Rearrangements, Expression, and Clinical Significance of MYB and MYBL1 in Adenoid Cystic Carcinoma: A Multi-Institutional Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Material
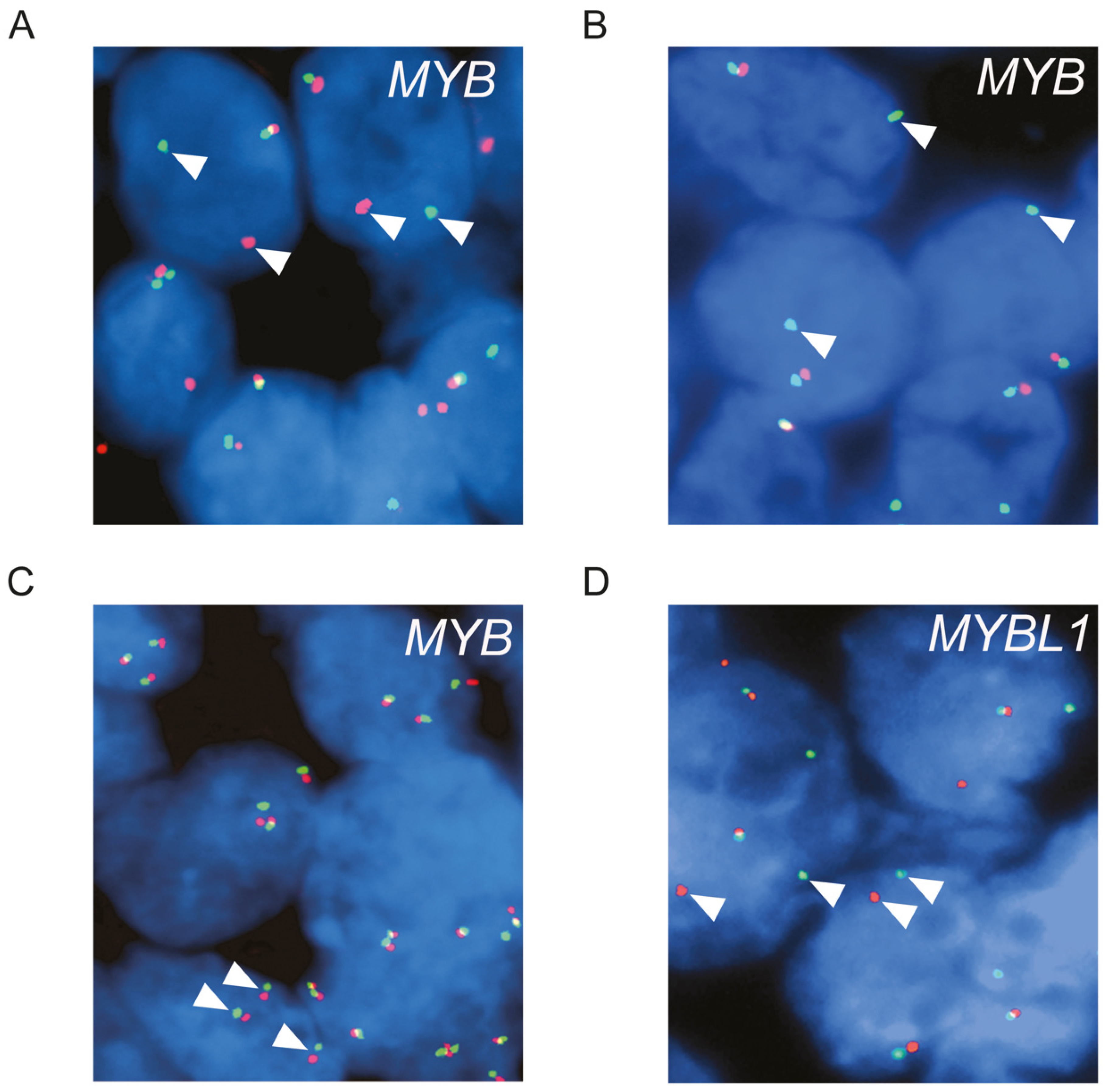
2.2. Fluorescence In Situ Hybridization (FISH) Analysis
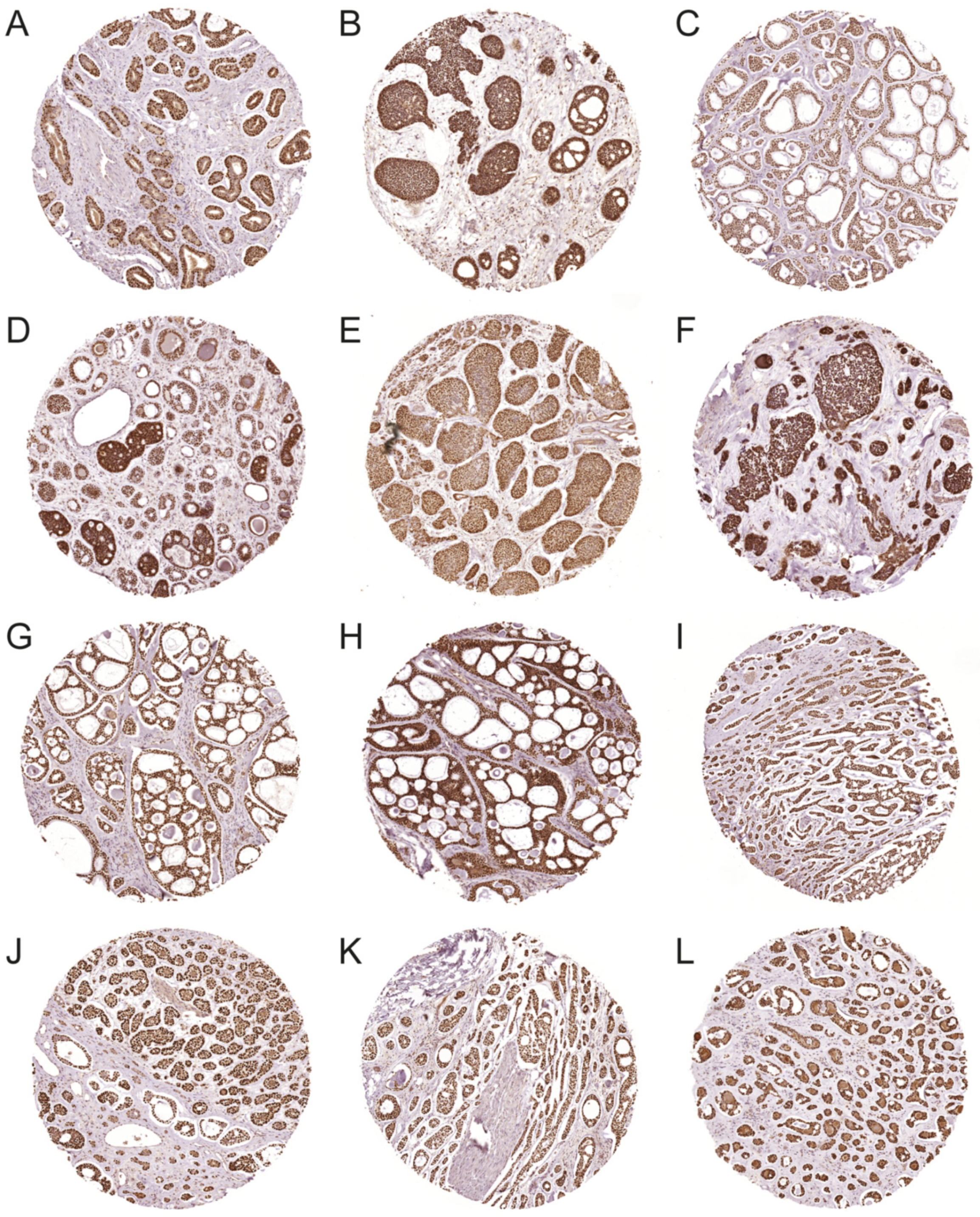
2.3. Immunohistochemistry (IHC)
2.4. Statistical Analyses
3. Results
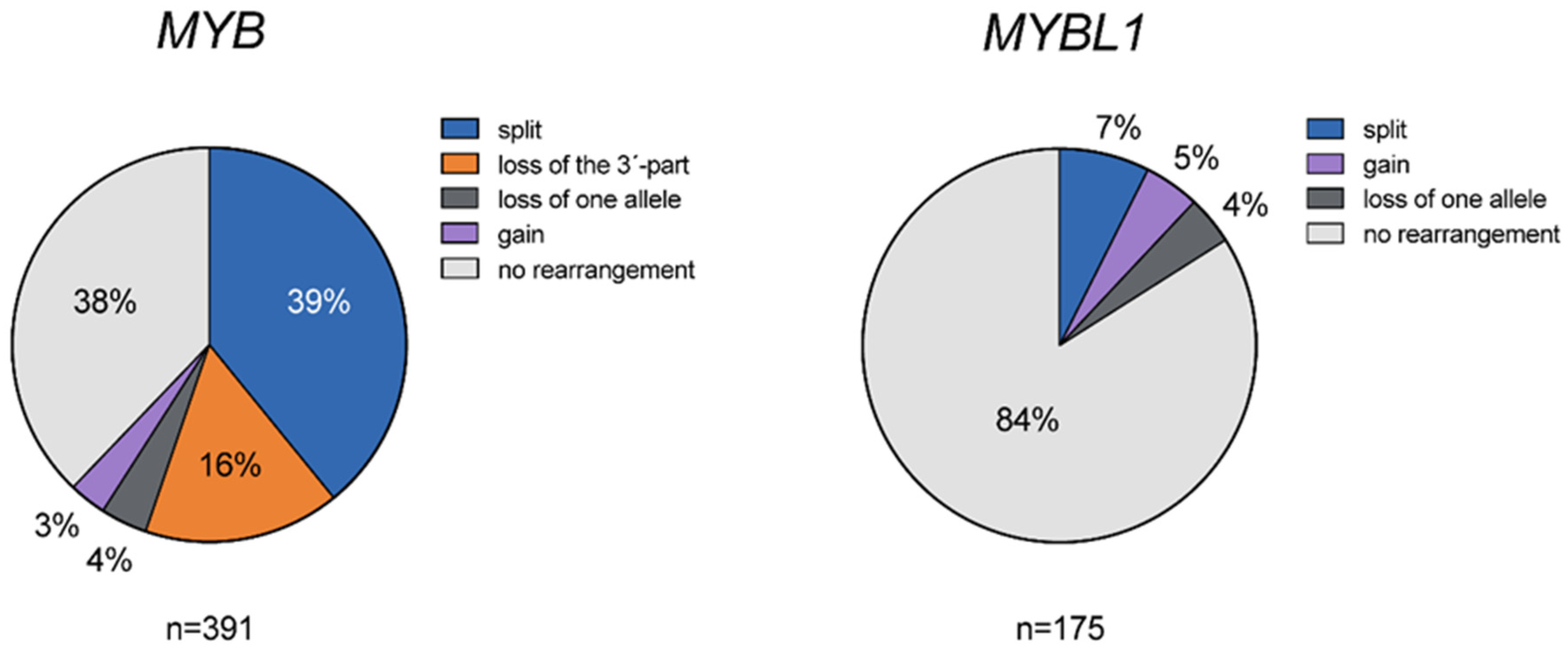
3.1. Genomic Alterations of MYB and MYBL1 in ACC
3.2. Expression of MYB and MYBL1 Oncoproteins in ACC
3.3. Clinical Significance of MYB and MYBL1 Rearrangements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stenman, G.; Licitra, L.; Said-Al-Naief, N.; van Zante, A.; Yarbrough, W.G. Adenoid cystic carcinoma. In World Health Organization Classification of Head and Neck Tumours; El-Naggar, A.K., Chan, J.K.C., Grandis, J.R., Takata, T., Slootweg, P.J., Eds.; IARC Press: Lyon, France, 2017; pp. 164–165. [Google Scholar]
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; Poorten, V.V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz, M.; Takes, R.P.; et al. Adenoid cystic carcinoma of the head and neck—An update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.B., 2nd; Kanner, W.A.; Fehr, A.; Andren, Y.; Moskaluk, C.A.; Loning, T.; Stenman, G.; Frierson, H.F., Jr. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod. Pathol. 2011, 24, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Ho, A.L.; Fury, M.G.; Sherman, E.; Pfister, D.G. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: A systematic review. Lancet Oncol. 2011, 12, 815–824. [Google Scholar] [CrossRef]
- Xu, B.; Drill, E.; Ho, A.; Ho, A.; Dunn, L.; Prieto-Granada, C.N.; Chan, T.; Ganly, I.; Ghossein, R.; Katabi, N. Predictors of Outcome in Adenoid Cystic Carcinoma of Salivary Glands: A Clinicopathologic Study with Correlation Between MYB Fusion and Protein Expression. Am. J. Surg. Pathol. 2017, 41, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Licitra, L.; Locati, L.; Raben, D.; Persson, F.; Stenman, G. Salivary Gland Cancer: An Update on Present and Emerging Therapies. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef]
- Stenman, G.; Sandros, J.; Dahlenfors, R.; Juberg-Ode, M.; Mark, J. 6q- and loss of the Y chromosome—Two common deviations in malignant human salivary gland tumors. Cancer Genet. Cytogenet. 1986, 22, 283–293. [Google Scholar] [CrossRef]
- Persson, M.; Andren, Y.; Mark, J.; Horlings, H.M.; Persson, F.; Stenman, G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl. Acad. Sci. USA 2009, 106, 18740–18744. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.K.; Stenman, G. The landscape of gene fusions and somatic mutations in salivary gland neoplasms—Implications for diagnosis and therapy. Oral Oncol. 2016, 57, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.K.; Afshari, M.K.; Andrén, Y.; Wick, M.J.; Stenman, G. Targeting the Oncogenic Transcriptional Regulator MYB in Adenoid Cystic Carcinoma by Inhibition of IGF1R/AKT Signaling. J. Natl. Cancer Inst. 2017, 109, djx017. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.K.; Åman, P.; Stenman, G. IGF2/IGF1R Signaling as a Therapeutic Target in MYB-Positive Adenoid Cystic Carcinomas and Other Fusion Gene-Driven Tumors. Cells 2019, 8, 913. [Google Scholar] [CrossRef] [Green Version]
- Mertens, F.; Johansson, B.; Fioretos, T.; Mitelman, F. The emerging complexity of gene fusions in cancer. Nat. Cancer 2015, 15, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Brayer, K.J.; Frerich, C.A.; Kang, H.; Ness, S.A. Recurrent Fusions in MYB and MYBL1 Define a Common, Transcription Factor–Driven Oncogenic Pathway in Salivary Gland Adenoid Cystic Carcinoma. Cancer Discov. 2016, 6, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Mitani, Y.; Liu, B.; Rao, P.H.; Borra, V.J.; Zafereo, M.; Weber, R.S.; Kies, M.; Lozano, G.; Futreal, P.A.; Caulin, C.; et al. Novel MYBL1 Gene Rearrangements with Recurrent MYBL1–NFIB Fusions in Salivary Adenoid Cystic Carcinomas Lacking t(6;9) Translocations. Clin. Cancer Res. 2016, 22, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Drier, Y.; Cotton, M.J.; Williamson, K.E.; Gillespie, S.M.; Ryan, R.J.H.; Kluk, M.J.; Carey, C.D.; Rodig, S.J.; Sholl, L.M.; Afrogheh, A.H.; et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat. Genet. 2016, 48, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, M.; Andrén, Y.; Moskaluk, C.A.; Frierson, H.F., Jr.; Cooke, S.L.; Futreal, P.A.; Kling, T.; Nelander, S.; Nordkvist, A.; Persson, F.; et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosom. Cancer 2012, 51, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.T.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; Van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar] [CrossRef]
- Rettig, E.M.; Talbot, C.C., Jr.; Sausen, M.; Jones, S.; Bishop, J.A.; Wood, L.D.; Tokheim, C.; Niknafs, N.; Karchin, R.; Fertig, E.J.; et al. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev. Res. 2016, 9, 265–274. [Google Scholar] [CrossRef] [Green Version]
- De Almeida-Pinto, Y.D.; Costa, S.F.D.S.; de Andrade, B.A.B.; Altemani, A.; Vargas, P.A.; Abreu, L.G.; Fonseca, F.P. t(6;9)(MYB-NFIB) in head and neck adenoid cystic carcinoma: A systematic review with meta-analysis. Oral Dis. 2019, 25, 1277–1282. [Google Scholar] [CrossRef]
- Clauditz, T.S.; Gontarewicz, A.; Lebok, P.; Tsourlakis, M.-C.; Grob, T.J.; Muenscher, A.; Sauter, G.; Bokemeyer, C.; Knecht, R.; Wilczak, W. Epidermal growth factor receptor (EGFR) in salivary gland carcinomas: Potentials as therapeutic target. Oral Oncol. 2012, 48, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Clauditz, T.S.; Gontarewicz, A.; Wang, C.-J.; Münscher, A.; Laban, S.; Tsourlakis, M.C.; Knecht, R.; Sauter, G.; Wilczak, W. 11q21 Rearrangement is a Frequent and Highly Specific Genetic Alteration in Mucoepidermoid Carcinoma. Diagn. Mol. Pathol. 2012, 21, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Clauditz, T.S.; Gontarewicz, A.; Bokemeyer, C.; Sauter, G.; Knecht, R.; Münscher, A.; Wilczak, W. Abundant expression of mTOR kinase in salivary gland tumors—Potentials as therapy target? J. Oral Pathol. Med. 2013, 42, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Szanto, P.A.; Luna, M.A.; Tortoledo, M.E.; White, R.A. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 1984, 54, 1062–1069. [Google Scholar] [CrossRef]
- Mitani, Y.; Li, J.; Rao, P.H.; Zhao, Y.-J.; Bell, D.; Lippman, S.M.; Weber, R.S.; Caulin, C.; El-Naggar, A.K. Comprehensive Analysis of the MYB-NFIB Gene Fusion in Salivary Adenoid Cystic Carcinoma: Incidence, Variability, and Clinicopathologic Significance. Clin. Cancer Res. 2010, 16, 4722–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitani, Y.; Rao, P.H.; Futreal, P.A.; Roberts, D.B.; Stephens, P.J.; Zhao, Y.-J.; Zhang, L.; Mitani, M.; Weber, R.S.; Lippman, S.M.; et al. Novel Chromosomal Rearrangements and Break Points at the t(6;9) in Salivary Adenoid Cystic Carcinoma: Association with MYB–NFIB Chimeric Fusion, MYB Expression, and Clinical Outcome. Clin. Cancer Res. 2011, 17, 7003–7014. [Google Scholar] [CrossRef] [Green Version]
- Rettig, E.M.; Tan, M.; Ling, S.; Yonescu, R.; Bishop, J.A.; Fakhry, C.; Ha, P.K. MYB rearrangement and clinicopathologic characteristics in head and neck adenoid cystic carcinoma. Laryngoscope 2015, 125, E292–E299. [Google Scholar] [CrossRef] [Green Version]
- Ho, A.L.; Dunn, L.; Sherman, E.J.; Fury, M.G.; Baxi, S.S.; Chandramohan, R.; Dogan, S.; Morris, L.G.T.; Cullen, G.; Haque, S.; et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann. Oncol. 2016, 27, 1902–1908. [Google Scholar] [CrossRef]
- Fujii, K.; Murase, T.; Beppu, S.; Saida, K.; Takino, H.; Masaki, A.; Ijichi, K.; Kusafuka, K.; Iida, Y.; Onitsuka, T.; et al. MYB, MYBL1, MYBL2 and NFIB gene alterations and MYC overexpression in salivary gland adenoid cystic carcinoma. Histopathology 2017, 71, 823–834. [Google Scholar] [CrossRef]
- Andreasen, S.; Tan, Q.; Agander, T.K.; Steiner, P.; Bjørndal, K.; Høgdall, E.; Larsen, S.R.; Erentaite, D.; Olsen, C.H.; Ulhøi, B.P.; et al. Adenoid cystic carcinomas of the salivary gland, lacrimal gland, and breast are morphologically and genetically similar but have distinct microRNA expression profiles. Mod. Pathol. 2018, 31, 1211–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togashi, Y.; Dobashi, A.; Sakata, S.; Sato, Y.; Baba, S.; Seto, A.; Mitani, H.; Kawabata, K.; Takeuchi, K. MYB and MYBL1 in adenoid cystic carcinoma: Diversity in the mode of genomic rearrangement and transcripts. Mod. Pathol. 2018, 31, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Lombardo, K.A.; Oliai, B.R.; Ha, P.K.; Bishop, J.A. MYB RNA In Situ Hybridization Facilitates Sensitive and Specific Diagnosis of Adenoid Cystic Carcinoma Regardless of Translocation Status. Am. J. Surg. Pathol. 2020, 45, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Mitelman, F.; Johansson, B.; Mertens, F. (Eds.) Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. 2022. Available online: https://mitelmandatabase.isb-cgc.org (accessed on 10 June 2022).
- Lahortiga, I.; De Keersmaecker, K.; Van Vlierberghe, P.; Graux, C.; Cauwelier, B.; Lambert, F.; Mentens, N.; Beverloo, H.B.; Pieters, R.; Speleman, F.; et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat. Genet. 2007, 39, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Lao, X.; Liang, Y. The value of MYB as a prognostic marker for adenoid cystic carcinoma: Meta-analysis. Head Neck 2019, 41, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Sandros, J.; Mark, J.; Happonen, R.P.; Stenman, G. Specificity of 6q- markers and other recurrent deviations in human malignant salivary gland tumors. Anticancer Res. 1988, 8, 637–643. [Google Scholar]
- Sandros, J.; Stenman, G.; Mark, J. Cytogenetic and molecular observations in human and experimental salivary gland tumors. Cancer Genet. Cytogenet. 1990, 44, 153–167. [Google Scholar] [CrossRef]
- Queimado, L.; Reis, A.; Fonseca, I.; Martins, C.; Lovett, M.; Soares, J.; Parreira, L. A refined localization of two deleted regions in chromosome 6q associated with salivary gland carcinomas. Oncogene 1998, 16, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, S.; Yu, Y.; Rumpel, C.A.; Frierson, H.F.; Moskaluk, C.A. Chromosome 6 deletion and candidate tumor suppressor genes in adenoid cystic carcinoma. Cancer Lett. 2006, 236, 309–317. [Google Scholar] [CrossRef]
- von Holstein, S.L.; Fehr, A.; Persson, M.; Therkildsen, M.H.; Prause, J.U.; Heegaard, S.; Stenman, G. Adenoid Cystic Carcinoma of the Lacrimal Gland: MYB Gene Activation, Genomic Imbalances, and Clinical Characteristics. Ophthalmology 2013, 120, 2130–2138. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roberts, D.; Godwin, A.K.; Schultz, D.C.; Sonoda, G.; Testa, J.R.; Hamilton, T.C. Identification of a zinc-finger gene at 6q25: A chromosomal region implicated in development of many solid tumors. Oncogene 1997, 14, 1973–1979. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, A. LOT1 (ZAC1/PLAGL1) and its family members: Mechanisms and functions. J. Cell. Physiol. 2006, 210, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, S.; Taylor, B.S.; Meng, S.; Fang, F.; Yilmaz, E.; Vivanco, I.; Janakiraman, M.; Schultz, N.; Hanrahan, A.J.; Pao, W.; et al. Somatic mutations of the Parkinson’s disease–associated gene PARK2 in glioblastoma and other human malignancies. Nat. Genet. 2009, 42, 77–82. [Google Scholar] [CrossRef]
- Visser, S.; Yang, X. LATS tumor suppressor: A new governor of cellular homeostasis. Cell Cycle 2010, 9, 3892–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, A.A.D.; Hergovich, A. The NDR/LATS protein kinases in immunology and cancer biology. Semin. Cancer Biol. 2018, 48, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Brayer, K.J.; Mitani, Y.; Burns, E.A.; Rao, P.H.; Bell, D.; Williams, M.D.; Ferrarotto, R.; Pytynia, K.B.; El-Naggar, A.K.; et al. Oncogenic Orphan Nuclear Receptor NR4A3 Interacts and Cooperates with MYB in Acinic Cell Carcinoma. Cancers 2020, 12, 2433. [Google Scholar] [CrossRef]

| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | HR 95% CI | p-Value | HR | HR 95% CI | p-Value | |
| MYB—loss of the 3′-part (n = 278) | 1.791 | 1.187–2.703 | 0.006 | 1.633 | 1.032–2.584 | 0.036 |
| MYB—split (n = 278) | 0.962 | 0.686–1.349 | 0.822 | |||
| Age (n = 365) | 1.027 | 1.017–1.038 | <0.001 | 1.028 | 1.014–1.043 | <0.001 |
| Sex (n = 366) | 1.110 | 0.827–1.491 | 0.487 | |||
| Perineural invasion (n = 180) | 0.770 | 0.397–1.492 | 0.439 | |||
| Grade (n = 304) | ||||||
| 1 (n = 127) | reference | reference | ||||
| 2 (n = 104) | 1.369 | 0.904–2.071 | 0.138 | 1.440 | 0.886–2.339 | 0.141 |
| 3 (n = 73) | 3.751 | 2.480–5.673 | <0.001 | 4.481 | 2.760–7.275 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persson, M.; Andersson, M.K.; Mitani, Y.; Brandwein-Weber, M.S.; Frierson, H.F., Jr.; Moskaluk, C.; Fonseca, I.; Ferrarotto, R.; Boecker, W.; Loening, T.; et al. Rearrangements, Expression, and Clinical Significance of MYB and MYBL1 in Adenoid Cystic Carcinoma: A Multi-Institutional Study. Cancers 2022, 14, 3691. https://doi.org/10.3390/cancers14153691
Persson M, Andersson MK, Mitani Y, Brandwein-Weber MS, Frierson HF Jr., Moskaluk C, Fonseca I, Ferrarotto R, Boecker W, Loening T, et al. Rearrangements, Expression, and Clinical Significance of MYB and MYBL1 in Adenoid Cystic Carcinoma: A Multi-Institutional Study. Cancers. 2022; 14(15):3691. https://doi.org/10.3390/cancers14153691
Chicago/Turabian StylePersson, Marta, Mattias K. Andersson, Yoshitsugu Mitani, Margaret S. Brandwein-Weber, Henry F. Frierson, Jr., Christopher Moskaluk, Isabel Fonseca, Renata Ferrarotto, Werner Boecker, Thomas Loening, and et al. 2022. "Rearrangements, Expression, and Clinical Significance of MYB and MYBL1 in Adenoid Cystic Carcinoma: A Multi-Institutional Study" Cancers 14, no. 15: 3691. https://doi.org/10.3390/cancers14153691






