Benefits of Physical Activity during and after Thyroid Cancer Treatment on Fatigue and Quality of Life: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Information Sources and Search Strategy
2.4. Selection Process, Data Collection Process and Effect Measures
2.5. Synthesis Methods
2.6. Evaluation of Study Quality
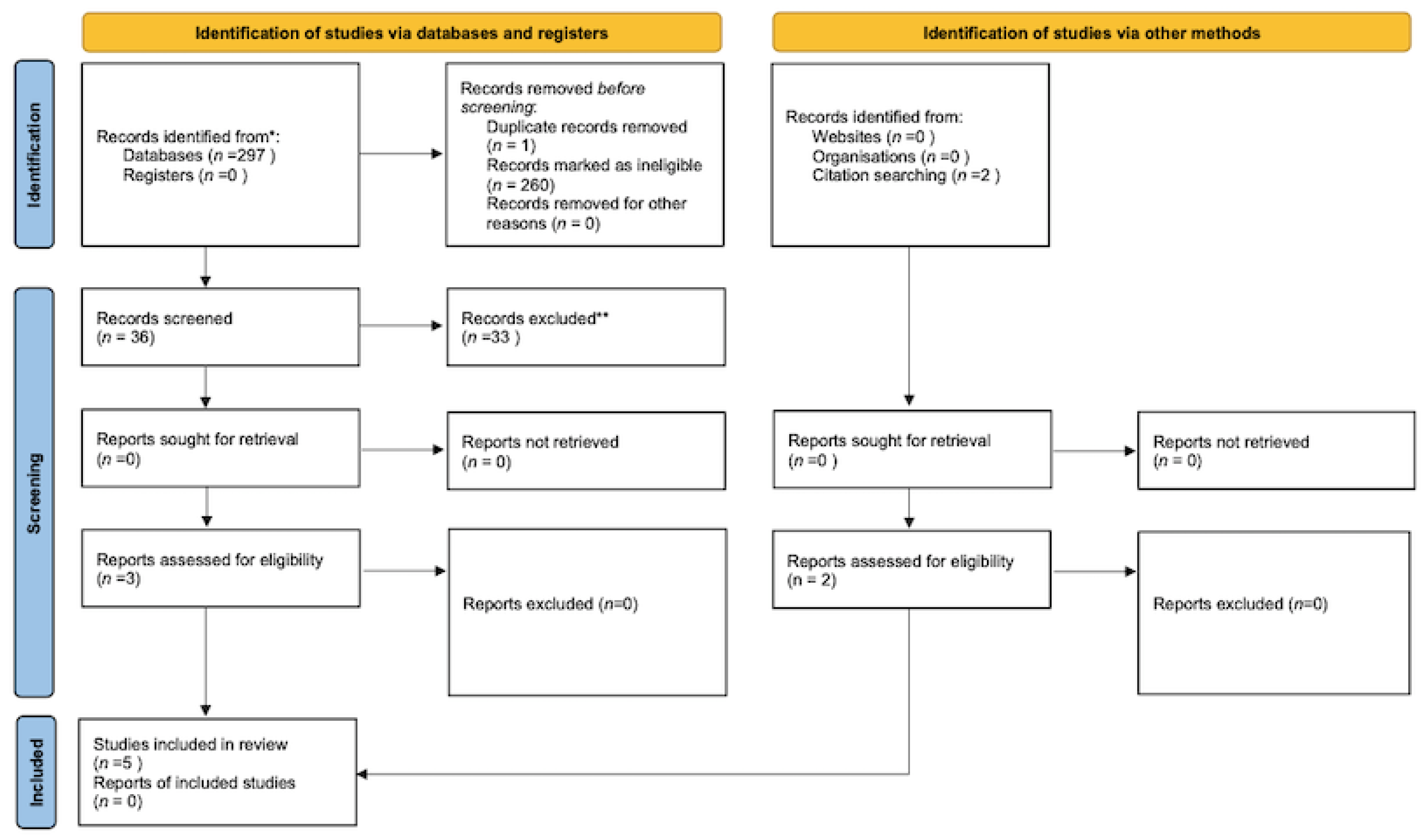
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Qol | quality of life |
| HRQoL | health-related quality of life |
| SCH | subclinical hyperthyroidism patients |
| SCH-Tr | trained patients |
| SCH-Sed | untrained patients |
| EU | euthyroid subjects |
| scTox | subclinical thyrotoxicosis |
| scTox-Tr | patients with subclinical thyrotoxicosis who adhered to the exercise intervention |
| scTox-Sed | patients with subclinical thyrotoxicosis who did not adhere to the exercise intervention |
| CG | control group |
| EG | experimental group |
| CFS | Chalder Fatigue Scale |
| WHOQOL-Bref | short version of the WHO quality of life questionnaire |
| SF-36 | Short Form 36 Health Survey |
| BFI | brief fatigue inventory |
| IPAQ-7 | International Physical Activity Questionnaire |
| EORTC QLQ-C30 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire. |
References
- SEER Cancer Statistics Review 1975–2016. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 30 March 2022).
- Street, W. Cancer Facts & Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019; 76p. [Google Scholar]
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009, 115, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.R.; Tucker, P. Incidence Trends for Papillary Thyroid Carcinoma and Their Correlation with Thyroid Surgery and Thyroid Fine-Needle Aspirate Cytology. Thyroid 2006, 16, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Thun, M.; Linet, M.S.; Cerhan, J.R.; Haiman, C.A.; Schottenfeld, D. Cancer Epidemiology and Prevention, 4th ed.; Oxford University Press: Oxford, UK, 2018; 1328p. [Google Scholar]
- Kitahara, C.M.; McCullough, M.L.; Franceschi, S.; Rinaldi, S.; Wolk, A.; Neta, G.; Olov Adami, H.; Anderson, K.; Andreotti, G.; Beane Freeman, L.E.; et al. Anthropometric Factors and Thyroid Cancer Risk by Histological Subtype: Pooled Analysis of 22 Prospective Studies. Thyroid 2016, 26, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s thyroid-cancer “epidemic”—Screening and overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, R.; Zhang, L.; Kebebew, E. Thyroid cancer gender disparity. Future Oncol. 2010, 6, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Batrinos, M.L. The problem of exogenous subclinical hyperthyroidism. Hormones 2006, 5, 119–125. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Ryder-Burbidge, C.J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Kao, T.-W.; Peng, T.-C.; Chang, Y.-W. Gender differences in the association between physical activity and health-related quality of life among community-dwelling elders. Aging Clin. Exp. Res. 2021, 33, 901–908. [Google Scholar] [CrossRef]
- Rossing, M.A.; Remler, R.; Voigt, L.F.; Wicklund, K.G.; Daling, J.R. Recreational physical activity and risk of papillary thyroid cancer (United States). Cancer Causes Control 2001, 112, 881–885. [Google Scholar] [CrossRef]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical activity and survival after breast cancer diagnosis. JAMA 2005, 293, 2479–2486. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.M.; Bennett, J.A.; Leo, M.C.; Torgrimson-Ojerio, B.; Luoh, S.-W.; Schwartzet, A. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: A randomized controlled trial. Osteoporos. Int. 2013, 24, 1637–1646. [Google Scholar] [CrossRef]
- De Luca, V.; Minganti, C.; Borrione, P.; Grazioli, E.; Cerulli, C.; Guerra, E.; Bonifacino, A.; Parisi, A. Effects of concurrent aerobic and strength training on breast cancer survivors: A pilot study. Public Health 2016, 136, 126–132. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Carrera-Ruiz, Á.; Díez-Fernández, D.M.; Esteban-Simón, A.; Maldonado-Quesada, M.; Moreno-Poza, N.; García-Martínez, M.D.M.; Alcaraz-García, C.; Vázquez-Sousa, R.; Moreno-Martos, H.; et al. Effects of a 12-week resistance and aerobic exercise program on muscular strength and quality of life in breast cancer survivors: Study protocol for the EFICAN randomized controlled trial. Medicine 2019, 98, e17625. [Google Scholar] [CrossRef]
- Rendeiro, J.A.; Rodrigues, C.A.M.P.; de Barros Rocha, L.; Rocha, R.S.B.; da Silva, M.L.; da Costa Cunha, K. Physical exercise and quality of life in patients with prostate cancer: Systematic review and meta-analysis. Support Care Cancer 2021, 29, 4911–4919. [Google Scholar] [CrossRef]
- Vigário, P.S.; Chachamovitz, D.S.; Cordeiro, M.F.; Teixeira, P.F.; Castro, C.L.; Oliveira, F.P.; Vaisman, M. Effects of physical activity on body composition and fatigue perception in patients on thyrotropin-suppressive therapy for differentiated thyroid carcinoma. Thyroid 2011, 21, 695–700. [Google Scholar] [CrossRef]
- Vigario, P.; de Oliveira Chachamovitz, D.S.; Teixeira, P.d.F.d.S.; Rocque, M.d.L.; Santos, M.L.d.; Vaisman, M. Exercise is associated with better quality of life in patients on TSH suppressive therapy with levothyroxine for differentiated thyroid carcinoma. Arq. Bras. Endocrinol. Metabol. 2014, 58, 274–281. [Google Scholar] [CrossRef][Green Version]
- Alhashemi, A.; Jones, J.M.; Goldstein, D.P.; Mina, D.S.; Thabane, L.; Sabiston, C.M.; Chang, E.K.; Brierley, J.D.; Sawka, A.M. An Exploratory Study of Fatigue and Physical Activity in Canadian Thyroid Cancer Patients. Thyroid 2017, 27, 1156–1163. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, M.; Ren, Y.; Liu, Q.; Zhao, G.; Cao, C.; Wang, H. Health-related quality of life of community thyroid cancer survivors in Hangzhou, China. Thyroid 2018, 28, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Gu, M.O.; Jung, J.H.; Hahm, J.R.; Kim, S.K.; Kim, J.H.; Woo, S.H. Efficacy of a Home-Based Exercise Program After Thyroidectomy for Thyroid Cancer Patients. Thyroid 2018, 28, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Castagna, M. New insight in the follow-up strategies of differentiated thyroid cancer. J. Endocrinol. Investig. 2012, 35, 36–39. [Google Scholar]
- Myers, J. The health benefits and economics of physical activity. Curr. Sports Med. Rep. 2008, 7, 314–316. [Google Scholar] [CrossRef]
- Fiore, M.; Cristaldi, A.; Okatyeva, V.; Lo Bianco, S.; Oliveri Conti, G.; Zuccarello, P.; Copat, C.; Caltabiano, R.; Cannizzaro, M.; Ferrante, M. Physical Activity and Thyroid Cancer Risk: A Case-Control Study in Catania (South Italy). Int. J. Environ. Res. Public Health 2019, 16, 1428. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwellet, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2009; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 28 May 2022).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Puzin, S.N.; Pogosyan, G.E.; Shurgaya, M.A.; Idrisova, L.S.; Lyalina, I.V.; Philippov, V.V. The gender and age characteristics of morbidity of thyroid cancer. Probl. Sotsialnoi Gig. Zdr. Istor. Med. 2020, 28, 928–933. [Google Scholar] [CrossRef]
- Finkel, D.; Andel, R.; Pedersen, N.L. Gender Differences in Longitudinal Trajectories of Change in Physical, Social, and Cognitive/Sedentary Leisure Activities. J. Gerontol. B Psychol. Sci. Soc. Sci. 2018, 73, 1491–1500. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; James, B.; Nagar, S.; Kaplan, S.; Seng, V.; Ahsan, H.; Angelos, P.; Kaplan, E.L.; Guerrero, M.A.; Kuo, J.H.; et al. Risk Factors for Decreased Quality of Life in Thyroid Cancer Survivors: Initial Findings from the North American Thyroid Cancer Survivorship Study. Thyroid 2015, 25, 1313–1321. [Google Scholar] [CrossRef]
- Bassett, A.J.; Ahlmen, A.; Rosendorf, J.M.; Romeo, A.A.; Erickson, B.J.; Bishop, M.E. The Biology of Sex and Sport. JBJS Rev. 2020, 8, e0140. [Google Scholar] [CrossRef]

| No. | Author, Year [Reference] | Place | Study Design | Excercise Type | Sample Size | Patient Characteristics | Questionnaire/Scale b | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | P. Vigario, 2011 [21] | Rio de Janeiro, Brazil | Prospective study | Training composed of 60 min of aerobic activity performed on a treadmill under the supervision of a physical education instructor and stretching exercises twice a week for a total of 12 weeks | Patients with scTox n = 36 (34 women, 2 men) scTox-Tr (n = 19) scTox-Sed (n = 17) CG n = 48 (41 women, 7 men) | Age scTox 48.0 (43.0–51.0) vs. CG 50.5 (40.2–56.0) (p > 0.05) Sex (male; %) scTox 5.6 vs. CG 14.6 (p > 0.05) Menopause (n; %) scTox 15 (41.7) vs. CG 22 (45.8) (p > 0.05) BMI (kg/m2) scTox 27.4(22.1–30.1) vs. CG 27.1 (23.4–30.3) (p > 0.05) Thyroid cancer histotype: DTC Both ScTox and CG patients had a sedentary lifestyle for at least 6 months before the study | CFS | CFS Baseline vs. after 3 months scTox-Tr 30.0 (24.0–35.0) vs. 17.0 (14.0–24.0) p < 0.05 scTox-Sed 27.0 (23.5–34.0 vs. 32.0 (23.5–36.5) p < 0.05 Muscular pain Baseline vs. after 3 months scTox-Tr 6.0 (4.0–6.0) vs. 3.0 (2.0–4.0) p < 0.05 scTox-Sed 5.0 (4.0–6.0) vs. 5.0 (4.0–6.0) p > 0.05 Qualification of fatigue Baseline vs. after 3 months scTox-Tr 5.0 (4.0–6.0) vs. 2.0 (2.0–4.0) p < 0.05 scTox-Sed 5.0 (4.0–6.5) vs. 5.0 (3.0–6.0) p > 0.05 |
| 2 | P. Vigario, 2014 [22] | Rio de Janeiro, Brazil | Prospective phase study | Training composed of 60 min of aerobic activity performed on a treadmill under the supervision of a physical education instructor and stretching exercises twice a week for a total of 12 weeks | SCH n = 33 (31 women, 2 men) SCH-Tr (n = 16) SCH-Sed (n = 17) EU n = 49 (42 women, 7 men) | Age (years): SCH 48 vs. EU 50 (p = 0.37) Sex (male; %) SCH 6.1 vs. EU 14.3 (p = 0.29) Menopause (yes; %) SCH 42.4 vs. EU 46.9 (p = 0.25) BMI (kg/m2) SCH 26.6 vs. EU 27 (p = 0.51) Thyroid cancer histotype: DTC SCH and EU sedentary lifestyle for at least six months prior the beginning of the study | WHOQoL-Bref Physical Psychological Social Environmental Physical Psychological Social Environmental SF-36 Physical function Role—physical Bodily pain General health Vitality Social functioning Role—emotional Mental health Physical function Role—physical Bodily pain General health Vitality Social functioning Role—emotional Mental health | Baseline SCH-Tr 12.3 vs. SCH-Sed 12.6; p = 0.01 SCH-Tr 13.7 vs. SCH-Sed 13.3; p = 0.59 SCH-Tr 14.7 vs. SCH-Sed 14.3; p = 0.70 SCH-Tr 11.8 vs. SCH-Sed 12; p = 0.20 After 3 months SCH-Tr 15.7 vs. SCH-Sed 12.6; p < 0.05 SCH-Tr 14.3 vs. SCH-Sed 13.3; p < 0.05 SCH-Tr 14.7 vs. SCH-Sed 14.7; p > 0.05 SCH-Tr 15.3 vs. SCH-Sed 12.0 p > 0.05 Baseline SCH-Tr 55 vs. SCH-Sed 62.5; p < 0.01 SCH-Tr 50 vs. SCH-Sed 50; p < 0.01 SCH-Tr 46 vs. SCH-Sed 51; p 0.01 SCH-Tr 57 vs. SCH-Sed 52; p < 0.01 SCH-Tr 45 vs. SCH-Sed 40; p < 0.01 SCH-Tr 75 vs. SCH-Sed 50; p < 0.13 SCH-Tr 33.3 vs. SCH-Sed 33.3; p < 0.01 SCH-Tr 56 vs. SCH-Sed 44; p < 0.01 After 3 months SCH-Tr 82.5 vs. SCH-Sed 60; p < 0.05 SCH-Tr 100 vs. SCH-Sed 50; p > 0.05 SCH-Tr 68 vs. SCH-Sed 51; p > 0.05 SCH-Tr 74.5 vs. SCH-Sed 52; p > 0.05 SCH-Tr 60 vs. SCH-Sed 45.0, p < 0.05 SCH-Tr 75 vs. SCH-Sed 62.5 p > 0.05 SCH-Tr 100 vs. SCH-Sed 33.3 p > 0.05 SCH-Tr 68 vs. SCH-Sed 52.2 p < 0.05 |
| 3 | A. Alhashemi, 2017 [23] | Toronto, Canada | Cross-sectional study | Various levels and intensities of physical activity (e.g., vigorous, moderate, walking) | Tot. n = 205 (152 women, 53 men) | Age, years (mean ± SD) 52.5 (13.5) Gender: Female: 152/205 (74.1%) Male: 53/205 (25.9%) Histotype: 91.5% DTC | BIF IPAQ-7 | Mean Global Fatigue Score ± SD 3.5 ± 2.4 out of 10 (10 is worst) Prevalence of moderate–severe fatigue (score 4.1–10): 41.4% (84/203) Weekly minutes spent performing physical activity vigorous, 0 (0–180); moderate, 60 (0–240); walking, 210 (90–840) |
| 4 | T. Wang, 2017 [24] | Hangzhou, China | Population-based survey | 30 min of moderate physical activity at least 5 days a week | Tot. n = 2755 EG n = 965 (772 women, 193 men) CG n = 1790 (Age- and sex-matched reference population) | Gender (n; %) Male (193; 20) Female (772; 80) Age (years): 49.7 ± 12.3 BMI (kg/m2): 23.2 ± 3.1 EG Thyroid cancer histotype (n;%) Papillary thyroid cancer: (881; 92.1%) Follicular thyroid cancer: (20; 2.1%) Undifferentiated thyroid cancer: (55; 5.8%) | SF-36 EORTC QLQ-C30 | Age (years), 14–24; female, 8 PCS: F (49.1 ± 11.4) MCS:F (46.8 ± 11.1) a Age (years), 25–34; female, 83; male, 30 _PCS: F (51.7 ± 8.7), M (52.8 ± 8.1) MCS: F (53.8 ± 7.9), M (52.4 ± 10.1) a Age(years) 35–44; female 166; male, 50 PCS: F (50.3 ± 9.8) a M (52.8 ± 6.6) MCS: F (51.5 ± 9.8) a M (53.7 ± 9.4) Age (years) 45–54; female, 97; male, 65 _PCS: F (48.2 ± 12.0) a M (50.2 ± 9.3) MCS: F (49.5 ± 9.9) a M (53.6 ± 10.1) Age (years) 55–64; female, 239; male, 32 PCS: F (48.6 ± 10.4)s M (51.1 ± 9.1) MCS: F (49.6 ± 9.0) a M (52.2 ± 8.7) Age (years) > 65; female, 79; male, 16 _PCS:; F (45.4 ± 12.1) M (46.9 ± 10.1) MCS: F (47.8 ± 10.4) M (51.0 ± 7.9) Global quality of life (mean ± SD) 73.2 ± 19.2 Functioning scale (mean ± SD) Role 94.5 ± 14.2 Physical 91.9 ± 11.2 Social 93.7 ± 14.5 Cognitive 90.8 ± 14.6 Emotional 91.6 ± 13.1 Symptom scale (item) (mean ± SD) Fatigue 14.8 ± 18.1 Pain 5.4 ± 12.8 Nausea/vomiting 1.5 ± 6.7 Dyspnea 6.9 ± 15.2 Loss of appetite 4.9 ± 12.9 Insomnia 12.9 ± 22.2 Constipation 5.8 ± 16.3 Diarrhea 2.8 ± 10.6 Financial difficulties 5.5 ± 15.5 |
| 5 | K. Kim, 2018 [25] | Gyeongsang, Korea | Quasi-experimental study with a non-equivalent control group | A home-based exercise program: 12 weeks of aerobic exercise (walking for 3–5 days a week for at least 150 min a week), resistance exercise (upper- and lower-body exercise twice a week, more than two sets per session) and flexibility exercises (5 min for 12 weeks before and after aerobic and resistance exercises) | Tot. n = 43 EG n = 22 (18 women, 4 men) CG n = 21 (18 women, 3 men) | Age EG 49.4 vs. CG 50.6 (p = 0.67) Gender EG (4 men, 18 women) vs. CG (3 men 3, 18 women) (p = 1.00) BMI EG 23.5 vs. CG 24.3 (p = 0.31) EG Thyroid papillary carcinoma EG and CG Lack of regular exercise before the study | BIF EORTC QLQ-C30 | Fatigue Pre exercise vs. post exercise EG 4.48 ± 1.46 vs. 3.52 ± 1.74 (p = 0.002) CG 4.37 ± 1.95 vs. 5.12 ± 1.71 (p = 0.001) Functional quality of life Pre exercise vs. post exercise EG 70.51 ± 12.33 vs. 82.73 ± 10.4 (p = 0.001) CG 71.85 ± 16.84 vs. 69.10 ± 14.79 (p = 0.06) Symptom quality of life Pre exercise vs. post exercise EG 78.03 ± 18.28 vs. 84.85 ± 13.76 (p = 0.01) CG 75.00 ± 21.08 vs. 67.46 ± 19.70 (p = 0.02) Overall health quality of life Pre exercise vs. post exercise EG 55.30 ± 18.64 vs. 72.35 ± 15.30 (p = 0.001) CG 56.35 ± 15.12 vs. 52.38 ± 18.85 (p = 0.23) |
| Author, Year | Selection | Comparability | Outcome | a Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativenes of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Outcome | Demonstration that Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of the Outcome | Was Follow-Up Long Enough for Outcome to Occur? | Adequacy of Follw-Up of Cohorts | ||
| P. Vigario, 2011 [21] | * | * | ** | * | 0 | 0 | * | * | 7/9 |
| P. Vigario, 2014 [22] | * | * | ** | * | 0 | 0 | * | * | 7/9 |
| K. Kim, 2018 [25] | * | * | * | 0 | 0 | * | * | 5/9 | |
| Author, Year | Selection | Comparability | Outcome | a Total | ||||
|---|---|---|---|---|---|---|---|---|
| Representativenes of Sample | Sample Size | Non-Respondents | Ascertainment of Exposure | The Subjects in Different Outcome Groups Are Comparable, Based on the Study Design or Analysis. Confounding Factors Are Controlled | Assessment of the Outcome | Statistical Test | ||
| A. Alhashemi, 2017 [23] | * | * | 0 | ** | * | 0 | * | 6/10 |
| T. Wang, 2017 [24] | * | * | 0 | ** | * | 0 | * | 6/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, M.; Distefano, G.; Distefano, C.; Copat, C.; Grasso, A.; Oliveri Conti, G.; Cristaldi, A.; Fiore, M. Benefits of Physical Activity during and after Thyroid Cancer Treatment on Fatigue and Quality of Life: A Systematic Review. Cancers 2022, 14, 3657. https://doi.org/10.3390/cancers14153657
Ferrante M, Distefano G, Distefano C, Copat C, Grasso A, Oliveri Conti G, Cristaldi A, Fiore M. Benefits of Physical Activity during and after Thyroid Cancer Treatment on Fatigue and Quality of Life: A Systematic Review. Cancers. 2022; 14(15):3657. https://doi.org/10.3390/cancers14153657
Chicago/Turabian StyleFerrante, Margherita, Giulia Distefano, Carlo Distefano, Chiara Copat, Alfina Grasso, Gea Oliveri Conti, Antonio Cristaldi, and Maria Fiore. 2022. "Benefits of Physical Activity during and after Thyroid Cancer Treatment on Fatigue and Quality of Life: A Systematic Review" Cancers 14, no. 15: 3657. https://doi.org/10.3390/cancers14153657
APA StyleFerrante, M., Distefano, G., Distefano, C., Copat, C., Grasso, A., Oliveri Conti, G., Cristaldi, A., & Fiore, M. (2022). Benefits of Physical Activity during and after Thyroid Cancer Treatment on Fatigue and Quality of Life: A Systematic Review. Cancers, 14(15), 3657. https://doi.org/10.3390/cancers14153657










