Intra-Operative Electron Radiation Therapy: An Update of the Evidence Collected in 40 Years to Search for Models for Electron-FLASH Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
IOeRT: 40 Years of Clinical Results: Evidence-Based Data from IOeRT in 6 ESTRO/ACROP Guidelines 2020
3. Results
4. Discussion
4.1. IOeRT Radiobiology: The Conundrum of Boosting versus Single High-Dose Fraction
4.2. IOeRT: Roll-Out of the Clinical Applications
4.3. IOeRT: Research and Clinical Perspectives with FLASH-IOeRT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 17 May 2022).
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2022, 49, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; Montay-Gruel, P.; Limoli, C.; Germond, J.F. All Irradiations that are Ultra-High Dose Rate may not be FLASH: The Critical Importance of Beam Parameter Characterization and In Vivo Validation of the FLASH Effect. Radiat. Res. 2020, 194, 571–572. Available online: https://bioone.org/journals/radiation-research/volume-194/issue-6/RADE-20-00141.1/All-Irradiations-that-are-Ultra-High-Dose-Rate-may-not/10.1667/RADE-20-00141.1.full (accessed on 17 May 2022). [CrossRef]
- Schüler, E.; Acharya, M.; Montay-Gruel, P.; Loo, B.W.; Vozenin, M.C.; Maxim, P.G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med. Phys. 2022, 49, 2082–2095. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Gonçalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Prades, J.; Remue, E.; Van Hoof, E.; Borras, J.M. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy 2015, 119, 464–474. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Calvo, F.A. Intraoperative irradiation: Precision medicine for quality cancer control promotion. Radiat. Oncol. 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Pascau, J.; Santos Miranda, J.A.; Calvo, F.A.; Bouché, A.; Morillo, V.; González-San Segundo, C.; Ferrer, C.; Tarjuelo, J.L.; Desco, M. An Innovative Tool for Intraoperative Electron Beam Radiotherapy Simulation and Planning: Description and Initial Evaluation by Radiation Oncologists. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e287–e295. [Google Scholar] [CrossRef] [PubMed]
- García-Vázquez, V.; Marinetto, E.; Guerra, P.; Valdivieso-Casique, M.F.; Calvo, F.Á.; Alvarado-Vásquez, E.; Sole, C.V.; Vosburgh, K.G.; Desco, M.; Pascau, J. Assessment of intraoperative 3D imaging alternatives for IOERT dose estimation. Z. Med. Phys. 2017, 27, 218–231. [Google Scholar] [CrossRef]
- Krengli, M.; Terrone, C.; Ballarè, A.; Loi, G.; Tarabuzzi, R.; Marchioro, G.; Beldì, D.; Mones, E.; Bolchini, C.; Volpe, A.; et al. Intraoperative radiotherapy during radical prostatectomy for locally advanced prostate cancer: Technical and dosimetric aspects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1073–1077. [Google Scholar] [CrossRef]
- López-Tarjuelo, J.; Morillo-Macías, V.; Bouché-Babiloni, A.; Boldó-Roda, E.; Lozoya-Albacar, R.; Ferrer-Albiach, C. Implementation of an intraoperative electron radiotherapy in vivo dosimetry program. Radiat. Oncol. 2016, 11, 41. [Google Scholar] [CrossRef]
- Guerra, P.; Udías, J.M.; Herranz, E.; Santos-Miranda, J.A.; Herraiz, J.L.; Valdivieso, M.F.; Rodríguez, R.; Calama, J.A.; Pascau, J.; Calvo, F.A.; et al. Feasibility assessment of the interactive use of a Monte Carlo algorithm in treatment planning for intraoperative electron radiation therapy. Phys. Med. Biol. 2014, 59, 7159–7179. [Google Scholar] [CrossRef]
- López-Tarjuelo, J.; Bouché-Babiloni, A.; Santos-Serra, A.; Morillo-Macías, V.; Calvo, F.A.; Kubyshin, Y.; Ferrer-Albiach, C. Failure mode and effect analysis oriented to risk-reduction interventions in intraoperative electron radiation therapy: The specific impact of patient transportation, automation, and treatment planning availability. Radiother. Oncol. 2014, 113, 283–289. [Google Scholar] [CrossRef]
- Calvo, F.A.; Sole, C.V.; Serrano, J.; Rodriguez, M.; Marcos, F.; Muñoz-Calero, A.; Zorrilla, J.; Lopez-Baena, J.A.; Diaz-Zorita, B.; García-Sabrido, J.L.; et al. Postchemoradiation laparoscopic resection and intraoperative electron-beam radiation boost in locally advanced rectal cancer: Long-term outcomes. J. Cancer Res. Clin. Oncol. 2013, 139, 1825–1833. [Google Scholar] [CrossRef]
- Calvo, F.A. (Department of Oncology, Clínica Universidad de Navarra, Madrid, Spain). Personal communication, 2022.
- García-Vázquez, V.; Marinetto, E.; Santos-Miranda, J.A.; Calvo, F.A.; Desco, M.; Pascau, J. Feasibility of integrating a multi-camera optical tracking system in intra-operative electron radiation therapy scenarios. Phys. Med. Biol. 2013, 58, 8769–8782. [Google Scholar] [CrossRef]
- Calvo, F.A.; Sallabanda, M.; Sole, C.V.; Gonzalez, C.; Murillo, L.A.; Martinez-Villanueva, J.; Santos, J.A.; Serrano, J.; Alavrez, A.; Blanco, J.; et al. Intraoperative radiation therapy opportunities for clinical practice normalization: Data recording and innovative development. Rep. Pract. Oncol. Radiother. 2014, 19, 246–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
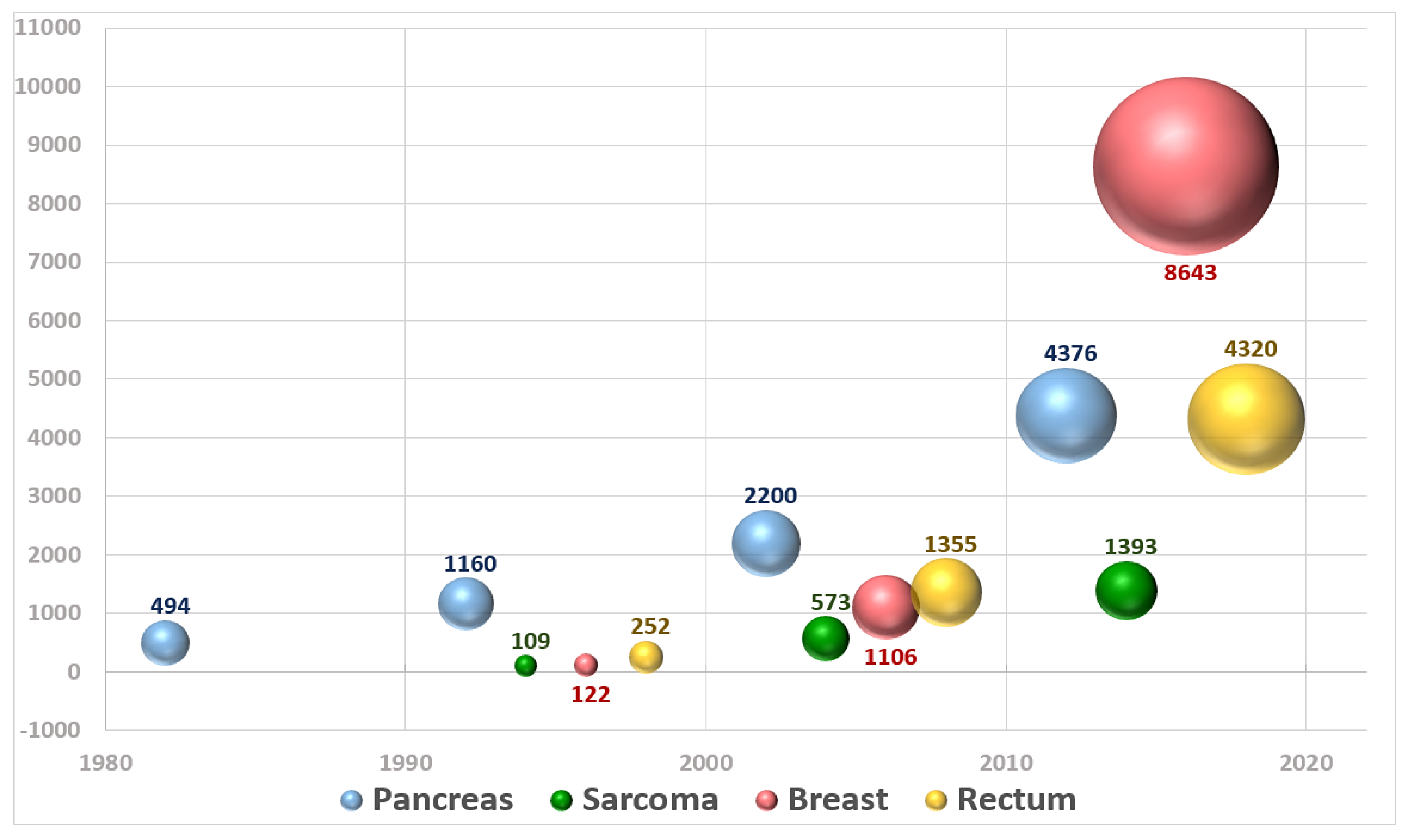
- Sole, C.V.; Calvo, F.A.; Ferrer, C.; Pascau, J.; Marsiglia, H. Bibliometrics of intraoperative radiotherapy: Analysis of technology, practice and publication tendencies. Strahlenther. Onkol. 2014, 190, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Fastner, G.; Gaisberger, C.; Kaiser, J.; Scherer, P.; Ciabattoni, A.; Petoukhova, A.; Sperk, E.; Poortmans, P.; Calvo, F.A.; Sedlmayer, F.; et al. ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy with electrons (IOERT) in breast cancer. Radiother. Oncol. 2020, 149, 150–157. [Google Scholar] [CrossRef]
- Calvo, F.A.; Asencio, J.M.; Roeder, F.; Krempien, R.; Poortmans, P.; Hensley, F.W.; Krengli, M. ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy in borderline-resected pancreatic cancer. Clin. Transl. Radiat. Oncol. 2020, 23, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.A.; Krengli, M.; Asencio, J.M.; Serrano, J.; Poortmans, P.; Roeder, F.; Krempien, R.; Hensley, F.W. ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy in unresected pancreatic cancer. Radiother. Oncol. 2020, 148, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.A.; Sole, C.V.; Rutten, H.J.; Dries, W.J.; Lozano, M.A.; Cambeiro, M.; Poortmans, P.; González-Bayón, L. ESTRO/ACROP IORT recommendations for intraoperative radiation therapy in locally recurrent rectal cancer. Clin. Transl. Radiat. Oncol. 2020, 24, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.A.; Sole, C.V.; Rutten, H.J.; Poortmans, P.; Asencio, J.M.; Serrano, J.; Aristu, J.; Roeder, F.; Dries, W.J. ESTRO/ACROP IORT recommendations for intraoperative radiation therapy in primary locally advanced rectal cancer. Clin. Transl. Radiat. Oncol. 2020, 25, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F.; Morillo, V.; Saleh-Ebrahimi, L.; Calvo, F.A.; Poortmans, P.; Ferrer Albiach, C. Intraoperative radiation therapy (IORT) for soft tissue sarcoma—ESTRO IORT Task Force/ACROP recommendations. Radiother. Oncol. 2020, 150, 293–302. [Google Scholar] [CrossRef]
- Sindelar, W.F.; Kinsella, T.J. Normal tissue tolerance to intraoperative radiotherapy. Surg. Oncol. Clin. N. Am. 2003, 12, 925–942. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Willett, C.G.; Calvo, F.A.; Harrison, L.B. Intraoperatile Irradiation Techniques and Results, 2nd ed.; Humana Press: New York, NY, USA, 2011. [Google Scholar]
- Cambeiro, M.; Calvo, F.A.; Aristu, J.J.; Jimenez, M.M.; San-Julian, M.; Alcalde, J.; Hernandez-Lizoain, J.L.; Jurado, M.; Martínez-Monge, R. Salvage surgery and radiotherapy including intraoperative electron radiotherapy in isolated locally recurrent tumors: Predictors of outcome. Radiother. Oncol. 2015, 116, 316–322. [Google Scholar] [CrossRef]
- Veronesi, U.; Orecchia, R.; Luini, A.; Galimberti, V.; Zurrida, S.; Intra, M.; Veronesi, P.; Arnone, P.; Leonardi, M.C.; Ciocca, M.; et al. Intraoperative radiotherapy during breast conserving surgery: A study on 1822 cases treated with electrons. Breast Cancer Res. Treat. 2010, 124, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.C.; Maisonneuve, P.; Mastropasqua, M.G.; Morra, A.; Lazzari, R.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, U.; Orecchia, R. How Do the ASTRO Consensus Statement Guidelines for the Application of Accelerated Partial Breast Irradiation Fit Intraoperative Radiotherapy? A Retrospective Analysis of Patients Treated at the European Institute of Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.C.; Maisonneuve, P.; Mastropasqua, M.G.; Morra, A.; Lazzari, R.; Dell’Acqua, V.; Ferrari, A.; Rotmensz, N.; Sangalli, C.; Luini, A.; et al. Accelerated partial breast irradiation with intraoperative electrons: Using GEC–ESTRO recommendations as guidance for patient selection. Radiother. Oncol. 2013, 106, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Orecchia, R.; Veronesi, U.; Maisonneuve, P.; Galimberti, V.E.; Lazzari, R.; Veronesi, P.; Jereczek-Fossa, B.A.; Cattani, F.; Sangalli, C.; Luini, A.; et al. Intraoperative irradiation for early breast cancer (ELIOT): Long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021, 22, 597–608. [Google Scholar] [CrossRef]
- Meattini, I.; Becherini, C.; Boersma, L.; Kaidar-Person, O.; Marta, G.N.; Montero, A.; Offersen, B.V.; Aznar, M.C.; Belka, C.; Brunt, A.M.; et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022, 23, e21–e31. [Google Scholar] [CrossRef]
- Van Loon, J.; Grutters, J.; Macbeth, F. Evaluation of novel radiotherapy technologies: What evidence is needed to assess their clinical and cost effectiveness, and how should we get it? Lancet Oncol. 2012, 13, e169–e177. [Google Scholar] [CrossRef]
- Rodin, D.; Aggarwal, A.; Lievens, Y.; Sullivan, R. Balancing Equity and Advancement: The Role of Health Technology Assessment in Radiotherapy Resource Allocation. Clin. Oncol. (R Coll. Radiol). 2017, 29, 93–98. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Lancellotta, V.; Colloca, G.; Marazzi, F.; Masiello, V.; Garganese, G.; Kovács, G.; Valentini, V.; Gambacorta, M.A. Could a Personalized Strategy Using Accelerated Partial Breast Irradiation be an Advantage for Elderly Patients? A Systematic Review of the Literature and Multidisciplinary Opinion. J. Oncol. 2020, 2020, 3928976. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Özşahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
| Cancer Site/Type/Status | # pts (%) | # REF (%) | IOERT DOSE Gy | Local Control | OS 5y |
|---|---|---|---|---|---|
| Pancreas Unresected | 2307 (12%) | 36 (31%) | 15–25 | 41–71% | 0–6% |
| Pancreas Resected | 2087 (10%) | 33 (28%) | 10–20 | 73–94% | 20–35% |
| Rectal Locally Advanced | 2590 (13%) | 18 (15%) | 10–15 | 75–100% | 64–84% |
| Rectal Oligo-Recurrent | 1730 (9%) | 10 (8%) | 12.5–20 | 44–72% | 25–52% |
| Soft Tissue Sarcoma Extremity | 922 (5%) | 14 (12%) | 10–20 | 58–100% | 69–82% |
| Soft Tissue Sarcoma Retroperitoneal | 871 (4%) | 24 (20%) | 10–20 | 40–90% | 38–74% |
| Breast Cancer (Partial Breast RT) | 4229 (22%) | 12 (10%) | 21–23 | 91–100% | 94–100% |
| Breast cancer (BOOST) | 4414 (23%) | 9 (7%) | 10–15 | 89–100% | 75–97% |
| IOeRT ESTRO Cancer Models Guidelines (Ref.) | ESTRO Dose IOeRT Recommendation (Gy) | Normal Tissues at Risk within IOeRT Target | FLASH Dose Escalation Proposal (Gy) |
|---|---|---|---|
| Unresected pancreas [27] | 15–20 | pancreatic tumor and parenchyma bile duct duodenum vascular structures vertebrae | 25–30–35 |
| Post-resected pancreas [26] | R0 10–12.5 R1 12.5–15 R2 15–20 | vascular structures vascular sutures * retroperitoneal tissue | R0 17.5–20–25 R1 20–25–30 R2 25–30 |
| Extremity sarcomas [30] | R0 10 R1 15 ** R2 20 ** | peripheral nerves muscle vascular structures bone | R0 15–20–25 R1 20–25–30 R2 25–30 |
| Retroperitoneal sarcomas [30] | Close margins 10–12.5 Involved margins 12–15 Gross residual 15–20 | peripheral nerves muscle vascular structures bone ureter * vascular suture * | Close margins 15–20–25 Involved margins 20–25–30 Gross residual 25–30 |
| Primary advanced rectal cancer [29] | R0 10–12.5 R1 12.5–15 R2 15–20 | bone peripheral nerves vascular structures soft tissue | R0 17.5–23–28 R1 20–25–30 R2 25–30 |
| Locally recurrent rectal cancer [28] | R0 12.5–15 R1 15–20 R2 15–20 | bone peripheral nerves vascular structures soft tissues vascular sutures * ureter previous irradiated tissues * | R0 17.5–23–28 R1 20–25–30 R2 20–25–30 * 20–25–30 |
| Breast cancer partial irradiation [25] | 21 | breast parenchyma reconstructed | 26–30 |
| Breast cancer boost [25] | 9–12 | breast parenchyma reconstructed chest wall components:
| 17–22–30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, F.A.; Serrano, J.; Cambeiro, M.; Aristu, J.; Asencio, J.M.; Rubio, I.; Delgado, J.M.; Ferrer, C.; Desco, M.; Pascau, J. Intra-Operative Electron Radiation Therapy: An Update of the Evidence Collected in 40 Years to Search for Models for Electron-FLASH Studies. Cancers 2022, 14, 3693. https://doi.org/10.3390/cancers14153693
Calvo FA, Serrano J, Cambeiro M, Aristu J, Asencio JM, Rubio I, Delgado JM, Ferrer C, Desco M, Pascau J. Intra-Operative Electron Radiation Therapy: An Update of the Evidence Collected in 40 Years to Search for Models for Electron-FLASH Studies. Cancers. 2022; 14(15):3693. https://doi.org/10.3390/cancers14153693
Chicago/Turabian StyleCalvo, Felipe A., Javier Serrano, Mauricio Cambeiro, Javier Aristu, Jose Manuel Asencio, Isabel Rubio, Jose Miguel Delgado, Carlos Ferrer, Manuel Desco, and Javier Pascau. 2022. "Intra-Operative Electron Radiation Therapy: An Update of the Evidence Collected in 40 Years to Search for Models for Electron-FLASH Studies" Cancers 14, no. 15: 3693. https://doi.org/10.3390/cancers14153693
APA StyleCalvo, F. A., Serrano, J., Cambeiro, M., Aristu, J., Asencio, J. M., Rubio, I., Delgado, J. M., Ferrer, C., Desco, M., & Pascau, J. (2022). Intra-Operative Electron Radiation Therapy: An Update of the Evidence Collected in 40 Years to Search for Models for Electron-FLASH Studies. Cancers, 14(15), 3693. https://doi.org/10.3390/cancers14153693







