Precision Medicine in Metastatic Colorectal Cancer: Targeting ERBB2 (HER-2) Oncogene
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Biology of HER2 Receptor
3. Diagnosis of HER2-Positive in mCRC
4. Clinical Features of Patients with HER2-Positive mCRC
5. Clinical Trials for Patients with ERBB2-Positive mCRC
5.1. Monoclonal Antibodies
5.2. Tyrosine Kinase Inhibitors (TKIs)
5.3. Antibody–Drug Conjugates (ADCs)
5.4. Ongoing Clinical Trials and Novel Anti-ERBB2 Therapies
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| ADCs | antibody-drug conjugates |
| BRAF CEP17 | v-raf murine sarcoma viral oncogene homolog B1 centromere enumeration probe for chromosome 17 |
| CNS | central nervous system |
| CRC | colorectal cancer |
| ctDNA | circulating tumor DNA |
| EGF EGFR EREG | epidermal growth factor epidermal growth factor receptor epiregulin |
| ESMO | European Society of Medical Oncology |
| FISH | fluorescence in situ hybridization |
| FOLFIRI | 5-fluouracil/leucovorin/irinotecan |
| FOLFOX | 5-fluouracil/leucovorin/oxaliplatin |
| FOLFOXIRI | 5-fluouracil/leucovorin/oxaliplatin/irinotecan |
| HER2 | human epidermal growth factor receptor 2 |
| IHC | immunochemistry |
| LV | leucovorin |
| m MAPK | month mitogen-activated protein kinase |
| mCRC | metastatic colorectal cancer |
| mPFS | median progression-free survival |
| NCCN | National Comprehensive Cancer Network |
| NGS | next-generation sequencing |
| NK | natural killer |
| NTRK | neurotrophic tyrosine receptor kinase |
| ORR | overall response rates |
| OS PI3K | overall survival phosphoinositide 3-kinase |
| PFS PTEN | progression-free survival phosphatase and tensin homolog |
| QT | chemotherapy |
| RAS RTK STAT | rat sarcoma virus receptor tyrosine kinase signal transducer and activator of transcription |
| T-DM1 | trastuzumab emastine |
| TD | trastuzumab deruxtecan |
| TKIs | tyrosine kinase inhibitors |
| VEGF | anti-vascular endothelial growth factor |
| WT | wild-type |
References
- Keum, N.; Giovannucci, E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Gastroenterol. Rev./Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Grothey, A.; Cutsem, E.V.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Vitiello, P.P.; Marsoni, S.; Siena, S.; Tabernero, J.; Trusolino, L.; Bernards, R.; Bardelli, A. Precision Oncology in Metastatic Colorectal Cancer—From Biology to Medicine. Nat. Rev. Clin. Oncol. 2021, 18, 506–525. [Google Scholar] [CrossRef]
- Mateo, J.; Steuten, L.; Aftimos, P.; André, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering Precision Oncology to Patients with Cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in Patients with Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Boni, V.; Drilon, A.; Deeken, J.; Garralda, E.; Chung, H.; Kinoshita, I.; Oh, D.; Patel, J.; Xu, R.; Norenberg, R.; et al. SO-29 Efficacy and Safety of Larotrectinib in Patients with Tropomyosin Receptor Kinase Fusion-Positive Gastrointestinal Cancer: An Expanded Dataset. Ann. Oncol. 2021, 32, S214–S215. [Google Scholar] [CrossRef]
- Patel, M.; Siena, S.; Demetri, G.; Doebele, R.; Chae, Y.; Conkling, P.; Garrido-Laguna, I.; Longo, F.; Rolfo, C.; Sigal, D.; et al. O-3 Efficacy and Safety of Entrectinib in NTRK Fusion-Positive Gastrointestinal Cancers: Updated Integrated Analysis of Three Clinical Trials (STARTRK-2, STARTRK-1 and ALKA-372-001). Ann. Oncol. 2020, 31, 232–233. [Google Scholar] [CrossRef]
- Cohen, R.; Pudlarz, T.; Delattre, J.-F.; Colle, R.; André, T. Molecular Targets for the Treatment of Metastatic Colorectal Cancer. Cancers 2020, 12, 2350. [Google Scholar] [CrossRef]
- Nader-Marta, G.; Martins-Branco, D.; de Azambuja, E. How We Treat Patients with Metastatic HER2-Positive Breast Cancer. ESMO Open 2022, 7, 100343. [Google Scholar] [CrossRef]
- Schettini, F.; Prat, A. Dissecting the Biological Heterogeneity of HER2-Positive Breast Cancer. Breast 2021, 59, 339–350. [Google Scholar] [CrossRef]
- Kahraman, S.; Yalcin, S. Recent Advances in Systemic Treatments for HER-2 Positive Advanced Gastric Cancer. OTT 2021, 14, 4149–4162. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X.; Wei, X.; Tang, C.; Zhang, W. HER2-Targeted Therapies in Gastric Cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188549. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Amatu, A.; Porcu, L.; Ghezzi, S.; Lonardi, S.; Leone, F.; Bergamo, F.; Fenocchio, E.; Martinelli, E.; Borelli, B.; et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist 2019, 24, 1395–1402. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Y.; Chang, W.; Ren, L.; Tang, W.; Zheng, P.; Wu, Q.; Liu, T.; Liu, Y.; Wei, Y.; et al. HER2 Positivity as a Biomarker for Poor Prognosis and Unresponsiveness to Anti-EGFR Therapy in Colorectal Cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 993–1002. [Google Scholar] [CrossRef]
- Greally, M.; Kelly, C.M.; Cercek, A. HER2: An Emerging Target in Colorectal Cancer. Curr. Probl. Cancer 2018, 42, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Lum, C.; Latham, S.; Tipping Smith, S.; Prenen, H.; Segelov, E. Refractory Metastatic Colorectal Cancer: Current Challenges and Future Prospects. CMAR 2020, 12, 5819–5830. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the Human Epidermal Growth Factor Receptor 2 (HER2) Oncogene in Colorectal Cancer. Ann. Oncol. 2018, 29, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- La Salvia, A.; Lopez-Gomez, V.; Garcia-Carbonero, R. HER2-Targeted Therapy: An Emerging Strategy in Advanced Colorectal Cancer. Expert Opin. Investig. Drugs 2019, 28, 29–38. [Google Scholar] [CrossRef]
- Bitar, L.; Zouein, J.; Haddad, F.G.; Eid, R.; Kourie, H.R. HER2 in Metastatic Colorectal Cancer: A New to Target to Remember. Biomark. Med. 2021, 15, 135–138. [Google Scholar] [CrossRef]
- Strickler, J.H.; Yoshino, T.; Graham, R.P.; Siena, S.; Bekaii-Saab, T. Diagnosis and Treatment of ERBB2-Positive Metastatic Colorectal Cancer: A Review. JAMA Oncol. 2022, 8, 760–769. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Lau, D.K.; Chau, I. HER2 Targeted Therapy in Colorectal Cancer: New Horizons. Cancer Treat. Rev. 2022, 105, 102363. [Google Scholar] [CrossRef]
- Yarden, Y.; Pines, G. The ERBB Network: At Last, Cancer Therapy Meets Systems Biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e3. [Google Scholar] [CrossRef]
- Sawada, K.; Nakamura, Y.; Yamanaka, T.; Kuboki, Y.; Yamaguchi, D.; Yuki, S.; Yoshino, T.; Komatsu, Y.; Sakamoto, N.; Okamoto, W.; et al. Prognostic and Predictive Value of HER2 Amplification in Patients With Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2018, 17, 198–205. [Google Scholar] [CrossRef]
- Park, J.S.; Yoon, G.; Kim, H.J.; Park, S.Y.; Choi, G.S.; Kang, M.K.; Kim, J.G.; Jang, J.-S.; Seo, A.N. HER2 Status in Patients with Residual Rectal Cancer after Preoperative Chemoradiotherapy: The Relationship with Molecular Results and Clinicopathologic Features. Virchows Arch. 2018, 473, 413–423. [Google Scholar] [CrossRef]
- Richman, S.D.; Southward, K.; Chambers, P.; Cross, D.; Barrett, J.; Hemmings, G.; Taylor, M.; Wood, H.; Hutchins, G.; Foster, J.M.; et al. HER2 Overexpression and Amplification as a Potential Therapeutic Target in Colorectal Cancer: Analysis of 3256 Patients Enrolled in the QUASAR, FOCUS and PICCOLO Colorectal Cancer Trials. J. Pathol. 2016, 238, 562–570. [Google Scholar] [CrossRef]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 Scoring System for Colorectal Cancer: Results from a Validation Study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef]
- Kavuri, S.M.; Jain, N.; Galimi, F.; Cottino, F.; Leto, S.M.; Migliardi, G.; Searleman, A.C.; Shen, W.; Monsey, J.; Trusolino, L.; et al. HER2 Activating Mutations Are Targets for Colorectal Cancer Treatment. Cancer Discov. 2015, 5, 832–841. [Google Scholar] [CrossRef]
- Osako, T.; Miyahara, M.; Uchino, S.; Inomata, M.; Kitano, S.; Kobayashi, M. Immunohistochemical Study of C-ErbB-2 Protein in Colorectal Cancer and the Correlation with Patient Survival. Oncology 1998, 55, 548–555. [Google Scholar] [CrossRef]
- Nowak, J.A. HER2 in Colorectal Carcinoma. Surg. Pathol. Clin. 2020, 13, 485–502. [Google Scholar] [CrossRef]
- Fujii, S.; Magliocco, A.M.; Kim, J.; Okamoto, W.; Kim, J.E.; Sawada, K.; Nakamura, Y.; Kopetz, S.; Park, W.-Y.; Tsuchihara, K.; et al. International Harmonization of Provisional Diagnostic Criteria for ERBB2-Amplified Metastatic Colorectal Cancer Allowing for Screening by Next-Generation Sequencing Panel. JCO Precis. Oncol. 2020, 4, 6–19. [Google Scholar] [CrossRef]
- Yagisawa, M.; Sawada, K.; Nakamura, Y.; Fujii, S.; Yuki, S.; Komatsu, Y.; Yoshino, T.; Sakamoto, N.; Taniguchi, H. Prognostic Value and Molecular Landscape of HER2 Low-Expressing Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2021, 20, 113–120.e1. [Google Scholar] [CrossRef]
- Cenaj, O.; Ligon, A.H.; Hornick, J.L.; Sholl, L.M. Detection of ERBB2 Amplification by Next-Generation Sequencing Predicts HER2 Expression in Colorectal Carcinoma. Am. J. Clin. Pathol. 2019, 152, 97–108. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-Targeted Therapy with Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Siravegna, G.; Lazzari, L.; Crisafulli, G.; Sartore-Bianchi, A.; Mussolin, B.; Cassingena, A.; Martino, C.; Lanman, R.B.; Nagy, R.J.; Fairclough, S.; et al. Radiologic and Genomic Evolution of Individual Metastases during HER2 Blockade in Colorectal Cancer. Cancer Cell 2018, 34, 148–162.e7. [Google Scholar] [CrossRef]
- Siravegna, G.; Sartore-Bianchi, A.; Nagy, R.J.; Raghav, K.; Odegaard, J.I.; Lanman, R.B.; Trusolino, L.; Marsoni, S.; Siena, S.; Bardelli, A. Plasma HER2 ( ERBB2 ) Copy Number Predicts Response to HER2-Targeted Therapy in Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 3046–3053. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating Tumor DNA-Guided Treatment with Pertuzumab plus Trastuzumab for HER2-Amplified Metastatic Colorectal Cancer: A Phase 2 Trial. Nat. Med. 2021, 27, 1899–1903. [Google Scholar] [CrossRef]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.-J.; Marshall, J.L. Comparative Molecular Analyses of Left-Sided Colon, Right-Sided Colon, and Rectal Cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.C.G.; Sveen, A.; Høland, M.; Alagaratnam, S.; Berg, M.; Danielsen, S.A.; Nesbakken, A.; Søreide, K.; Lothe, R.A. Gene Expression Profiles of CMS2-Epithelial/Canonical Colorectal Cancers Are Largely Driven by DNA Copy Number Gains. Oncogene 2019, 38, 6109–6122. [Google Scholar] [CrossRef] [PubMed]
- Sveen, A.; Bruun, J.; Eide, P.W.; Eilertsen, I.A.; Ramirez, L.; Murumägi, A.; Arjama, M.; Danielsen, S.A.; Kryeziu, K.; Elez, E.; et al. Colorectal Cancer Consensus Molecular Subtypes Translated to Preclinical Models Uncover Potentially Targetable Cancer Cell Dependencies. Clin. Cancer Res. 2018, 24, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Aprile, G.; De Maglio, G.; Menis, J.; Casagrande, M.; Tuniz, F.; Pisa, E.; Fontanella, C.; Skrap, M.; Beltrami, A.; Fasola, G.; et al. HER-2 Expression in Brain Metastases from Colorectal Cancer and Corresponding Primary Tumors: A Case Cohort Series. IJMS 2013, 14, 2370–2387. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Lonardi, S.; Aglietta, M.; Martino, C.; Ciardiello, F.; Marsoni, S.; Siena, S. Central Nervous System as Possible Site of Relapse in ERBB2 -Positive Metastatic Colorectal Cancer: Long-Term Results of Treatment With Trastuzumab and Lapatinib. JAMA Oncol. 2020, 6, 927. [Google Scholar] [CrossRef]
- Li, J.-L.; Lin, S.-H.; Chen, H.-Q.; Liang, L.-S.; Mo, X.-W.; Lai, H.; Zhang, J.; Xu, J.; Gao, B.-Q.; Feng, Y.; et al. Clinical Significance of HER2 and EGFR Expression in Colorectal Cancer Patients with Ovarian Metastasis. BMC Clin. Pathol. 2019, 19, 3. [Google Scholar] [CrossRef]
- Kapitanović, S.; Radosević, S.; Kapitanović, M.; Andelinović, S.; Ferencić, Z.; Tavassoli, M.; Primorać, D.; Sonicki, Z.; Spaventi, S.; Pavelic, K.; et al. The Expression of P185(HER-2/Neu) Correlates with the Stage of Disease and Survival in Colorectal Cancer. Gastroenterology 1997, 112, 1103–1113. [Google Scholar] [CrossRef]
- Ingold Heppner, B.; Behrens, H.-M.; Balschun, K.; Haag, J.; Krüger, S.; Becker, T.; Röcken, C. HER2/Neu Testing in Primary Colorectal Carcinoma. Br. J. Cancer 2014, 111, 1977–1984. [Google Scholar] [CrossRef]
- Laurent-Puig, P.; Balogoun, R.; Cayre, A.; Le Malicot, K.; Tabernero, J.; Mini, E.; Folprecht, G.; van Laethem, J.-L.; Thaler, J.; Petersen, L.N.; et al. ERBB2 Alterations a New Prognostic Biomarker in Stage III Colon Cancer from a FOLFOX Based Adjuvant Trial (PETACC8). Ann. Oncol. 2016, 27, vi151. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, R.; Jiang, J.; Zeng, X.; Zou, S. Correlated Non-Nuclear COX2 and Low HER2 Expression Confers a Good Prognosis in Colorectal Cancer. Saudi J. Gastroenterol. 2018, 24, 301. [Google Scholar] [CrossRef]
- Conradi, L.-C.; Styczen, H.; Sprenger, T.; Wolff, H.A.; Rödel, C.; Nietert, M.; Homayounfar, K.; Gaedcke, J.; Kitz, J.; Talaulicar, R.; et al. Frequency of HER-2 Positivity in Rectal Cancer and Prognosis. Am. J. Surg. Pathol. 2013, 37, 522–531. [Google Scholar] [CrossRef]
- Kruszewski, W.J.; Rzepko, R.; Ciesielski, M.; Szefel, J.; Zieliński, J.; Szajewski, M.; Jasiński, W.; Kawecki, K.; Wojtacki, J. Expression of HER2 in Colorectal Cancer Does Not Correlate with Prognosis. Dis. Markers 2010, 29, 207–212. [Google Scholar] [CrossRef][Green Version]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Corà, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A Molecularly Annotated Platform of Patient-Derived Xenografts (“Xenopatients”) Identifies HER2 as an Effective Therapeutic Target in Cetuximab-Resistant Colorectal Cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Clonal Evolution and Resistance to EGFR Blockade in the Blood of Colorectal Cancer Patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 Signaling Causes Resistance to the EGFR-Directed Therapeutic Antibody Cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Takegawa, N.; Yonesaka, K.; Sakai, K.; Ueda, H.; Watanabe, S.; Nonagase, Y.; Okuno, T.; Takeda, M.; Maenishi, O.; Tsurutani, J.; et al. HER2 Genomic Amplification in Circulating Tumor DNA from Patients with Cetuximab-Resistant Colorectal Cancer. Oncotarget 2016, 7, 3453–3460. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.W.; Niedzwiecki, D.; Hollis, D.; Mayer, R. Phase II Trial of 5-Fluorouracil (5-FU), Leucovorin (LV), Oxaliplatin (Ox), and Trastuzumab (T) for Patients with Metastatic Colorectal Cancer (CRC) Refractory to Initial Therapy. Proc. Am. Soc. Clin. Oncol. 2003, 22, abstr-3584. [Google Scholar]
- Ramanathan, R.K.; Hwang, J.J.; Zamboni, W.C.; Sinicrope, F.A.; Safran, H.; Wong, M.K.; Earle, M.; Brufsky, A.; Evans, T.; Troetschel, M.; et al. Low Overexpression of HER-2/Neu in Advanced Colorectal Cancer Limits the Usefulness of Trastuzumab (Herceptin®) and Irinotecan as Therapy. A Phase II Trial. Cancer Investig. 2004, 22, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus Trastuzumab for HER2-Amplified Metastatic Colorectal Cancer (MyPathway): An Updated Report from a Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Gupta, R.; Garrett-Mayer, E.; Halabi, S.; Mangat, P.K.; D’Andre, S.D.; Meiri, E.; Shrestha, S.; Warren, S.L.; Ranasinghe, S.; Schilsky, R.L. Pertuzumab plus Trastuzumab (P+T) in Patients (Pts) with Colorectal Cancer (CRC) with ERBB2 Amplification or Overexpression: Results from the TAPUR Study. JCO 2020, 38, 132. [Google Scholar] [CrossRef]
- Okamoto, W.; Nakamura, Y.; Kato, T.; Esaki, T.; Komoda, M.; Kato, K.; Komatsu, Y.; Masuishi, T.; Nishina, T.; Sawada, K.; et al. Pertuzumab plus Trastuzumab and Real-World Standard of Care (SOC) for Patients (Pts) with Treatment Refractory Metastatic Colorectal Cancer (MCRC) with HER2 (ERBB2) Amplification (Amp) Confirmed by Tumor Tissue or CtDNA Analysis (TRIUMPH, EPOC1602). JCO 2021, 39, 3555. [Google Scholar] [CrossRef]
- Tosi, F.; Sartore-Bianchi, A.; Lonardi, S.; Amatu, A.; Leone, F.; Ghezzi, S.; Martino, C.; Bencardino, K.; Bonazzina, E.; Bergamo, F.; et al. Long-Term Clinical Outcome of Trastuzumab and Lapatinib for HER2-Positive Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2020, 19, 256–262.e2. [Google Scholar] [CrossRef]
- Yuan, Y.; Fu, X.; Ying, J.; Yang, L.; Fang, W.; Han, W.; Zhang, S. Dual-Targeted Therapy with Pyrotinib and Trastuzumab for HER2-Positive Advanced Colorectal Cancer: Preliminary Results from a Multicenter Phase 2 Trial. JCO 2021, 39, e15554. [Google Scholar] [CrossRef]
- Strickler, J.H.; Zemla, T.; Ou, F.-S.; Cercek, A.; Wu, C.; Sanchez, F.A.; Hubbard, J.; Jaszewski, B.; Bandel, L.; Schweitzer, B.; et al. Trastuzumab and Tucatinib for the Treatment of HER2 Amplified Metastatic Colorectal Cancer (MCRC): Initial Results from the MOUNTAINEER Trial. Ann. Oncol. 2019, 30, v200. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and Trastuzumab Emtansine in Patients with HER2-Amplified Metastatic Colorectal Cancer: The Phase II HERACLES-B Trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab Deruxtecan (DS-8201) in Patients with HER2-Expressing Metastatic Colorectal Cancer (DESTINY-CRC01): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Coussens, L.; Yang-Feng, T.L.; Liao, Y.-C.; Chen, E.; Gray, A.; McGrath, J.; Seeburg, P.H.; Libermann, T.A.; Schlessinger, J.; Francke, U.; et al. Tyrosine Kinase Receptor with Extensive Homology to EGF Receptor Shares Chromosomal Location with Neu Oncogene. Science 1985, 230, 1132–1139. [Google Scholar] [CrossRef]
- Richard, S.; Selle, F.; Lotz, J.-P.; Khalil, A.; Gligorov, J.; Soares, D.G. Pertuzumab and Trastuzumab: The Rationale Way to Synergy. An. Acad. Bras. Ciênc. 2016, 88, 565–577. [Google Scholar] [CrossRef]
- Esteban-Villarrubia, J.; Soto-Castillo, J.J.; Pozas, J.; San Román-Gil, M.; Orejana-Martín, I.; Torres-Jiménez, J.; Carrato, A.; Alonso-Gordoa, T.; Molina-Cerrillo, J. Tyrosine Kinase Receptors in Oncology. IJMS 2020, 21, 8529. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Lee, J.J.; George, T.J.; Wade, J.L.; Stella, P.J.; Wang, D.; Sama, A.R.; Piette, F.; Pogue-Geile, K.L.; Kim, R.S.; et al. Neratinib-Plus-Cetuximab in Quadruple-WT (KRAS, NRAS, BRAF, PIK3CA) Metastatic Colorectal Cancer Resistant to Cetuximab or Panitumumab: NSABP FC-7, A Phase Ib Study. Clin. Cancer Res. 2021, 27, 1612–1622. [Google Scholar] [CrossRef]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing Antibody-Drug Conjugates (ADCs) in HER2-Positive Breast Cancer: State of the Art and Future Directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, Pharmacokinetics, and Antitumour Activity of Trastuzumab Deruxtecan (DS-8201), a HER2-Targeting Antibody–Drug Conjugate, in Patients with Advanced Breast and Gastric or Gastro-Oesophageal Tumours: A Phase 1 Dose-Escalation Study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef]
- Siena, S.; Raghav, K.; Masuishi, T.; Yamaguchi, K.; Nishina, T.; Elez, E.; Rodriguez, J.; Chau, I.; Di Bartolomeo, M.; Kawakami, H.; et al. 386O Exploratory Biomarker Analysis of DESTINY-CRC01, a Phase II, Multicenter, Open-Label Study of Trastuzumab Deruxtecan (T-DXd, DS-8201) in Patients (Pts) with HER2-Expressing Metastatic Colorectal Cancer (MCRC). Ann. Oncol. 2021, 32, S532. [Google Scholar] [CrossRef]
- Karan, C.; Tan, E.; Sarfraz, H.; Knepper, T.C.; Walko, C.M.; Felder, S.; Kim, R.; Sahin, I.H. Human Epidermal Growth Factor Receptor 2–Targeting Approaches for Colorectal Cancer: Clinical Implications of Novel Treatments and Future Therapeutic Avenues. JCO Oncol. Pract. 2022, OP-21. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Gray, R.; Chen, A.; Li, S.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Mitchell, E.P.; Iafrate, A.J.; Sklar, J.; et al. The Molecular Analysis for Therapy Choice (NCI-MATCH) Trial: Lessons for Genomic Trial Design. JNCI J. Natl. Cancer Inst. 2020, 112, 1021–1029. [Google Scholar] [CrossRef]
- Strickler, J.H.; Ng, K.; Cercek, A.; Fountzilas, C.; Sanchez, F.A.; Hubbard, J.M.; Wu, C.; Siena, S.; Tabernero, J.; Van Cutsem, E.; et al. MOUNTAINEER:Open-Label, Phase II Study of Tucatinib Combined with Trastuzumab for HER2-Positive Metastatic Colorectal Cancer (SGNTUC-017, Trial in Progress). JCO 2021, 39, TPS153. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Lee, J.J.; George, T.J.; Yothers, G.; Kolevska, T.; Yost, K.J.; Wade, J.L.; Buchschacher, G.L.; Stella, P.J.; Shipstone, A.; et al. NSABP FC-11: A Phase II Study of Neratinib (N) plus Trastuzumab (T) or n plus Cetuximab (C) in Patients (Pts) with “Quadruple Wild-Type (WT)” (KRAS/NRAS/BRAF/PIK3CA WT) Metastatic Colorectal Cancer (MCRC) Based on HER2 Status—Amplified (Amp), Non-Amplified (Non-Amp), WT, or Mutated (Mt). JCO 2019, 37, TPS716. [Google Scholar] [CrossRef]
- Jacobs, S.A.; George, T.J.; Kolevska, T.; Wade, J.L.; Zera, R.; Buchschacher, G.L.; Al Baghdadi, T.; Shipstone, A.; Lin, D.; Yothers, G.; et al. NSABP FC-11: A Phase II Study of Neratinib (N) plus Trastuzumab (T) or N plus Cetuximab (C) in Patients (Pts) with “Quadruple Wild-Type” Metastatic Colorectal Cancer (MCRC) Based on HER2 Status. JCO 2022, 40, 3564. [Google Scholar] [CrossRef]
- Raghav, K.P.S.; Yoshino, T.; Guimbaud, R.; Chau, I.; Van Den Eynde, M.; Maurel, J.; Tie, J.; Kim, T.W.; Yeh, K.-H.; Barrios, D.; et al. Trastuzumab Deruxtecan in Patients with HER2-Overexpressing Locally Advanced, Unresectable, or Metastatic Colorectal Cancer (MCRC): A Randomized, Multicenter, Phase 2 Study (DESTINY-CRC02). JCO 2021, 39, TPS3620. [Google Scholar] [CrossRef]
- Hausman, D.F.; Hamilton, E.P.; Beeram, M.; Thimmarayappa, J.; Ng, G.; Meric-Bernstam, F. Phase 1 Study of ZW25, a Bispecific Anti-HER2 Antibody, in Patients with Advanced HER2-Expressing Cancers. JCO 2017, 35, TPS215. [Google Scholar] [CrossRef]
- Xu, J.; Meng, Q.; Sun, H.; Zhang, X.; Yun, J.; Li, B.; Wu, S.; Li, X.; Yang, H.; Zhu, H.; et al. HER2-Specific Chimeric Antigen Receptor-T Cells for Targeted Therapy of Metastatic Colorectal Cancer. Cell Death Dis. 2021, 12, 1109. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sawada, K.; Fujii, S.; Yoshino, T. HER2-Targeted Therapy Should Be Shifted towards an Earlier Line for Patients with Anti-EGFR-Therapy Naïve, HER2-Amplified Metastatic Colorectal Cancer. ESMO Open 2019, 4, e000530. [Google Scholar] [CrossRef]
- Raghav, K.P. Sequencing Strategies in the Management of Metastatic Colorectal Cancer with HER2 Amplification. Clin. Adv. Hematol. Oncol. 2022, 20, 86–88. [Google Scholar]
- Baraibar, I.; Ros, J.; Mulet, N.; Salvà, F.; Argilés, G.; Martini, G.; Cuadra, J.L.; Sardo, E.; Ciardiello, D.; Tabernero, J.; et al. Incorporating Traditional and Emerging Biomarkers in the Clinical Management of Metastatic Colorectal Cancer: An Update. Expert Rev. Mol. Diagn. 2020, 20, 653–664. [Google Scholar] [CrossRef]
| Study (Year) | Patients | Stage | ERBB2 Positivity Criteria | Prognostic Significance |
| Yagisawa et al. (2021) [41] | 370 | IV | International harmonization | Better prognosis of ERBB-low patients |
| Sawada et al. (2018) [33] | 359 | I–IV | HERACLES | No differences in OS |
| Park et al. (2018) [34] | 145 | I–III | Modified HERACLES | No differences in survival |
| Richman et al. (2016) [35] | 3256 | I–IV | Gastric cancer scoring | No differences in OS or PFS |
| Laurent-Puig et al. (2016) [56] | 1804 | III | HERACLES + NGS | Lower DFS and OS |
| Heppner et al. (2014) [55] | 1645 | I–IV | Gastric cancer scoring | No significant trend to poorer OS |
| Conradi et al. (2013) [58] | 264 | II–IV | Gastric cancer scoring | Better DFS |
| Kruszewsky et al. (2010) [59] | 202 | I–IV | Membranous + cytoplasmic staining | No association with OS |
| Osako et al. (1998) [38] | 146 | Dukes A-D | Membranous + cytoplasmic staining | Poorer survival in cytoplasmic staining |
| Kapitanovic et al. (1997) [54] | 221 | Bening, premalignant and malignant lesions | Membranous staining | Strong staining correlates with poorer survival |
| Trial | Reference | Treatment | n | Prior Lines of Treatment | Mutational Status | mPFS (m) | ORR (%) |
|---|---|---|---|---|---|---|---|
| Trastuzumab + QT | |||||||
| Clark et al. | [64] | Trastuzumab + FOLFOX | <2 | NS | NR | 24 | |
| Ramanathan et al. | [65] | Trastuzumab + irinotecan | 9 | ≤1 | NS | NR | 71 |
| Monoclonal antibodies | |||||||
| MyPathway | [66] | Trastuzumab + pertuzumab | 57 | ≥1 | RAS WT | 2.9 | 32 |
| TAPUR | [67] | Trastuzumab + pertuzumab | 28 | ≥0 | NS | NR | 14 |
| TRIUMPH | [68] | Trastuzumab + pertuzumab | 27 (Tissue) | ≥1 | RAS WT | 4.0 | 30 |
| 25 (ctDNA) | 3.1 | 25 | |||||
| Monoclonal antibody + TKI | |||||||
| HERACLES-A | [43,69] | Trastuzumab + lapatinib | 35 | ≥2 | KRAS WT | 4.7 | 28 |
| Yuan et al. | [70] | Trastuzumab + pyrotinib | 11 | ≥2 | RAS WT and mutated | NR | 27 |
| MOUNTAINEER | [71] | Trastuzumab + tucatinib | 23 | ≥2 | RAS WT | 8.1 | 52 |
| ADCs | |||||||
| HERACLES-B | [72] | Pertuzumab + T-DM1 | 31 | ≥2 | RAS/BRAF WT | 4.1 | 10 |
| DESTINY-CRC01 | [73] | TD | 53 (Cohort A) | ≥2 | RAS/BRAF WT | 6.9 | 45 |
| Molecular biology |
|
| Diagnosis of HER2-positivity in mCRC |
|
| Clinical features of patients with HER2-positive mCRC |
|
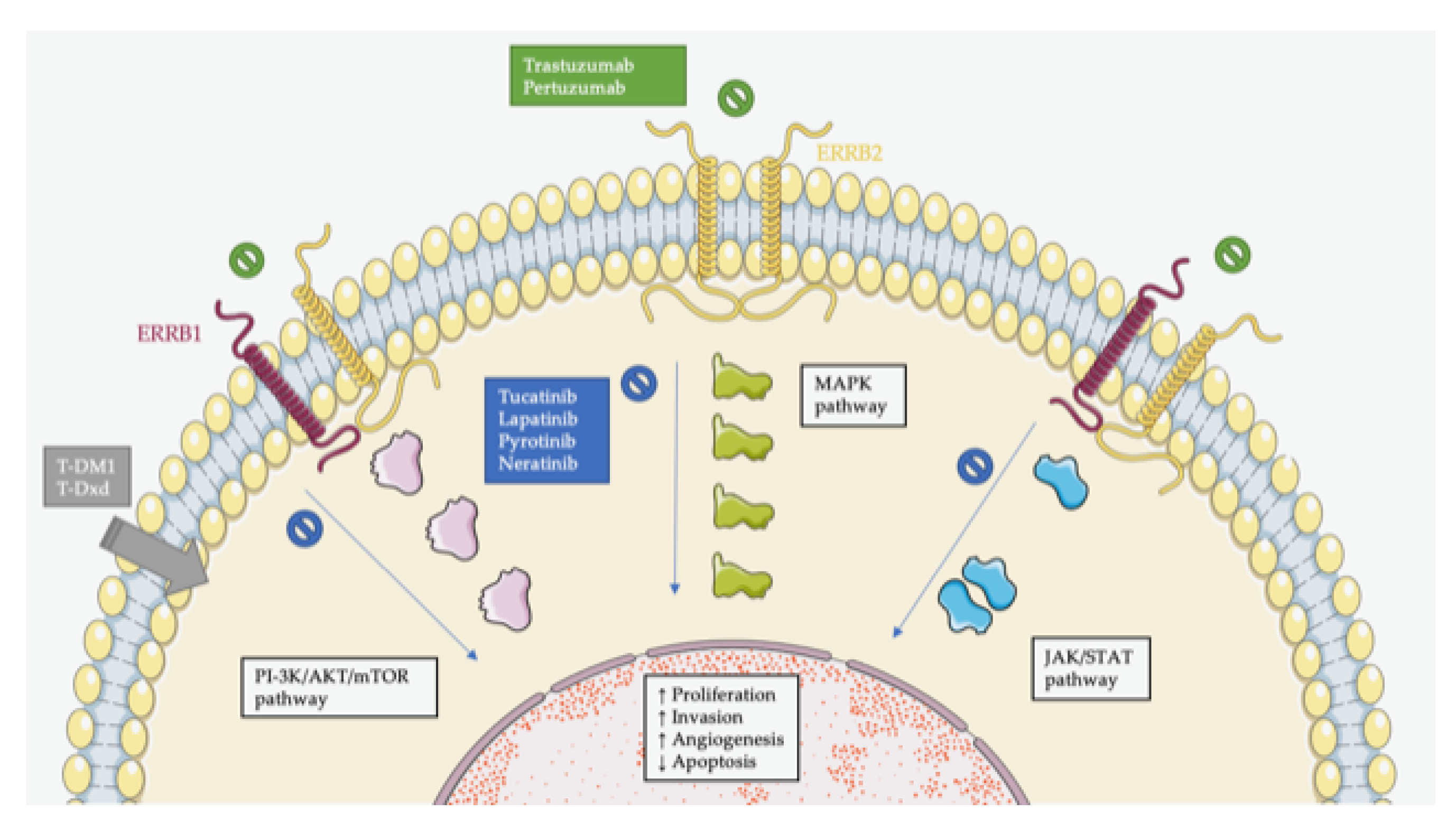
| Clinical trials for patients with ERBB2-positive mCRC |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Jiménez, J.; Esteban-Villarrubia, J.; Ferreiro-Monteagudo, R. Precision Medicine in Metastatic Colorectal Cancer: Targeting ERBB2 (HER-2) Oncogene. Cancers 2022, 14, 3718. https://doi.org/10.3390/cancers14153718
Torres-Jiménez J, Esteban-Villarrubia J, Ferreiro-Monteagudo R. Precision Medicine in Metastatic Colorectal Cancer: Targeting ERBB2 (HER-2) Oncogene. Cancers. 2022; 14(15):3718. https://doi.org/10.3390/cancers14153718
Chicago/Turabian StyleTorres-Jiménez, Javier, Jorge Esteban-Villarrubia, and Reyes Ferreiro-Monteagudo. 2022. "Precision Medicine in Metastatic Colorectal Cancer: Targeting ERBB2 (HER-2) Oncogene" Cancers 14, no. 15: 3718. https://doi.org/10.3390/cancers14153718
APA StyleTorres-Jiménez, J., Esteban-Villarrubia, J., & Ferreiro-Monteagudo, R. (2022). Precision Medicine in Metastatic Colorectal Cancer: Targeting ERBB2 (HER-2) Oncogene. Cancers, 14(15), 3718. https://doi.org/10.3390/cancers14153718






