking HLA genotyping and clarifying HLA impact on survival in tumor immunotherapy. Mol. Oncol. 2021, 15, 1764–1782. [Google Scholar] [CrossRef]
- Wichmann, G.; Lehmann, C.; Herchenhahn, C.; Kolb, M.; Hofer, M.; Wiegand, S.; Dietz, A. Development of a Human Leukocyte Antigen Score to Predict Progression-Free Survival in Head and Neck Squamous Cell Carcinoma Patients. Front. Oncol. 2018, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Madden, D.R. The three-dimensional structure of peptide-MHC complexes. Annu. Rev. Immunol. 1995, 13, 587–622. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef]
- Brown, S.D.; Warren, R.L.; Gibb, E.A.; Martin, S.D.; Spinelli, J.J.; Nelson, B.H.; Holt, R.A. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014, 24, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Johnson, B.A., III; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Schuler, P.J.; Boeckers, P.; Engers, R.; Boelke, E.; Bas, M.; Greve, J.; Dumitru, C.A.; Lehnerdt, G.F.; Ferris, R.L.; Andrade Filho, P.A.; et al. EGFR-specific T cell frequencies correlate with EGFR expression in head and neck squamous cell carcinoma. J. Transl. Med. 2011, 9, 168. [Google Scholar] [CrossRef][Green Version]
- Albers, A.E.; Qian, X.; Kaufmann, A.M.; Mytilineos, D.; Ferris, R.L.; Hoffmann, T.K.; DeLeo, A.B. Phenotype of p53 wild-type epitope-specific T cells in the circulation of patients with head and neck cancer. Sci. Rep. 2018, 8, 10716. [Google Scholar] [CrossRef]
- Koskinen, W.J.; Partanen, J.; Vaheri, A.; Aaltonen, L.M. HLA-DRB1, -DQB1 alleles in head and neck carcinoma patients. Tissue Antigens 2006, 67, 237–240. [Google Scholar] [CrossRef]
- Reinders, J.; Rozemuller, E.H.; Otten, H.G.; van der Veken, L.T.; Slootweg, P.J.; Tilanus, M.G. HLA and MICA associations with head and neck squamous cell carcinoma. Oral Oncol. 2007, 43, 232–240. [Google Scholar] [CrossRef]
| Variable | Category | n | % |
|---|---|---|---|
| Age (years) | <50 | 15 | 17.9 |
| 50–59 | 30 | 39.3 | |
| 60–69 | 27 | 28.6 | |
| 70–79 | 9 | 10.7 | |
| >80 | 3 | 3.6 | |
| Sex | Male | 65 | 77.4 |
| Female | 19 | 22.6 | |
| Localization | Hypopharynx | 12 | 14.3 |
| Larynx | 17 | 20.2 | |
| Oropharynx | 30 | 35.7 | |
| Oral cavity | 11 | 13.1 | |
| Nasal cavity/paranasal sinuses | 7 | 8.3 | |
| CUP | 7 | 8.3 | |
| T category | T1 | 13 | 15.5 |
| T2 | 28 | 33.3 | |
| T3 | 17 | 20.2 | |
| T4 | 17 | 20.2 | |
| Unknown/n.a. (CUP) | 9 | 10.7 | |
| N category | N0 | 36 | 43.4 |
| N1 | 5 | 6.0 | |
| N2 | 35 | 42.2 | |
| N3 | 7 | 8.4 | |
| Unknown | 1 | 1.2 | |
| UICC stage | I | 8 | 9.6 |
| II | 14 | 16.9 | |
| III | 11 | 13.3 | |
| IV | 50 | 60.2 | |
| Unknown | 1 | 1.2 | |
| Therapy | Surgery only | 40 | 47.6 |
| Surgery + aCRT | 17 | 20.2 | |
| Surgery + aRT | 27 | 32.1 |
| HLA-Type | Normal (% (n)) | Tumor (% (n)) | p Value |
|---|---|---|---|
| HLA-A | |||
| HLA-A*01 | 25.9 (45) | 26.2 (22) | 1.00 |
| HLA-A*02 | 51.1 (89) | 52.4 (44) | 0.89 |
| HLA-A*03 | 28.7 (50) | 20.2 (17) | 0.17 |
| HLA-A*11 | 8.6 (15) | 7.1 (6) | 0.81 |
| HLA-A*23 | 3.4 (6) | 1.2 (1) | 0.43 |
| HLA-A*24 | 19.0 (33) | 16.7 (14) | 0.73 |
| HLA-A*25 | 4.6 (8) | 13.1 (11) | 0.02 |
| HLA-A*26 | 6.3 (11) | 7.1 (6) | 0.79 |
| HLA-A*29 | 4.0 (7) | 4.8 (4) | 0.75 |
| HLA-A*30 | 4.6 (8) | 6.0 (5) | 0.76 |
| HLA-A*31 | 6.9 (12) | 2.4 (2) | 0.16 |
| HLA-A*32 | 3.4 (6) | 7.1 (6) | 0.21 |
| HLA-A*33 | 3.4 (6) | 6.0 (5) | 0.34 |
| HLA-A*68 | 12.1 (21) | 8.3 (7) | 0.40 |
| HLA-Type | Normal (% (n)) | Tumor (% (n)) | p Value |
|---|---|---|---|
| HLA-B | |||
| HLA-B*07 | 25.3 (44) | 20.2 (17) | 0.44 |
| HLA-B*08 | 25.3 (44) | 16.7 (14) | 0.15 |
| HLA-B*13 | 5.2 (9) | 11.9 (10) | 0.07 |
| HLA-B*14 | 3.4 (6) | 7.1 (6) | 0.21 |
| HLA-B*15 | 14.4 (25) | 16.7 (14) | 0.71 |
| HLA-B*18 | 4.6 (8) | 10.7 (9) | 0.10 |
| HLA-B*27 | 13.2 (23) | 6.0 (5) | 0.09 |
| HLA-B*35 | 13.2 (23) | 17.9 (15) | 0.35 |
| HLA-B*37 | 1.7 (3) | 1.2 (1) | 1.00 |
| HLA-B*38 | 4.6 (8) | 2.4 (2) | 0.51 |
| HLA-B*39 | 5.7 (10) | 1.2 (1) | 0.11 |
| HLA-B*40 | 17.2 (30) | 13.1 (11) | 0.47 |
| HLA-B*41 | 3.4 (6) | 1.2 (1) | 0.43 |
| HLA-B*42 | 0.0 (0) | 0.0 (0) | 1.00 |
| HLA-B*44 | 17.2 (30) | 26.2 (22) | 0.10 |
| HLA-B*45 | 0.6 (1) | 1.2 (1) | 0.55 |
| HLA-B*46 | 0.0 (0) | 0.0 (0) | 1.00 |
| HLA-B*47 | 0.0 (0) | 0.0 (0) | 1.00 |
| HLA-B*48 | 0.6 (1) | 0.0 (0) | 1.00 |
| HLA-B*49 | 2.3 (4) | 4.8 (4) | 0.28 |
| HLA-B*50 | 1.7 (3) | 2.4 (2) | 0.66 |
| HLA-B*51 | 10.3 (18) | 13.1 (11) | 0.53 |
| HLA-B*52 | 1.1 (2) | 3.6 (3) | 0.33 |
| HLA-B*53 | 0.0 (0) | 1.2 (1) | 0.33 |
| HLA-B*54 | 0.6 (1) | 0.0 (0) | 1.00 |
| HLA-B*55 | 6.3 (11) | 1.2 (1) | 0.11 |
| HLA-B*56 | 2.3 (4) | 2.4 (2) | 1.00 |
| HLA-B*57 | 5.2 (9) | 10.7 (9) | 0.12 |
| HLA-B*58 | 2.9 (5) | 2.4 (2) | 1.00 |
| HLA-B*59 | 0.6 (1) | 0.0 (0) | 1.00 |
| HLA-B*73 | 0.0 (0) | 0.0 (0) | 1.00 |
| HLA-Type | Normal (% (n)) | Tumor (% (n)) | p Value |
|---|---|---|---|
| HLA-C | |||
| HLA-C*01 | 10.9 (19) | 7.1 (6) | 0.38 |
| HLA-C*02 | 5.2 (9) | 9.5 (8) | 0.19 |
| HLA-C*03 | 30.5 (53) | 23.8 (20) | 0.30 |
| HLA-C*04 | 17.2 (30) | 23.8 (20) | 0.24 |
| HLA-C*05 | 12.1 (21) | 16.7 (14) | 0.34 |
| HLA-C*06 | 12.1 (21) | 25 (21) | 0.01 |
| HLA-C*07 | 59.2 (103) | 48.8 (41) | 0.14 |
| HLA-C*08 | 4.0 (7) | 7.1 (6) | 0.36 |
| HLA-C*12 | 9.8 (17) | 15.5 (13) | 0.21 |
| HLA-C*14 | 1.7 (3) | 4.8 (4) | 0.22 |
| HLA-C*15 | 4.0 (7) | 4.8 (4) | 0.75 |
| HLA-C*16 | 1.1 (2) | 3.6 (3) | 0.33 |
| HLA-C*17 | 1.7 (3) | 0.0 (0) | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyckhoff, G.; Herold-Mende, C.; Scherer, S.; Plinkert, P.K.; Warta, R. Human Leucocyte Antigens as Prognostic Markers in Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 3828. https://doi.org/10.3390/cancers14153828
Dyckhoff G, Herold-Mende C, Scherer S, Plinkert PK, Warta R. Human Leucocyte Antigens as Prognostic Markers in Head and Neck Squamous Cell Carcinoma. Cancers. 2022; 14(15):3828. https://doi.org/10.3390/cancers14153828
Chicago/Turabian StyleDyckhoff, Gerhard, Christel Herold-Mende, Sabine Scherer, Peter K. Plinkert, and Rolf Warta. 2022. "Human Leucocyte Antigens as Prognostic Markers in Head and Neck Squamous Cell Carcinoma" Cancers 14, no. 15: 3828. https://doi.org/10.3390/cancers14153828
APA StyleDyckhoff, G., Herold-Mende, C., Scherer, S., Plinkert, P. K., & Warta, R. (2022). Human Leucocyte Antigens as Prognostic Markers in Head and Neck Squamous Cell Carcinoma. Cancers, 14(15), 3828. https://doi.org/10.3390/cancers14153828






