Simple Summary
Mesothelioma is a deadly disease with few treatment options. Phytocannabinoids derived from the cannabis plant are garnering interest for their anti-cancer properties, however very little is known about their effects in mesothelioma. We aimed to assess whether phytocannabinoids have anti-cancer effects in mesothelioma and potential modes of action. We showed that several phytocannabinoids inhibited growth of mesothelioma cells, with two phytocannabinoids, cannabidiol (CBD) and cannabigerol (CBG), being the most potent. CBD and CBG also inhibited mesothelioma cell migration and invasion. Gene expression analysis highlighted signalling pathways that play a role in how CBD and CBG may exert their anti-cancer effects. CBD and CBG were unable to increase survival in a rat model of mesothelioma but this may be due to limitations in the drug delivery method.
Abstract
Mesothelioma is an aggressive cancer with limited treatment options and a poor prognosis. Phytocannabinoids possess anti-tumour and palliative properties in multiple cancers, however their effects in mesothelioma are unknown. We investigated the anti-cancer effects and potential mechanisms of action for several phytocannabinoids in mesothelioma cell lines. A panel of 13 phytocannabinoids inhibited growth of human (MSTO and H2452) and rat (II-45) mesothelioma cells in vitro, and cannabidiol (CBD) and cannabigerol (CBG) were the most potent compounds. Treatment with CBD or CBG resulted in G0/G1 arrest, delayed entry into S phase and induced apoptosis. CBD and CBG also significantly reduced mesothelioma cell migration and invasion. These effects were supported by changes in the expression of genes associated with the cell cycle, proliferation, and cell movement following CBD or CBG treatment. Gene expression levels of CNR1, GPR55, and 5HT1A also increased with CBD or CBG treatment. However, treatment with CBD or CBG in a syngeneic orthotopic rat mesothelioma model was unable to increase survival. Our data show that cannabinoids have anti-cancer effects on mesothelioma cells in vitro and alternatives of drug delivery may be needed to enhance their effects in vivo.
1. Introduction
Malignant mesothelioma is an aggressive cancer with a poor response to current therapies and, consequently, has a very grim prognosis. The median survival for malignant mesothelioma is only approximately 12 months and the 5-year survival rate is less than 10% [1,2]. Worldwide, over 38,000 people die each year from mesothelioma [3] with asbestos exposure linked to approximately 80% of all cases [4]. Fortunately, asbestos use has now been banned in many countries but its widespread use in thousands of construction-related products for over a century has left a deadly legacy in the community. Approximately 125 million people have experienced occupational exposure to asbestos, but a non-occupational wave is also emerging [5]. This, together with the 20–40-year latency period for disease development means that mesothelioma is not a disease of the past, but rather one that is and has been increasing in incidence. As most mesothelioma patients are diagnosed when their disease is advanced, systemic chemotherapy of pemetrexed with cisplatin is the standard of care and most effective treatment option [6,7]. However, failure of this treatment is inevitable and there are no proven second line options [2]. Therefore, there is an urgent unmet need for more effective treatments.
With the legalisation of medicinal cannabis around the world there has been a renewed interest in examining the therapeutic potential of the cannabinoids. Cannabis contains over 100 plant-derived cannabinoids (phytocannabinoids), including the main psychoactive constituent ∆9-tetrahydrocannabinol (THC) and the non-intoxicating compound cannabidiol (CBD). Although CBD and THC are the most studied, there is growing interest in the therapeutic potential of other phytocannabinoids, for example cannabigerol (CBG) and cannabidiolic acid (CBDA) [8,9,10,11,12]. The majority of research has focused on the use of THC in the symptomatic management of cancer due to its appetite stimulatory, analgesic, and anti-emetic effects [13,14]. However, evidence suggests that the major cannabinoids CBD and THC display anti-tumoural properties in pre-clinical models and while clinical trials are still pending, anecdotal evidence for efficacy does exist [15,16]. Cannabinoids were shown to decrease the growth and metastatic potential of cancer cells in a number of solid cancers, as well as haematological malignancies (reviewed in [17,18,19]). However, no prior studies have examined the anti-cancer effects of cannabinoids against mesothelioma. The overall aim of this project is to determine whether a panel of phytocannabinoids have anti-cancer properties in pre-clinical mesothelioma models in vitro and in vivo.
2. Materials and Methods
Cell culture: the human mesothelioma cell lines MSTO-211H (MSTO) and NCI-H2452 (H2452) were purchased from the ATCC. MSTO and H2452 cells are both derived from pleural mesothelioma patients [20,21]. MSTO cells display biphasic sarcomatoid and epithelioid features, while H2452 cells are epithelioid. The rat mesothelioma cell line II-45 (also biphasic) was kindly provided by A/Prof. Emanuela Felley-Bosco, Zurich University [22]. All cell lines were cultured in RPMI-1640 with L-glutamine (Gibco, Thermofisher Scientific, North Ryde, NSW, Australia) supplemented with 10% foetal bovine serum at 37 °C with 5% CO2, unless stated otherwise. All experiments were performed at least 3 times.
Phytocannabinoids: THC, CBD, CBDA, cannabigerolic acid (CBGA), cannabidivarin (CBDV), CBG, Δ9-tetrahydrocannabivarin (THCV), and cannabinol (CBN) were purchased from THC Pharm GmbH (Frankfurt, Germany). Cannabichromene (CBC) was synthesised by Lambert Initiative chemists [23]. Δ9-tetrahydrocannabinolic acid (THCA) was isolated from hemp extracts [24]. Cannabidivarinic acid (CBDVA), cannabichromenic acid (CBCA) and cannabichromevarinic acid (CBCVA) were synthesised by Professor Michael Kassiou at the University of Sydney (Australia). For all in vitro experiments, phytocannabinoids were dissolved in ethanol and added to cells in culture medium to a maximum concentration of 0.5%.
Cell viability/cytotoxicity: cells were seeded in triplicate in 96-well plates (Nunc MicroWell 96-Well Microplates, ThermoFisher Scientific) at a density of 5 × 103 cells/well for MSTO and H2452 cells, and 2 × 103 cells/well for II-45 cells. After 4 h, cells were treated with serial 2-fold dilutions of phytocannabinoids. After 72 h of treatment, cell viability was measured by standard MTT (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays [25].
Cell cycle assay: cell lines were seeded and treated for 24 h with the phytocannabinoids (at concentrations double that of the 72 h IC50; II-45 cells: CBD 15 µM and CBG 33.2 µM; MSTO cells: CBD 22 µM and CBG 32.9 µM; H2452 cells: CBD 20 µM and CBG 31 µM) or vehicle as control. After treatment, cells were harvested, washed with 1X PBS, fixed with 1 mL of ice-cold 70% ethanol and stored at −20 °C overnight. The fixed cells were stained and analysed using the MUSE® Cell Cycle Kit and the MUSE® Cell Analyser following the manufacturer’s instructions [26]. The percentage of cells in G0/G1, S and G2/M phases was assessed.
Apoptosis assay: cells were seeded and treated with the phytocannabinoid, or vehicle control for 24 h, as described for the cell cycle assay. After treatment, cells were harvested, stained, and analysed using the MUSE® Annexin V and Dead Cell Kit and the MUSE® Cell Analyser (Abacus dx, Cannon Hill, QLD, Australia) following the manufacturer’s instructions.
Transwell migration and invasion assays: the effect of phytocannabinoids on mesothelioma cell migration and invasion was assessed using transwell plates containing 8.0 μm pore size inserts (Sigma-Aldrich Pty. Ltd., Sydney, Australia). For migration assays cells were seeded in 350 µL serum free media in the upper chamber (1.25 × 104 for rat II-45 and human H2452 cells and 5 × 104 cells for human MSTO cells). For the invasion assays transwells pre-coated with Matrigel™ were used and cells were seeded in 500 µL serum free media in the MatrigelTM coated chamber (3 × 104 of rat II-45 cells and 2 × 105 of human H2452 cells). Media containing 10% FBS was added as a chemotactic agent to the lower chamber. Sub-lethal concentrations of cannabinoids were then added (or vehicle only for control). After treating for 24 h (with the exception of II-45 migration assays, which were treated for 6 h), media and non-migrated cells were removed with a cotton swab. Membranes were fixed in 70% ethanol and migrated/invaded cells were stained with ProlongTM Gold Antifade Mountant with DAPI (Thermofisher Scientific). Images of migrated/invaded cells were taken at 100× magnification with an Olympus IX70 fluorescence microscope (Olympus, Macquarie Park, NSW, Australia). The number of cells in 5 fields of view was calculated using CellProfiler [27] and graphs were plotted as percentage of migrated/invaded cells normalised to vehicle only control cells in GraphPad Prism (GraphPad Software, San Diego, CA, USA). It is noteworthy that MSTO cells did not invade, therefore invasion assays were unable to be performed.
RNA extraction and cDNA synthesis: II-45, MSTO and H2452 cells were seeded and left to adhere for 4 h. Cells were then treated with CBD, CBG, or a vehicle as described for the cell cycle assay. After the 24 h treatment, cells were counted, and viability measured using the MUSE® Count & Viability Kit (Abacus dx) following the manufacturer’s instructions. At harvesting, cells were greater than 80% viable. RNA was extracted using an RNeasy Mini RNA Isolation kit (Qiagen, Clayton, VIC, Australia) following the manufacturer’s instructions and quantified using the Qubit RNA BR assay kit (Life Technologies Australia Pty. Ltd., Mulgrave, VIC, Australia). Quality was assessed from the 260/280 ratio using the NanoDrop Spectrophotometer only accepting ratios > 1.6. cDNA was synthesised using the Superscript IV VILO Master Mix (Thermofisher Scientific) as per the manufacturer’s instructions.
TaqMan gene expression assays: TaqMan gene expression assays (Thermofisher Scientific) were used to quantify changes in gene expression for receptors known to interact with phytocannabinoids [28,29]. Taqman assays and identification numbers are listed in the Supplementary Material (Table S1). Assays were performed on the QuantStudio™ 12K Flex Real Time PCR System (Applied Biosystems, ThermoFisher Scientific) as per the manufacturer’s instructions. The results were analysed on QuantStudio 12K Flex Software v1.2.2. Fold change in gene expression for each target was determined using the 2−ΔΔCt method normalised to the housekeeping gene (TBP). Fold change (FC) in gene expression was calculated relative to vehicle-treated control cells.
TaqManTM OpenArrayTM Real-Time-PCR: the TaqManTM OpenArrayTM Human Cancer Panel (Thermofisher Scientific) was used to evaluate the expression of 624 known cancer-related genes in vehicle control, CBD, and CBG-treated MSTO and H2452 cells. Taqman assays included on the OpenArray are listed in Table S2. cDNA was prepared as described above prior to adding to the TaqManTM OpenArrayTM Real-time PCR Mastermix (Applied Biosystems) and distributed on a 384-well open array plate using the OpenArrayTM AccuFill™ System following the manufacturer’s instructions. The PCR was then run on the QuantStudio™ 12K Flex Real Time PCR System (Thermofisher Scientific). QuantStudio™ 12K Flex Software v1.2.2 (Thermofisher Scientific) was used to normalise gene expression results to a panel of housekeeping genes included on the TaqManTM OpenArrayTM Human Cancer Panel and calculate FC between treated versus control cells using the 2−ΔΔCt method. Gene expression results can be found in Table S3. A FC of >2 was used as the cut-off for determining differentially expressed genes (DEGs). Genes found to be FC > 2 and shared between the MSTO and H2452 cell lines are listed in Table S4.
Pathway enrichment analysis: to better understand the biological relevance of the identified DEGs, pathway enrichment analysis was performed. Data were analysed using ingenuity pathway analysis [30]. Molecules from the dataset that had a FC > 2 and were associated with a canonical pathway in the Ingenuity Knowledge Base were considered for the analysis. The significance of the association between the dataset and the canonical pathway was measured in two ways: (1) a ratio of the number of molecules from the dataset that map to the pathway divided by the total number of molecules that map to the canonical pathway; and (2) a right-tailed Fisher’s Exact Test was used to calculate a p-value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone. A comparison analysis was then used to identify shared canonical pathways between the MSTO and H2452 cells (−log (p-value) > 2). IPA z-scores predict activation or inhibition of the canonical pathways with z-scores of >2 considered significantly activated and <−2 considered significantly inhibited. Data presented represents the top shared canonical pathways with z-scores in the same direction in both cell lines, i.e., either activated or inhibited in both MSTO and H2452 cells with general cancer canonical pathways being removed for relevance. The full list of shared canonical pathways is available in Tables S5 and S6 of the Supplementary Material.
In vivo experiments: all procedures involving animals were carried out in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The protocol for this study was approved by the Royal North Shore Hospital Animal Care and Ethics Committee (protocol numbers RESP/17/30). Female Fischer 344 rats weighing 150–200 g were housed in groups of three at the Kearns Facility under standard conditions (12 h light/dark cycles, free access to food and water, nesting material with plastic huts, and environmental enrichment, such as soft wood sticks, straws, paper and tissue boxes).
Pharmacokinetic assay to determine the vehicle for the in vivo survival experiment: 20 mg/kg CBD was made up in either: (1) hemp seed oil; (2) ethanol/tween80/0.9% saline (at a 1:1:18 ratio) or DMSO/kolliphor/0.9% saline (at a 1:1:18 ratio) to determine which vehicle provided the highest plasma levels of exposure to CBD. Rats (n = 4 per group) were intraperitoneally (i.p.) injected with CBD in the different vehicles. Blood was taken at 15 min, 1 h, 2 h, and 4 h post i.p. injection into EDTA blood tubes. Plasma concentrations of CBD were measured using LC MS/MS and calculated as previously described [31,32]. Results are presented in Figure S1.
In vivo survival study: on day zero, female Fischer rats were pleurally engrafted with 100 μL of serum free media containing 1 × 104 II-45 cells. Pharmacological treatments began on day 1 and continued for 4 weeks. The positive control, a standard of care chemotherapy combination of 1 mg/kg cisplatin and 6.7 mg/kg pemetrexed, was administered i.p. on days 3, 6, 11, 15, 20, and 24. 40 mg/kg CBD and 100 mg/kg CBG were administered with single daily i.p. injections in the vehicle of DMSO:kolliphor:saline at a 1:1:18 ratio. Deiana et al. [33] compared the plasma pharmacokinetics of CBD and CBG in rats following i.p. injections and found that total plasma exposures (AUC 0–∞) were approximately 2.5-fold less in CBG compared to CBD dosed animals. CBD and CBG doses were thus selected based on extrapolated data from our pharmacokinetic experiment and that of Deiana et al. [33] to result in similar plasma concentrations (Cmax = 2–4 µM CBD and 2 µM CBG), as well as the maximum concentration able to be diluted in solvent. This approximates the maximal plasma concentrations that have been achieved in intractable epilepsy patients taking very high, oral doses of CBD, thus representing maximal clinically relevant doses that can be attained in humans with registered pharmaceutical CBD products [34]. Control animals received the vehicle only, using the same treatment schedule as CBD and CBG. Rats were euthanised at ethically approved endpoints (weight loss of >20% or difficulty in breathing) and blood, tissue, and tumour samples were collected. Tumours were scored on a scale of 1 to 5 based on volume and infiltration of the tumour into the pleural cavity, as described previously [35], with 1 indicating the tumour occupied up to 10% of the pleural cavity and 5 indicating the tumour occupied 40–50% of the pleural cavity.
Statistical analysis: GraphPad Prism (Version 9.3.1) was used for graphs and statistical analysis. For cell viability/cytotoxicity assays, non-linear (curve fit) regression algorithms were used to calculate the drug dose causing 50% growth inhibition (IC50 drug dose). For cell cycle, apoptosis assays and TaqMan gene expression assays, two-way ANOVA with Holm-Sidak’s multiple comparisons was used. For transwell migration and invasion assays, one-way ANOVA with Kruskal-Wallis multiple comparisons was used. For survival analysis, p-values were calculated using Log-rank (Mantel-Cox) test, relative to control treated animals.
3. Results
3.1. Phytocannabinoids Inhibit Viability of Mesothelioma Cells In Vitro
A panel of 13 phytocannabinoids was screened for their ability to inhibit the viability of mesothelioma cell lines. All phytocannabinoids reduced the viability of both rat (II-45) and human (MSTO and H2452) mesothelioma cell lines with the drug concentration causing 50% inhibition (IC50) shown in Figure 1. In general, the neutral cannabinoids (i.e., THC, CBN, CBD, CBG, and CBC) were more potent than the acid forms of the molecules (THCA, CBDA, CBGA, and CBCA). Overall, CBD and CBG most potently reduced the viability of the mesothelioma cell lines.
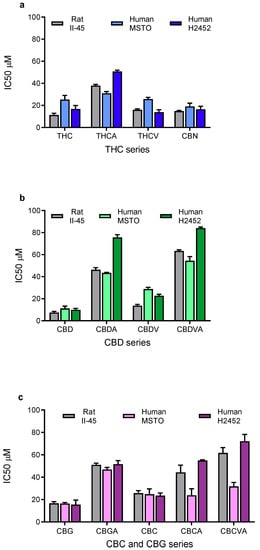
Figure 1.
Phytocannabinoids reduce the proliferation of mesothelioma cell lines. The drug concentration causing 50% growth inhibition (IC50) of (a) ∆9-tetrahydrocannabinol (THC) related, (b) cannabidiol (CBD) related, and (c) cannabichromene (CBC) and cannabigerol (CBG) related phytocannabinoids against rat II-45 and human MSTO and H2452 mesothelioma cell lines were determined. Cell viability was assessed using MTT assays in the presence of phytocannabinoid as indicated. Bars show the mean and standard deviation (SD) from at least three independent experiments.
3.2. CBD and CBG Induce G0/G1 Cell Cycle Arrest and Apoptosis in Mesothelioma Cell Lines
Having identified CBD and CBG as the most promising phytocannabinoids, assays were undertaken to ascertain whether these cannabinoids induced programmed cell death in the rat (II-45) and human (MSTO and H2452) mesothelioma cell lines. After 24 h of CBD or CBG treatment, cell cycle changes were identified with G0/G1 arrest and delayed entry into S Phase. Apoptosis was also induced by both CBD and CBG (Figure 2 and Figure 3).
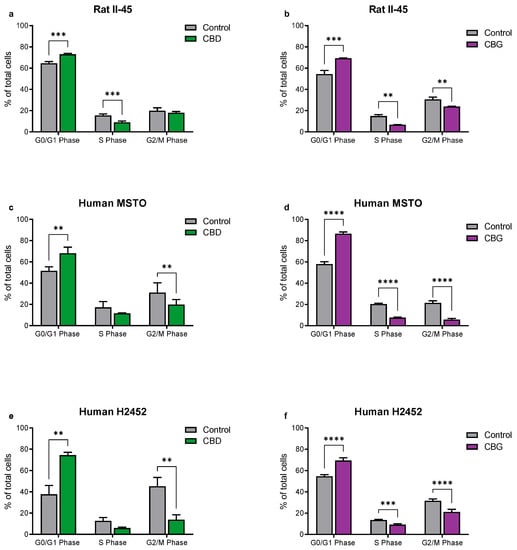
Figure 2.
The phytocannabinoids cannabidiol (CBD) and cannabigerol (CBG) induce cell cycle arrest in mesothelioma cell lines. The effects of CBD and CBG (2xIC50) on cell cycle were assessed in rat II-45 (a,b), human MSTO (c,d) and H2452 (e,f) mesothelioma cell lines. After 24 h of treatment with vehicle (control), CBD or CBG, cells were harvested, and assays performed. Bars show the mean and SD from three independent experiments. p-values were calculated using two-way ANOVA Holm-Sidak’s multiple comparisons. ** p < 0.01, *** p < 0.001, and **** p < 0.0001 relative to vehicle-treated control cells.
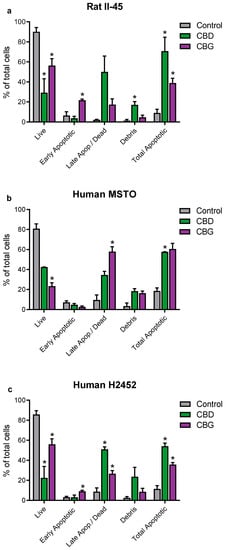
Figure 3.
The phytocannabinoids cannabidiol (CBD) and cannabigerol (CBG) induced apoptosis in mesothelioma cell lines. The effects of CBD and CBG (2xIC50) on apoptosis were assessed in rat II-45 (a), human MSTO (b), and H2452 (c) mesothelioma cell lines. After 24 h of treatment with vehicle (control), CBD or CBG, cells were harvested, and assays performed. Bars show the mean and SD from three independent experiments. p-values were calculated using two-way ANOVA Holm-Sidak’s multiple comparisons. * p < 0.05 relative to vehicle-treated control cells.
3.3. CBD and CBG Inhibit Migration and Invasion of Mesothelioma Cell Lines In Vitro
We then assessed whether CBD and CBG could inhibit migration and invasion of mesothelioma cell lines. Although similar to migration assays, invasion assays examine the potential of the mesothelioma cells to invade through an extracellular matrix, which is extremely important to their metastatic ability. Of note, the human MSTO cells were unable to invade through an extracellular matrix, therefore invasion assays were not examined in this cell line. Using sub-cytotoxic concentrations, both CBD and CBG significantly reduced the migratory and invasive potential of the rat II-45 and human H2452 mesothelioma cell lines, suggesting that these compounds may also slow the metastatic spread of mesothelioma cells (Figure 4).
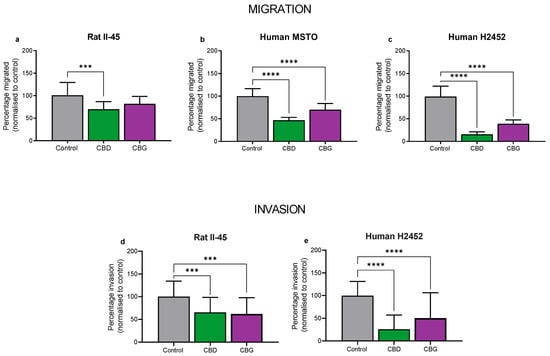
Figure 4.
Cannabidiol (CBD) and cannabigerol (CBG) reduced migration and invasion of mesothelioma cell lines. Rat II-45 (a,d) and human MSTO (b) and H2452 (c,e) mesothelioma cell lines were examined with CBD and CBG being tested at sub-cytotoxic concentrations (2 µM CBD for II-45, 6 µM for MSTO and H2452 cells, and 10 µM CBG for all cell lines). The number of migrated cells or cells that had invaded through the Matrigel per 5–10 fields of view was counted and analysed using CellProfiler. Bars show the mean and SD from three independent experiments. p-values were calculated using one-way ANOVA Kruskal-Wallis multiple comparisons. *** p < 0.001 and **** p < 0.0001 relative to vehicle treated control cells.
3.4. CBD and CBG Treatment Effects on Cannabinoid-Related Gene Targets in Mesothelioma Cell Lines
To begin to investigate mechanisms of action, we examined whether CBD and CBG treatment affected the gene expression of targets in mesothelioma cells implicated in the effects of cannabinoids, or the pathobiology of mesothelioma (Figure 5). CNR2 was not expressed in any of the mesothelioma cell lines and was not included in the analyses. In all three mesothelioma cell lines, both CBD and CBG robustly upregulated mRNA for the cannabinoid CB1 receptor (CNR1), G protein-coupled receptor 55 (GPR55) and the 5-HT1a receptor (HTR1A) (Figure 5). Notably, CBD and CBG upregulated these targets by as high as approximately 50-fold compared to vehicle-treated cells. CBD and CBG increased mRNA expression of transient receptor potential vanilloid type 1 (TRPV1) in all mesothelioma cell lines, except in CBD-treated H2452 cells. CBD and CBG did not consistently affect TRPV2 or peroxisome proliferator-activated receptor gamma (PPARG) expression. Interestingly, CBD and CBG increased expression of C-X-C chemokine receptor 4 (CXCR4) in all cell lines. CBD treatment was associated with downregulation of the endogenous agonist of CXCR4, C-X-C motif chemokine 12 (CXCL12), in all cell lines. Similarly CBG reduced CXCL12 expression in the II-45 and H2452 cells but increased its expression in the MSTO cells.
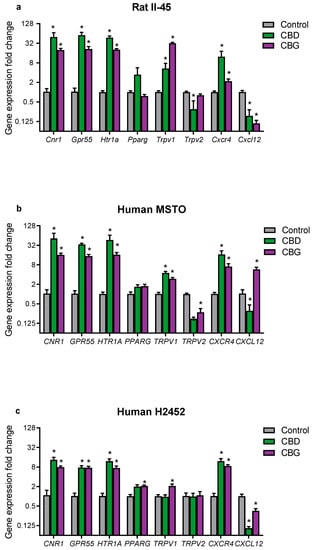
Figure 5.
The phytocannabinoids cannabidiol (CBD) and cannabigerol (CBG) affected mRNA expression of cannabinoid and mesothelioma-related targets in cultured mesothelioma cells. Gene expression was quantified by RT-qPCR in (a) rat II-45 and human (b) MSTO and (c) H2452 mesothelioma cells. FC in gene expression was calculated relative to untreated control cells using the 2-delta-delta Ct method after normalizing to TBP. Bars show the means and SD in gene expression relative to control (n = 3). Grey bars represent a fold change (FC) of one in control treated cells. p-values were calculated using two-way ANOVA Holm–Sidak’s multiple comparisons. * p < 0.01 relative to vehicle treated control cells and FC < 0.5 or >2.
3.5. Identification of Genes and Pathways Associated with CBD and CBG Treatment
A more extensive analysis of the molecular impacts of CBD and CBG on human mesothelioma cell lines was performed using gene expression arrays. The complete list of differentially expressed genes are available in Table S3. Figure 6a,b summarise the number of dysregulated genes (FC > 2) identified following CBD or CBG treatment. CBD treatment resulted in 181 and 255 dysregulated genes in the MSTO and H2452 cells, respectively. Of these, 94 were found to be shared in both cell lines. CBG treatment resulted in 184 and 223 dysregulated genes in the MSTO and H2452 cells, respectively. Of these, 116 were found to be shared in both cell lines. Table S4 lists shared genes with FC >2 in mRNA expression as a result of CBD and CBG treatment. Of note, CBD and CBG consistently decreased expression of key cell cycle genes, including those encoding cyclins (CCB1, CCB2, and CCNE1) and cyclin-dependent kinases (CDK1 and CDK2), as well as the anti-apoptotic gene BIRC5, and pro-metastatic gene ID1 and the DTL gene, involved in an array of cancer promoting pathways, including proliferation, migration, and invasion [36,37]. Additionally, CBD and CBG consistently increased expression of the pro-apoptotic gene BBC3 and of GDF-15, a gene involved in numerous biological functions and a promising prognostic marker in numerous cancers (reviewed in [38]). Pathway enrichment analysis was then performed using the shared differentially expressed genes to gain mechanistic insight into how CBD and CBG affect the mesothelioma cell lines (Tables S5 and S6). Figure 6c,d summarise the top shared canonical pathways predicted to be activated or inhibited with CBD or CBG treatment. Both CBD and CBG inhibited many pathways involved in cell cycle regulation, supporting our in vitro results. Additionally, both cannabinoids activated pathways involved in regulating intracellular calcium levels (e.g., Gαq, phospholipase C (PLC) and TEC kinase signalling) and inflammatory/immune responses (e.g., thrombin and NF-κB signalling). The inhibition of DNA repair pathways (base excision repair and nucleotide excision repair) by CBG was also noted.
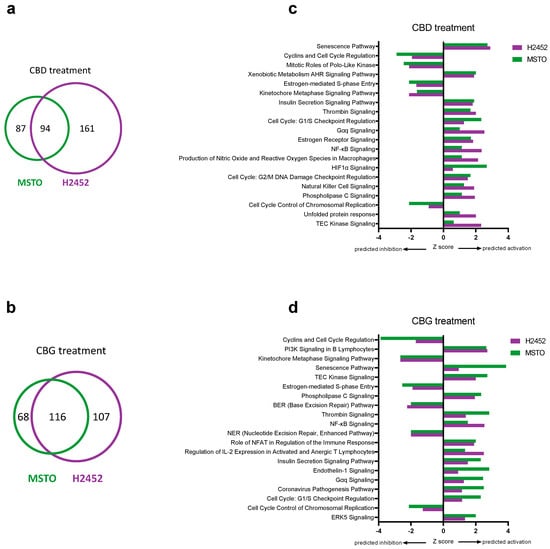
Figure 6.
Differentially expressed genes and pathway analysis associated with cannabidiol (CBD) and cannabigerol (CBG) treatment. The number of genes found to be differentially expressed (a,b) and top shared canonical pathways (c,d) as a result of CBD (a,c) and CBG (b,d) treatment in human MSTO and H2452 mesothelioma cell lines. Analysis was performed relative to vehicle treated control cells and only includes genes with FC > 2. Overlapping areas in Venn diagrams (a,b) indicate the number of shared genes found to be differentially expressed in the same direction in both cell lines. Z-scores (c,d) indicate predicted activation state of the top significantly enriched canonical pathways with values < 0 indicating inhibition and values > 0 indicating activation.
3.6. CBD and CBG Did Not Increase Survival in an Immunocompetent Rat Model of Mesothelioma
We then wished to observe whether the promising effects of CBD and CBG observed in vitro could be translated in vivo. We first aimed to determine which vehicle provided the optimal level of plasma exposure in rats using CBD as the indicative compound. 20 mg/kg CBD i.p. was administered in 3 different vehicles (hemp seed oil, ethanol/Tween80/saline or DMSO/kolliphor/saline). After injecting animals, blood was taken at 15 min, 1 h, 2 h, and 4 h post-injection and then assessed for plasma CBD concentrations using LC-MS/MS. Figure S1 shows that the DMSO/kolliphor/saline vehicle provided marginally superior plasma CBD exposures with a more prolonged half-life compared to the ethanol/Tween80/saline vehicle (AUC = 130 versus 150 µg per min/mL; t1/2 = 112 versus 210 min for ethanol versus DMSO vehicles, respectively). Hemp seed oil resulted in very low plasma CBD concentrations. The DMSO/kolliphor/saline vehicle was thus used for the in vivo survival study.
A syngeneic orthotopic rat mesothelioma model [39] was used to assess whether daily treatment for 4 weeks with 40 mg/kg CBD or 100 mg/kg CBG i.p. compared to standard of care chemotherapy (1 mg/kg cisplatin + 6.7 mg/kg pemetrexed) improved survival (Figure 7). Although survival was the primary endpoint of this study, severity of pleural dissemination at endpoint was also assessed. No statistical differences were observed between the groups (data not shown). As expected, treatment with cisplatin + pemetrexed significantly prolonged survival compared to vehicle-treated animals (median survival 28 days vs. 23 days, respectively; p < 0.05), however neither CBD nor CBG prolonged survival.
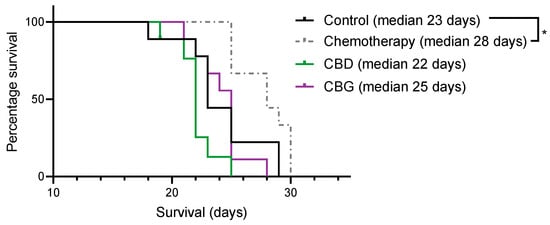
Figure 7.
Pemetrexed + cisplatin but not cannabidiol (CBD) or cannabigerol (CBG) treatment prolongs survival in a rat syngeneic orthotopic model of pleural mesothelioma. Rats were pleurally engrafted with mesothelioma cells and then treated with control (vehicle), cisplatin + pemetrexed (chemotherapy), 40 mg/kg CBD or 100 mg/kg CBG for 4 weeks and overall survival was assessed. Data were analysed using Log-rank (Mantel-Cox) test. * p < 0.05 versus vehicle control.
4. Discussion
As failure of standard of care treatment is inevitable in mesothelioma, there is an urgent unmet need for more effective treatments. With the legalisation of medicinal cannabis around the world there has been an increasing focus on the plant cannabinoids and their medicinal potential, including in the treatment of cancer. Cannabinoids display anti-cancer properties, including anti-proliferative, anti-migratory, anti-invasive, and anti-angiogenic effects in preclinical cancer models (reviewed in [17,18,19]). These anti-cancer effects have been observed in an array of different cancers, including breast, prostate, brain, lymph, blood, pancreas, bladder, and skin (reviewed in [40]). However, the present study is the first to investigate anticancer effects of phytocannabinoids in preclinical mesothelioma models. We screened a panel of 13 phytocannabinoids of which CBD and CBG had the most potent and consistent anti-proliferative effects across rat and human mesothelioma cell lines. The anti-proliferative effects of CBD and CBG were associated with cell cycle arrest and apoptosis. Moreover, CBD and CBG reduced the migration and invasiveness of the mesothelioma cells in vitro. However, systemic administration of CBD and CBG in vivo at levels of exposure relevant to human dosing [31,32,34] was ineffective in prolonging the lifespan of rats in a syngeneic orthotopic mesothelioma model.
In this study, we screened a comprehensive panel of cannabinoids that are found in cannabis, some of which are abundant in specific cannabis strains. This included both acidic and neutral forms of the phytocannabinoids. The acidic forms (e.g., CBDA) are enzymatically biosynthesised in the plant and then decarboxylated via exposure to heat or air into the neutral cannabinoids (e.g., CBD). Most prior research has focused on the neutral forms, such as THC and CBD, however there is emerging evidence that the acidic compounds also have therapeutic potential [24]. Here, we show that the acidic cannabinoids display consistently lower potency than the neutral cannabinoids in reducing the proliferation of mesothelioma cells in vitro. Compounds with a carboxylic acid moiety are notorious for having poor membrane permeability, thus it is possible that the reduced anti-proliferative potency of the acids versus the neutral cannabinoids may be due to diminished access of the acid forms to intracellular anti-cancer targets [41]. This might be further exacerbated by active transport by drug efflux proteins expressed in mesothelioma cells [35,42,43], as some acidic cannabinoids appear to be substrates of ABC transporters. For example, CBDA is an excellent substrate of ABCG2 (breast cancer resistance protein), and CBCA is a substrate of ABCB1 (P-glycoprotein) [44,45].
CBD and CBG were found to be the most potent at decreasing cell viability with IC50 values within the low µm range in all three cell lines. Both these compounds do not appear to have THC-like intoxicating effects which makes them more favourable candidates for therapeutic development [11]. Hence, we focused on CBD and CBG to more comprehensively explore their anti-cancer potential and modes of action. Both phytocannabinoids showed induction of G0/G1 arrest, as well as delayed entry into S-phase, coinciding with decreased expression of key cell cycle genes including those encoding cyclins (CCNB1, CCNB2, and CCNE1) and cyclin-dependent kinases (CDK1 and CDK2). These results are consistent with prior studies which have reported arrest at the G1/S transition via downregulation of cell cycle regulators [46]. Additionally, we also found increased expression of GDF-15, previously identified as one of the most up-regulated genes in response to CBD treatment [47]. It is hypothesised that upregulation of GDF-15 combined with decreased expression of FOXM1 act together to form a CBD-dependent antiproliferative pathway across numerous cancer types [47]. The present results provide evidence for the first time that the cannabinoids CBD and CBG have anti-proliferative effects in mesothelioma cells that are associated with cell cycle arrest.
As escape from programmed cell death or apoptosis is an important survival mechanism used by cancers, we also investigated whether CBD and CBG could induce apoptosis in mesothelioma cells. Both CBD and CBG induced apoptosis in all three mesothelioma cell lines which corresponded with expression changes in intrinsic apoptotic pathway genes, including increased expression of BBC3 (which encodes BCL2 binding component 3) and decreased expression of BIRC5 (encoding baculoviral inhibitor of apoptosis (IAP) repeat containing 5). Our results agree with numerous prior studies that have reported both CBD and CBG have pro-apoptotic effects in various cancer cell lines [48,49,50]. However, the pro-apoptotic effects of CBD and CBG observed here did not correspond with any increase in reactive oxygen species (ROS) (Figure S2a,c,e), as has been shown in some prior studies [51]. Autophagy is another cellular process induced in many cancers by phytocannabinoids including CBD [18,52]. Interestingly, in our study autophagy was not induced by either CBD or CBG (Figure S2b,d,f), contrary to most cancer studies. However, mesothelioma cells have been noted to have high basal levels of autophagy [53] and, therefore, induction may be difficult to identify.
Although distant metastasis in mesothelioma is rare, locally invasive disease occurs in approximately 50% of patients [54,55], contributing to the high mortality of the disease. Therefore, new therapies which can also slow metastatic spread and local invasion are highly sought after. Our results are similar to several other cancer studies, showing that CBD has potent anti-migratory and anti-invasive potential in vitro and in vivo [56,57,58]. Our results here provide only the second report that CBG has anti-invasive activity, which was recently reported using glioblastoma cell lines [59]. To the best of our knowledge this is the first report that CBG has anti-migratory effects in vitro in any type of cancer. The mechanism responsible for these effects is unknown, however we did observe both CBD and CBG treatment reduced expression of the pro-metastatic gene ID1 (inhibitor of DNA binding 1) in mesothelioma cells. CBD has previously been shown to decrease expression of ID1 in various cancers [60]. Future studies are needed to probe the mechanism of action of the anti-migratory and anti-invasive effects of the cannabinoids in mesothelioma.
The mechanism of action for phytocannabinoids CBD and CBG is complex with multiple different receptors proposed to be involved [18]. To provide some preliminary insight into the possible mode of action of CBD and CBG, we analysed whether treatment with these cannabinoids altered mRNA expression of various targets implicated in the effects of CBD and CBG. We found that both CBD and CBG produced a dramatic increase in the expression of genes encoding the cannabinoid CB1 receptor, GPR55, and the 5-HT1A receptor in all three mesothelioma cell lines. Moreover, we observed that the cannabinoids also increased the mRNA expression of the chemokine receptor CXCR4. Notably, a prior study showed the combination of CBD and THC was required to inhibit expression of CXCR4 that was associated with anti-migratory effects in multiple myeloma cells [61]. Here, we observed induction of CXCR4 following treatment with higher cannabinoid concentrations, however different effects on CXCR4 expression might be observed at lower cannabinoid concentrations that reduce migration and invasion.
We then performed gene pathway analyses to further probe possible mechanisms associated with CBD and CBG treatment on mesothelioma cell lines. Not surprisingly, pathways associated with regulation of the cell cycle were consistently affected by both CBD and CBG. Pathway analysis also revealed disruption of cellular calcium homeostasis through stimulation of Gαq and PLC signalling. These results may be related to the cannabinoid-induced upregulation of GPR55 we observed here, as GPR55 signals through Gαq and PLC [62]. Moreover, CBG was found to stimulate nuclear factor of activated T cells (NFAT) signalling, a group of transcription factors which is also activated via GPR55 [62]. Both CBD and CBG consistently activated NF-κB, another transcription factor linked to the expression of various inflammatory mediators, including cytokines and chemokines (including CXCL12-CXCR4), but also cell cycle regulators, anti-apoptotic factors, and adhesion molecules [63,64]. Intriguingly, CBD and CBG also affected senescence pathways in both human mesothelioma cell lines, which warrants more detailed examination in future studies.
Given our in vitro data showing that CBD and CBG have potent anti-tumour effects in vitro, we examined the effect of both cannabinoids in a syngeneic orthotopic rat model of mesothelioma [22]. This model is an immunocompetent model that takes into account potential cannabinoid effects on the immune microenvironment, thus offering an advantage over prior research which has largely utilised immunodeficient animals. To provide greater clinical relevance, we also administered doses of the cannabinoids that equate to maximal plasma levels of exposure observed in humans following high CBD doses [31,32]. We were unable to show any survival benefit for rats treated with daily i.p. doses of CBD (40 mg/kg) or CBG (100 mg/kg) in the II45 rat model. This is in contrast to previous studies investigating CBD in mouse models of other cancer types, such as glioma, breast, and lung, which have all demonstrated that CBD reduces tumour growth and metastasis in vivo [65,66]. Although observing reductions in tumour volume is important, the examination of survival in preclinical rodent models of cancer is arguably a more clinically relevant endpoint. Consistent with our present results, a study that examined the effects of CBD (100 mg/kg i.p. administered daily) in a mouse pancreatic cancer xenograft model failed to show any statistically significant improvement in survival [67].
Studies that have demonstrated reduced tumour volumes in mouse xenograft models required repeated dosing of CBD (typically near daily or daily dosing) at between 1 and 25 mg/kg i.p. When considering interspecies differences in body surface area, this amounts to CBD doses of 0.5–12.5 mg/kg in rats, which are lower than the dose used in the present study. CBG has not been widely investigated in vivo, however one study demonstrated that CBG (3 and 10 mg/kg i.p. administered once daily for 5 days) slows tumour growth in a mouse xenograft model of colorectal cancer [51]. Again, these doses are much lower than the dose used in the present study. The argument could then be made that our cannabinoid doses were too high. However, the predicted plasma concentrations that were likely attained here remained 3-fold lower than the IC50 concentrations required to reduce proliferation in vitro. It is therefore likely we were unable to reach a sufficient therapeutic level to produce a significant survival benefit. Our findings highlight the potential difficulty of administering cannabinoids in sufficient quantity for therapeutic benefit in in vivo models of mesothelioma. Future studies may benefit from the use of alternative methods of drug delivery to maximise therapeutic exposures, such as using novel formulation strategies, for example lipid nanoparticles [68]. Overall, the lack of survival benefit seen in our rat model may be due to the aggressive and rapid growth of the II45 cell line in vivo, combined with a failure to reach sufficient drug plasma concentrations.
5. Conclusions
Our data present the first report that plant cannabinoids have anti-proliferative effects on mesothelioma cells, that was associated with apoptosis, rather than autophagy or production of ROS. CBD and CBG were the most potent cannabinoids and also inhibited mesothelioma cell migration and invasion. We were unable to show an anti-tumour effect in vivo, potentially due to insufficient plasma concentrations being reached. Thus alternative drug delivery methods may be needed to clinically translate these findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14153813/s1; Figure S1: pharmacokinetics of CBD using three standard vehicles; Figure S2: the phytocannabinoids CBD and CBG do not induce reactive oxygen species or autophagy in cultured mesothelioma cancer cell lines; Table S1: TaqMan assays used in the study; Table S2: list of TaqMan assays on Open Arrays; Table S3: Open Array gene expression results; Table S4: Open Array shared genes FC; Table S5: CBD comparisons IPA data; Table S6: CBG comparisons IPA data.
Author Contributions
Conceptualization, E.K.C., A.L.H., I.S.M. and J.C.A.; Formal analysis, E.K.C., A.L.H., L.L.A., R.P.K. and J.C.A.; Methodology, E.K.C., A.L.H., L.L.A., R.P.K. and J.C.A.; Resources, V.M.H., I.S.M. and J.C.A.; Supervision, V.M.H. and J.C.A.; Writing—original draft, E.K.C., A.L.H. and J.C.A.; Writing—review and editing, E.K.C., A.L.H., L.L.A., R.P.K., V.M.H., I.S.M. and J.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was part funded by the Lambert Initiative for Cannabinoid Therapeutics and the Bill Walsh Translational Cancer Research Laboratory through philanthropic donations. Emily K. Colvin was supported by the Proud Family Fellowship.
Institutional Review Board Statement
The animal study protocol was approved by the Royal North Shore Hospital Animal Care and Ethics Committee (protocol code RESP/17/30; date of approval 16 March 2017).
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials. Raw data can be provided upon reasonable request.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. I.S.M. and J.C.A. receive salary support from the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded centre for medicinal cannabis research at the University of Sydney. J.C.A. and I.S.M. receive research funding from the Australian National Health and Medical Re-search Council (NHMRC) (GNT1161571). J.C.A. and I.S.M. have served as expert witnesses in various medicolegal cases involving cannabis and the cannabinoids. J.C.A. has received consulting fees from Creo Inc. and Medicinal Cannabis Industry Australia (MCIA). I.S.M. acts as a consultant to Kinoxis Therapeutics and the MCIA, and has received honoraria from Janssen. J.C.A., A.L.H., E.K.C., L.L.A. V.M.H. and I.S.M. hold patents on cannabinoid therapies (PCT/AU2018/05089 and PCT/AU2019/050554).
References
- Brims, F. Epidemiology and Clinical Aspects of Malignant Pleural Mesothelioma. Cancers 2021, 13, 4094. [Google Scholar] [CrossRef] [PubMed]
- Mutti, L.; Peikert, T.; Robinson, B.W.S.; Scherpereel, A.; Tsao, A.S.; de Perrot, M.; Woodard, G.A.; Jablons, D.M.; Wiens, J.; Hirsch, F.R.; et al. Scientific Advances and New Frontiers in Mesothelioma Therapeutics. J. Thorac. Oncol. 2018, 13, 1269–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odgerel, C.O.; Takahashi, K.; Sorahan, T.; Driscoll, T.; Fitzmaurice, C.; Yoko, O.M.; Sawanyawisuth, K.; Furuya, S.; Tanaka, F.; Horie, S.; et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup. Environ. Med. 2017, 74, 851–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roe, O.D.; Stella, G.M. Malignant pleural mesothelioma: History, controversy and future of a manmade epidemic. Eur. Respir. Rev. 2015, 24, 115–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, N.J.; Franklin, P.J.; Reid, A.; de Klerk, N.H.; Threlfall, T.J.; Shilkin, K.; Musk, B. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med. J. Aust. 2011, 195, 271–274. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Clarke, C.; Henderson, D.; Musk, A.W.; Fong, K.; Nowak, A.; Loneragan, R.; McCaughan, B.; Boyer, M.; Feigen, M.; et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J. Thorac. Dis. 2013, 5, E254–E307. [Google Scholar] [CrossRef]
- Anderson, L.L.; Low, I.K.; Banister, S.D.; McGregor, I.S.; Arnold, J.C. Pharmacokinetics of Phytocannabinoid Acids and Anticonvulsant Effect of Cannabidiolic Acid in a Mouse Model of Dravet Syndrome. J. Nat. Prod. 2019, 82, 3047–3055. [Google Scholar] [CrossRef] [Green Version]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (−)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Russo, E.B.; Cuttler, C.; Cooper, Z.D.; Stueber, A.; Whiteley, V.L.; Sexton, M. Survey of Patients Employing Cannabigerol-Predominant Cannabis Preparations: Perceived Medical Effects, Adverse Events, and Withdrawal Symptoms. Cannabis Cannabinoid Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Assareh, N.; Arnold, J.C. The Cannabis Constituent Cannabigerol Does Not Disrupt Fear Memory Processes or Stress-Induced Anxiety in Mice. Cannabis Cannabinoid Res. 2022, 7, 294–303. [Google Scholar] [CrossRef]
- Abrams, D.I. Integrating cannabis into clinical cancer care. Curr. Oncol. 2016, 23, S8–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimison, P.; Mersiades, A.; Kirby, A.; Lintzeris, N.; Morton, R.; Haber, P.; Olver, I.; Walsh, A.; McGregor, I.; Cheung, Y.; et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: A randomised, placebo-controlled, phase II crossover trial. Ann. Oncol. 2020, 31, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Liew, K.L.; Capuano, E.; Yung, B. Lung cancer patient who had declined conventional cancer treatment: Could the self-administration of ‘CBD oil’ be contributing to the observed tumour regression? BMJ Case Rep. 2021, 14, e244195. [Google Scholar] [CrossRef]
- Sule-Suso, J.; Watson, N.A.; van Pittius, D.G.; Jegannathen, A. Striking lung cancer response to self-administration of cannabidiol: A case report and literature review. SAGE Open Med. Case Rep. 2019, 7, 2050313X19832160. [Google Scholar] [CrossRef]
- Malhotra, P.; Casari, I.; Falasca, M. Therapeutic potential of cannabinoids in combination cancer therapy. Adv. Biol. Regul. 2021, 79, 100774. [Google Scholar] [CrossRef]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef]
- Ladin, D.A.; Soliman, E.; Griffin, L.; Van Dross, R. Preclinical and Clinical Assessment of Cannabinoids as Anti-Cancer Agents. Front. Pharmacol. 2016, 7, 361. [Google Scholar] [CrossRef] [Green Version]
- Bepler, G.; Koehler, A.; Kiefer, P.; Havemann, K.; Beisenherz, K.; Jaques, G.; Gropp, C.; Haeder, M. Characterization of the state of differentiation of six newly established human non-small-cell lung cancer cell lines. Differentiation 1988, 37, 158–171. [Google Scholar] [CrossRef]
- Sekido, Y.; Pass, H.I.; Bader, S.; Mew, D.J.; Christman, M.F.; Gazdar, A.F.; Minna, J.D. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995, 55, 1227–1231. [Google Scholar] [PubMed]
- Craighead, J.E.; Akley, N.J.; Gould, L.B.; Libbus, B.L. Characteristics of tumors and tumor cells cultured from experimental asbestos-induced mesotheliomas in rats. Am. J. Pathol. 1987, 129, 448–462. [Google Scholar] [PubMed]
- Anderson, L.L.; Ametovski, A.; Lin Luo, J.; Everett-Morgan, D.; McGregor, I.S.; Banister, S.D.; Arnold, J.C. Cannabichromene, Related Phytocannabinoids, and 5-Fluoro-cannabichromene Have Anticonvulsant Properties in a Mouse Model of Dravet Syndrome. ACS Chem. Neurosci. 2021, 12, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.J.; Anderson, L.L.; Low, I.K.; Luo, J.L.; Kevin, R.C.; Zhou, C.; McGregor, I.S.; Arnold, J.C. Evaluation of the Possible Anticonvulsant Effect of Delta(9)-Tetrahydrocannabinolic Acid in Murine Seizure Models. Cannabis Cannabinoid Res. 2022, 7, 46–57. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Hudson, A.L.; Donaghy, H.; Stoner, S.P.; Wheeler, H.R.; Diakos, C.I.; Howell, V.M. Differential effects of radiation fractionation regimens on glioblastoma. Radiat. Oncol. 2022, 17, 17. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinformatics 2021, 22, 433. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Kovalchuk, I. Cannabinoids as anticancer therapeutic agents. Cell Cycle 2020, 19, 961–989. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Marone, G.; Galdiero, M.R.; Varricchi, G.; Loffredo, S. The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 2021, 10, 1282. [Google Scholar] [CrossRef]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Anderson, L.L.; Low, I.K.; McGregor, I.S.; Arnold, J.C. Interactions between cannabidiol and Delta(9) -tetrahydrocannabinol in modulating seizure susceptibility and survival in a mouse model of Dravet syndrome. Br. J. Pharmacol. 2020, 177, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Absalom, N.L.; Abelev, S.V.; Low, I.K.; Doohan, P.T.; Martin, L.J.; Chebib, M.; McGregor, I.S.; Arnold, J.C. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia 2019, 60, 2224–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Contin, M.; Mohamed, S.; Santucci, M.; Lodi, M.A.M.; Russo, E.; Mecarelli, O.; Cbd Lice Italy Study Group. Cannabidiol in Pharmacoresistant Epilepsy: Clinical Pharmacokinetic Data From an Expanded Access Program. Front. Pharmacol. 2021, 12, 637801. [Google Scholar] [CrossRef]
- Hudson, A.L.; Weir, C.; Moon, E.; Harvie, R.; Klebe, S.; Clarke, S.J.; Pavlakis, N.; Howell, V.M. Establishing a panel of chemo-resistant mesothelioma models for investigating chemo-resistance and identifying new treatments for mesothelioma. Sci. Rep. 2014, 4, 6152. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ng, E.K.; Ng, Y.P.; Wong, C.Y.; Yu, J.; Jin, H.; Cheng, V.Y.; Go, M.Y.; Cheung, P.K.; Ebert, M.P.; et al. Identification of retinoic acid-regulated nuclear matrix-associated protein as a novel regulator of gastric cancer. Br. J. Cancer 2009, 101, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Wang, Q.; Lei, Z.; Feng, M.; Zhao, Z.; Wang, Y.; Wei, G. DTL promotes cancer progression by PDCD4 ubiquitin-dependent degradation. J. Exp. Clin. Cancer Res. 2019, 38, 350. [Google Scholar] [CrossRef] [Green Version]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Weir, C.J.; Hudson, A.L.; Peters, L.; Howell, V.M. Orthotopic Implantation and Peripheral Immune Cell Monitoring in the II-45 Syngeneic Rat Mesothelioma Model. J. Vis. Exp. 2015, 104, e53019. [Google Scholar] [CrossRef] [Green Version]
- Nahler, G. Cannabidiol and Other Phytocannabinoids as Cancer Therapeutics. Pharmaceut. Med. 2022, 36, 99–129. [Google Scholar] [CrossRef]
- Lassalas, P.; Gay, B.; Lasfargeas, C.; James, M.J.; Tran, V.; Vijayendran, K.G.; Brunden, K.R.; Kozlowski, M.C.; Thomas, C.J.; Smith, A.B., 3rd; et al. Structure Property Relationships of Carboxylic Acid Isosteres. J. Med. Chem. 2016, 59, 3183–3203. [Google Scholar] [CrossRef]
- Frei, C.; Opitz, I.; Soltermann, A.; Fischer, B.; Moura, U.; Rehrauer, H.; Weder, W.; Stahel, R.; Felley-Bosco, E. Pleural mesothelioma side populations have a precursor phenotype. Carcinogenesis 2011, 32, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Melaiu, O.; Cristaudo, A.; Melissari, E.; Di Russo, M.; Bonotti, A.; Bruno, R.; Foddis, R.; Gemignani, F.; Pellegrini, S.; Landi, S. A review of transcriptome studies combined with data mining reveals novel potential markers of malignant pleural mesothelioma. Mutat. Res. 2012, 750, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Etchart, M.G.; Bahceci, D.; Golembiewski, T.A.; Arnold, J.C. Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma cannabidiolic acid concentrations. Sci. Rep. 2021, 11, 14948. [Google Scholar] [CrossRef]
- Etchart, M.G.; Anderson, L.L.; Ametovski, A.; Jones, P.M.; George, A.M.; Banister, S.D.; Arnold, J.C. In vitro evaluation of the interaction of the cannabis constituents cannabichromene and cannabichromenic acid with ABCG2 and ABCB1 transporters. Eur. J. Pharmacol. 2022, 922, 174836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Desprez, P.Y.; Murase, R.; Limbad, C.; Woo, R.W.L.; Adrados, I.; Weitenthaler, K.; Soroceanu, L.; Salomonis, N.; McAllister, S.D. Cannabidiol Treatment Results in a Common Gene Expression Response Across Aggressive Cancer Cells from Various Origins. Cannabis Cannabinoid Res. 2021, 6, 148–155. [Google Scholar] [CrossRef]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Hamad, H.; Olsen, B.B. Cannabidiol Induces Cell Death in Human Lung Cancer Cells and Cancer Stem Cells. Pharmaceuticals 2021, 14, 1169. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Ramer, R. Cannabinoids as anticancer drugs: Current status of preclinical research. Br. J. Cancer 2022, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, N.; Ziltener, G.; Barbone, D.; Weder, W.; Stahel, R.A.; Broaddus, V.C.; Felley-Bosco, E. Inhibition of autophagy sensitizes malignant pleural mesothelioma cells to dual PI3K/mTOR inhibitors. Cell Death Dis. 2015, 6, e1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Yang, X. Simultaneous tongue metastasis from malignant pleural mesothelioma: Case report and literature review. Thorac. Cancer 2020, 12, 391–396. [Google Scholar] [CrossRef]
- Finn, R.S.; Brims, F.J.H.; Gandhi, A.; Olsen, N.; Musk, A.W.; Maskell, N.A.; Lee, Y.C.G. Postmortem findings of malignant pleural mesothelioma: A two-center study of 318 patients. Chest 2012, 142, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat 2011, 129, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Vaccani, A.; Massi, P.; Colombo, A.; Rubino, T.; Parolaro, D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br. J. Pharmacol. 2005, 144, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Ramer, R.; Bublitz, K.; Freimuth, N.; Merkord, J.; Rohde, H.; Haustein, M.; Borchert, P.; Schmuhl, E.; Linnebacher, M.; Hinz, B. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2012, 26, 1535–1548. [Google Scholar] [CrossRef] [Green Version]
- Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; Zvar Baskovic, B.; Majc, B.; Mlinar, M.; Bosnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef]
- McAllister, S.D.; Soroceanu, L.; Desprez, P.Y. The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. J. Neuroimmune Pharmacol. 2015, 10, 255–267. [Google Scholar] [CrossRef] [Green Version]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543–77557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.Y.; Lu, H.C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurosawa, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Sprent, J.; Ishimaru, N. NF-kappaB2 Controls the Migratory Activity of Memory T Cells by Regulating Expression of CXCR4 in a Mouse Model of Sjogren’s Syndrome. Arthritis Rheumatol. 2017, 69, 2193–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.E.; Arifin, S.A.; Fyffe, C.A.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Sebastian, V.; Benoit, J.P.; Torres-Suarez, A.I. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. Eur. J. Pharm. Biopharm. 2019, 134, 126–137. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).