Comparison between Endoscopic Submucosal Dissection and Surgery in Patients with Early Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
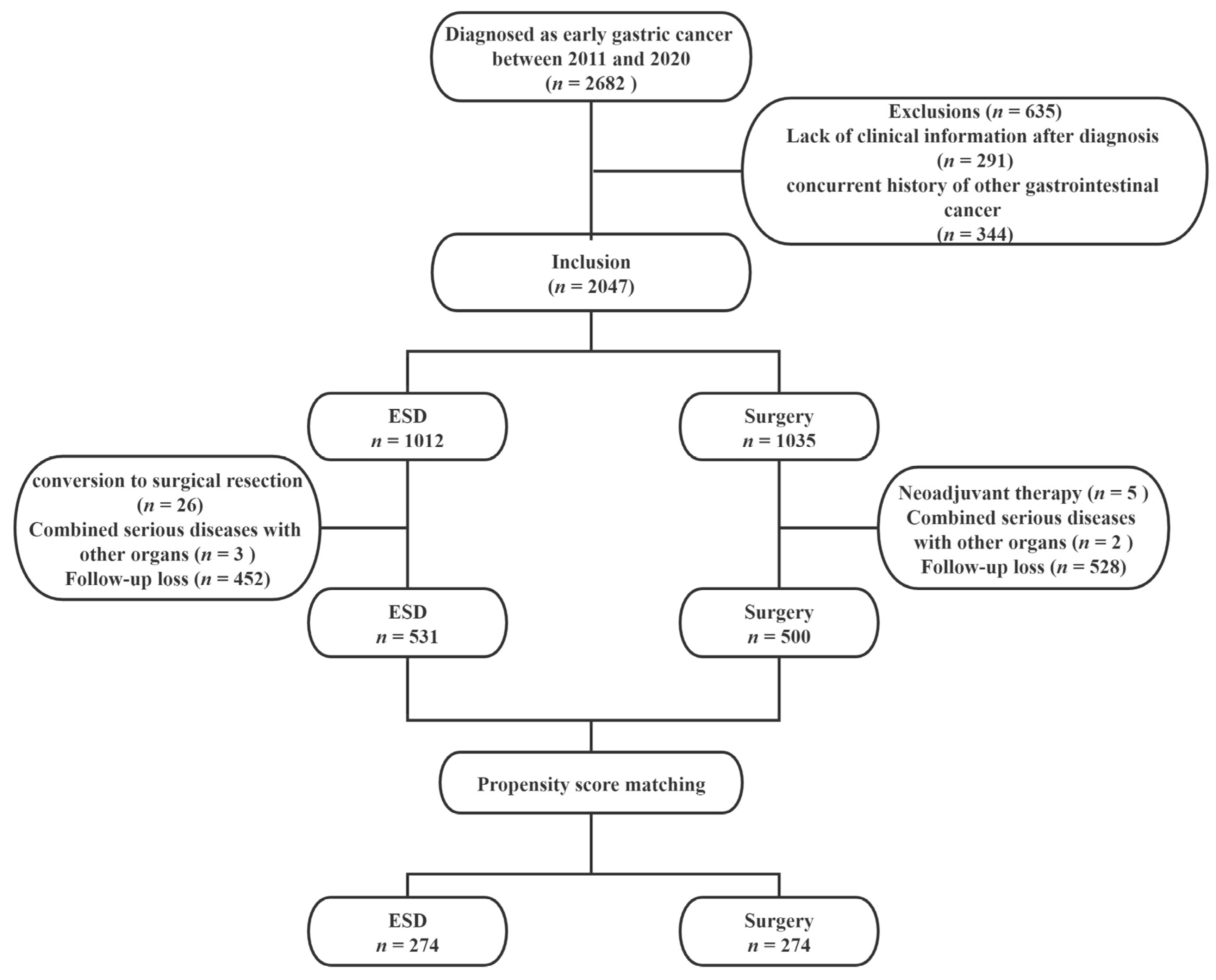
2.1. Patients
2.2. Data Collection
2.3. Treatment Procedures and Follow-Up
2.4. Definitions and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes and Treatment-Related Events
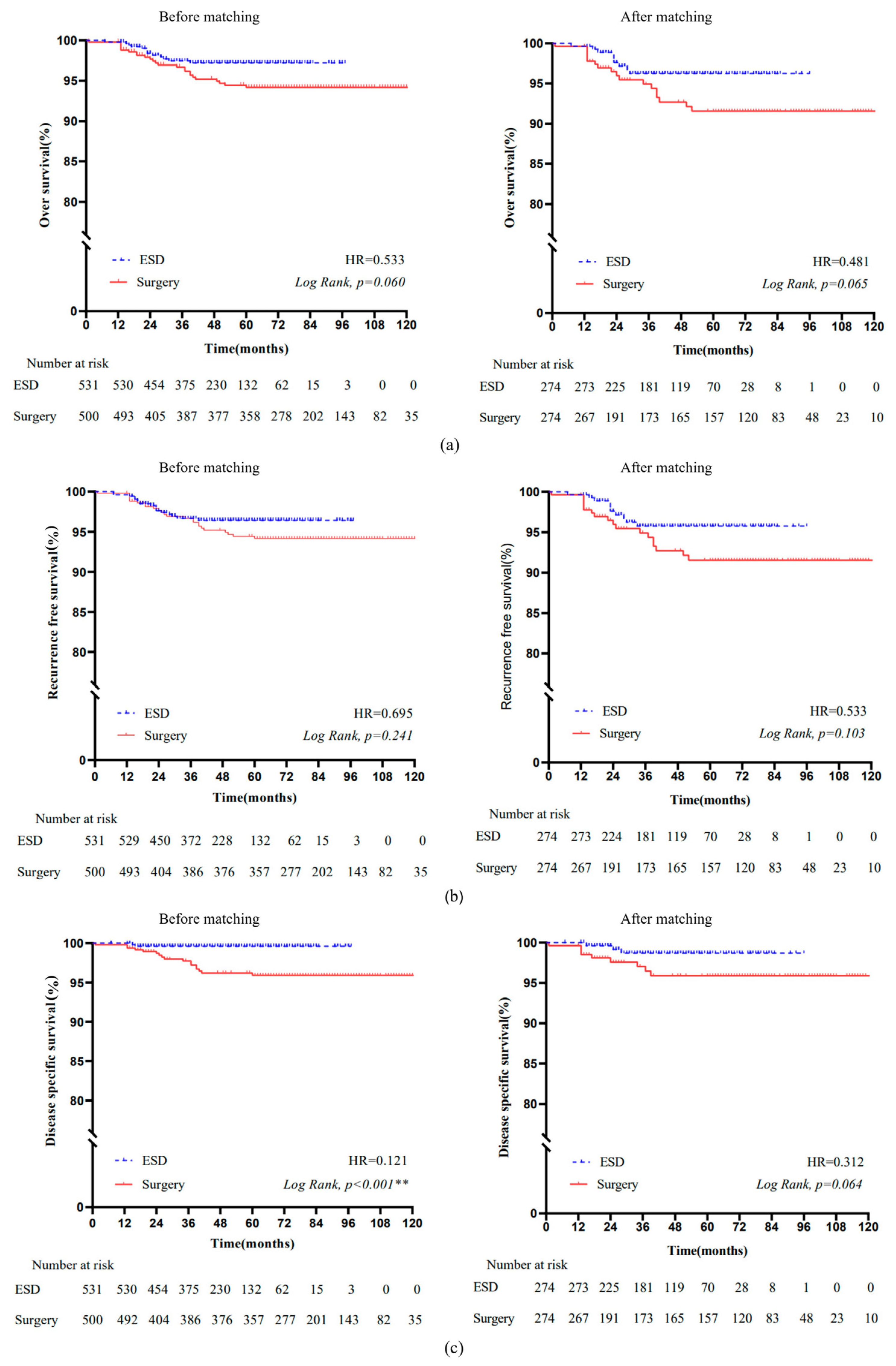
3.3. Survival Analysis
3.3.1. Overall Survival
3.3.2. Recurrence-Free Survival
3.4. Disease-Specific Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wang, S.; Feng, T.; Yan, M.; Yuan, F.; Zhu, Z.; Li, T.; Zhu, Z. Nomograms Involving HER2 for Predicting Lymph Node Metastasis in Early Gastric Cancer. Front Cell Dev. Biol. 2021, 9, 781824. [Google Scholar] [CrossRef] [PubMed]
- Draganov, P.V.; Wang, A.Y.; Othman, M.O.; Fukami, N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig. Endosc. 2021, 33, 4–20. [Google Scholar] [CrossRef]
- Fleischmann, C.; Probst, A.; Ebigbo, A.; Faiss, S.; Schumacher, B.; Allgaier, H.P.; Dumoulin, F.L.; Steinbrueck, I.; Anzinger, M.; Marienhagen, J.; et al. Endoscopic Submucosal Dissection in Europe: Results of 1000 Neoplastic Lesions From the German Endoscopic Submucosal Dissection Registry. Gastroenterology 2021, 161, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Fiorillo, C.; Longo, F.; Laterza, V.; Rosa, F.; Cina, C.; Menghi, R.; Tortorelli, A.P.; Barbaro, F.; Pecere, S.; et al. Propensity score-matched comparison of short- and long-term outcomes between surgery and endoscopic submucosal dissection (ESD) for intestinal type early gastric cancer (EGC) of the middle and lower third of the stomach: A European tertiary referral center experience. Surg. Endosc. 2021, 35, 2592–2600. [Google Scholar] [CrossRef]
- Pourmousavi, M.K.; Wang, R.; Kerdsirichairat, T.; Kamal, A.; Akshintala, V.S.; Hajiyeva, G.; Lopimpisuth, C.; Hanada, Y.; Kumbhari, V.; Singh, V.K.; et al. Comparable Cancer-Specific Mortality of Patients With Early Gastric Cancer Treated With Endoscopic Therapy vs Surgical Resection. Clin. Gastroenterol. Hepatol. 2020, 18, 2824–2832. [Google Scholar] [CrossRef]
- Li, H.; Feng, L.Q.; Bian, Y.Y.; Yang, L.L.; Liu, D.X.; Huo, Z.B.; Zeng, L. Comparison of endoscopic submucosal dissection with surgical gastrectomy for early gastric cancer: An updated meta-analysis. World J. Gastrointest. Oncol. 2019, 11, 161–171. [Google Scholar] [CrossRef]
- Choi, I.J.; Lee, J.H.; Kim, Y.I.; Kim, C.G.; Cho, S.J.; Lee, J.Y.; Ryu, K.W.; Nam, B.H.; Kook, M.C.; Kim, Y.W. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest. Endosc. 2015, 81, 333–341. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Markar, S.R.; Phillips, A.W.; Salti, G.I.; Dahdaleh, F.S. Local Endoscopic Resection is Inferior to Gastrectomy for Early Clinical Stage T1a and T1b Gastric Adenocarcinoma: A Propensity-Matched Study. Ann. Surg. Oncol. 2021, 28, 2992–2998. [Google Scholar] [CrossRef]
- Park, C.H.; Yang, D.H.; Kim, J.W.; Kim, J.H.; Kim, J.H.; Min, Y.W.; Lee, S.H.; Bae, J.H.; Chung, H.; Choi, K.D.; et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Intest. Res. 2021, 19, 127–157. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, A.; Brenkman, H.J.F.; Seesing, M.F.J.; Haverkamp, L.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; Stoot, J.; Tegels, J.J.W.; Wijnhoven, B.P.L.; Lagarde, S.M.; et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, Y.; Kikuchi, H.; Takeuchi, H. Function-Preserving Gastrectomy for Early Gastric Cancer. Cancers 2021, 13, 2683–2692. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef] [Green Version]
- Yim, K.; Jang, W.M.; Lee, S.H. Modified Tumor Budding as a Better Predictor of Lymph Node Metastasis in Early Gastric Cancer: Possible Real-World Applications. Cancers 2021, 13, 3405. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011, 14, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Japanese Gastric Cancer, A. Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1998, 1, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, N.; Bourke, M.J. ESD, not EMR, should be the first-line therapy for early gastric neoplasia. Gut 2020, 69, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Hirabayashi, S.; Oda, I.; Ono, H.; Nashimoto, A.; Isobe, Y.; Miyashiro, I.; Tsujitani, S.; Seto, Y.; Fukagawa, T.; et al. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer 2017, 20, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Draganov, P.V.; Aihara, H.; Karasik, M.S.; Ngamruengphong, S.; Aadam, A.A.; Othman, M.O.; Sharma, N.; Grimm, I.S.; Rostom, A.; Elmunzer, B.J.; et al. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology 2021, 160, 2317–2327. [Google Scholar] [CrossRef]
- Cao, S.; Zou, T.; Sun, Q.; Liu, T.; Fan, T.; Yin, Q.; Fan, X.; Jiang, J.; Raymond, D.; Wang, Y.; et al. Safety and long-term outcomes of early gastric cardiac cancer treated with endoscopic submucosal dissection in 499 Chinese patients. Ther. Adv. Gastroenterol. 2020, 13, 1756284820966929. [Google Scholar] [CrossRef]
- Abdelfatah, M.M.; Barakat, M.; Lee, H.; Kim, J.J.; Uedo, N.; Grimm, I.; Othman, M.O. The incidence of lymph node metastasis in early gastric cancer according to the expanded criteria in comparison with the absolute criteria of the Japanese Gastric Cancer Association: Asystematic review of the literature and meta-analysis. Gastrointest. Endosc. 2018, 87, 338–347. [Google Scholar] [CrossRef]
- Nishizawa, T.; Yahagi, N. Long-Term Outcomes of Using Endoscopic Submucosal Dissection to Treat Early Gastric Cancer. Gut Liver 2018, 12, 119–124. [Google Scholar] [CrossRef] [Green Version]
- The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [CrossRef] [Green Version]
- Libânio, D.; Braga, V.; Ferraz, S.; Castro, R.; Lage, J.; Pita, I.; Ribeiro, C.; Abreu De Sousa, J.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Prospective comparative study of endoscopic submucosal dissection and gastrectomy for early neoplastic lesions including patients’ perspectives. Endoscopy 2019, 51, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Ding, L.; Qiu, X.; Meng, F. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: A systematic review and meta-analysis. Int. J. Surg. 2020, 73, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.K.; Kim, G.H.; Lee, B.E.; Park, D.Y.; Song, G.A.; Kim, D.H.; Jeon, T.Y. Long-term outcome of endoscopic submucosal dissection is comparable to that of surgery for early gastric cancer: A propensity-matched analysis. Gastric Cancer 2018, 21, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.Y.; Zhong, Q.; Wang, W.; Chen, S.; Li, P.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Lu, J.; Cao, L.L.; et al. Prognosis of Young Survivors of Gastric Cancer in China and the U.S.: Determining Long-Term Outcomes Based on Conditional Survival. Oncologist 2019, 24, e260–e274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyo, J.H.; Lee, H.; Min, B.H.; Lee, J.H.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, K.M.; Ahn, J.H.; et al. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am. J. Gastroenterol. 2016, 111, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Li, S.; Bai, F.; Xie, H.; Shan, H.; Liu, Z.; Ma, T.; Tang, X.; Tang, H.; et al. Risk Factors of Lymph Node Metastasis and Its Prognostic Significance in Early Gastric Cancer: A Multicenter Study. Front Oncol. 2021, 11, 649035. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Gotoda, T.; Kanno, T.; Yuan, Y.; Koike, T.; Moayyedi, P.; Masamune, A. Prevalence and risk factors for lymph node metastasis after noncurative endoscopic resection for early gastric cancer: A systematic review and meta-analysis. J. Gastroenterol. 2020, 55, 742–753. [Google Scholar] [CrossRef]
- Kim, S.M.; Min, B.H.; Ahn, J.H.; Jung, S.H.; An, J.Y.; Choi, M.G.; Sohn, T.S.; Bae, J.M.; Kim, S.; Lee, H.; et al. Nomogram to predict lymph node metastasis in patients with early gastric cancer: A useful clinical tool to reduce gastrectomy after endoscopic resection. Endoscopy 2020, 52, 435–443. [Google Scholar] [CrossRef]
- Dahan, M.; Pauliat, E.; Liva-Yonnet, S.; Brischoux, S.; Legros, R.; Tailleur, A.; Carrier, P.; Charissoux, A.; Valgueblasse, V.; Loustaud-Ratti, V.; et al. What is the cost of endoscopic submucosal dissection (ESD)? A medico-economic study. United Eur. Gastroenterol. J. 2019, 7, 138–145. [Google Scholar] [CrossRef]
- Park, K.B.; Jeon, C.H.; Seo, H.S.; Jung, Y.J.; Song, K.Y.; Park, C.H.; Lee, H.H. Operative safety of curative gastrectomy after endoscopic submucosal dissection (ESD) for early gastric cancer—1:2 propensity score matching analysis: A retrospective single-center study (cohort study). Int. J. Surg. 2020, 80, 124–128. [Google Scholar] [CrossRef]
- Jung, D.H.; Youn, Y.H.; Kim, J.H.; Park, J.J.; Park, H. Secondary endoscopic submucosal dissection for locally recurrent or incompletely resected gastric neoplasms. World J. Gastroenterol. 2018, 24, 3776–3785. [Google Scholar] [CrossRef]
- Shimada, S.; Sawada, N.; Oae, S.; Seki, J.; Takano, Y.; Nakahara, K.; Takehara, Y.; Mukai, S.; Ishida, F.; Kudo, S.E. Impact of non-curative endoscopic submucosal dissection on short- and long-term outcome of subsequent laparoscopic gastrectomy for pT1 gastric cancer. Surg. Endosc. 2021, 36, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Lie, H.C.; Rueegg, C.S.; Fosså, S.D.; Loge, J.H.; Ruud, E.; Kiserud, C.E. Limited evidence of non-response bias despite modest response rate in a nationwide survey of long-term cancer survivors-results from the NOR-CAYACS study. J. Cancer Surviv. 2019, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
| ESD N = 531 | Surgery N = 500 | p-Value | ESD N = 274 | Surgery N = 274 | p-Value | |
|---|---|---|---|---|---|---|
| Age (mean ± SD; years) | 61.6 ± 10.7 | 61.4 ± 10.6 | 0.196 | 61.1 ± 10.7 | 61.6 ± 10.7 | 0.448 |
| Sex, n (%) | 0.506 | 0.851 | ||||
| Male | 368 (69.3%) | 356 (71.2%) | 192 (70.1%) | 194 (70.8%) | ||
| Female | 163 (30.7%) | 144 (28.8%) | 82 (29.9%) | 80 (29.2%) | ||
| Lifestyle, n (%) | ||||||
| Cigarette | 104 (19.6%) | 108 (21.6%) | 0.424 | 54 (19.7%) | 53 (19.3%) | 0.948 |
| Alcohol Positive family history, n (%) | 89 (16.8%) 22 (4.1%) | 89 (17.8%) 39 (7.8%) | 0.659 0.013 | 46 (16.8%) 13 (4.7%) | 44 (16.1%) 21 (6.6%) | 0.818 0.355 |
| Tumor location, n (%) | 0.005 | 0.390 | ||||
| Cardia | 209 (39.4%) | 143 (28.6%) | 102 (37.2%) | 94 (34.3%) | ||
| Fundus | 16 (3.0%) | 13 (2.6%) | 9 (3.3%) | 8 (2.9%) | ||
| Body | 91 (17.1%) | 100 (20.0%) | 49 (17.9%) | 54 (19.7%) | ||
| Antrum | 145 (27.3%) | 154 (30.8%) | 87 (31.7%) | 77 (28.1%) | ||
| Incisura angularis or pylorus | 70 (13.2%) | 90 (18.0%) | 27 (9.9%) | 41 (15.0%) | ||
| Size (mean ± SD; cm) | 2.6 ± 1.5 | 2.3 ± 1.1 | <0.001 ** | 2.6 ± 1.5 | 2.4 ± 1.2 | 0.103 |
| Tumor morphology, n (%) | 0.932 | 0.913 | ||||
| Elevated | 86 (16.2%) | 80 (16.0%) | 52 (19.0%) | 51 (18.6%) | ||
| Flat or depressed | 445 (83.8%) | 420 (84.0%) | 222 (81.0%) | 223 (81.4%) | ||
| Tumor infiltration, n (%) | 0.271 | 0.899 | ||||
| Mucosa | 450 (84.7%) | 411 (82.2%) | 239 (87.2%) | 238 (86.9%) | ||
| Submucosa | 81 (15.3%) | 89 (17.8%) | 35 (12.8%) | 36 (13.1%) | ||
| Ulceration, n (%) | 32 (6.0%) | 237 (47.4%) | <0.001 ** | 32 (11.7%) | 48 (17.5%) | 0.053 |
| Tumor differentiation grade, n (%) | <0.001 ** | 0.062 | ||||
| Well-differentiated | 363 (68.3%) | 137 (15.3%) | 122 (44.5%) | 114 (41.6%) | ||
| Moderately differentiated | 156 (29.4%) | 148 (15.3%) | 140 (51.1%) | 134 (48.9%) | ||
| Poorly differentiated | 12 (2.3%) | 215 (15.3%) | 12 (4.4%) | 26 (9.5%) | ||
| Lymphovascular invasion, n (%) | 16 (3.0%) | 32 (6.4%) | 0.010 | 12 (4.4%) | 13 (4.7%) | 0.838 |
| ESD N = 531 | Surgery N = 500 | p-Value | ESD N = 274 | Surgery N = 274 | p-Value | |
|---|---|---|---|---|---|---|
| Estimated blood loss (mL) | <0.001 ** | <0.001 ** | ||||
| ≤50 | 511 (96.2%) | 68 (13.6%) | 265 (96.7%) | 50 (18.2%) | ||
| >50 | 20 (3.8%) | 432 (86.4%) | 9 (3.6%) | 224 (86.3%) | ||
| Operative time (min), median (IQR) | 73.5 (55.0–118.0) | 195.5 (153.0–232.0) | <0.001 ** | 94.0 (60.0–120.0) | 197.0 (153.0–232.0) | <0.001 ** |
| Hospital duration (day), median (IQR) | 9.0 (8.0–11.0) | 17.0 (14.0–20.0) | <0.001 ** | 10.3 (8.0–11.0) | 19.1 (14.0–21.0) | <0.001 ** |
| Hospital cost (USD), median (IQR) | 3121.1 (2693.1–3658.0) | 6142.2 (5005.1–7211.7) | <0.001 ** | 3304.2 (2660.9–3438.9) | 6507.6 (5157.7–7357.6) | <0.001 ** |
| Resection margin | 0.092 | 0.082 | ||||
| R0 resection | 528 (99.4%) | 500 (100.0%) | 271 (98.9%) | 274 (100.0%) | ||
| R1 resection | 3 (0.6%) | 0 (0.0%) | 3 (1.1%) | 0 (0.0%) | ||
| Recurrence | 4 (0.8%) | 1 (0.2%) | 0.201 | 1 (0.4%) | 0 (0.0%) | 0.317 |
| Adverse events, n (%) | 7 (1.3%) | 26 (5.2%) | <0.001 ** | 1 (0.4%) | 16 (5.8%) | <0.001 ** |
| Adverse Events | Total N = 7 | Age | Sex | Location | Size (cm) | Infiltration | Estimated Blood Loss (mL) | Operative Time (min) | Hospital Duration (Day) | Hospital Cost (USD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gastropathy | 2 (28.6%) | 59 | Male | Cardia | 1.5 | Mucosa | 10 | 55 | 12 | 1203.9 |
| 53 | Male | Cardia | 1.0 | Mucosa | 5 | 70 | 9 | 3162.0 | ||
| Bleeding | 4 (57.1%) | 84 | Female | Body | 3.5 | Mucosa | 2 | 40 | 14 | 3964.1 |
| 67 | Male | Cardia | 1.5 | Mucosa | 10 | 60 | 12 | 3861.5 | ||
| 63 * | Male | Cardia | 4.0 | Mucosa | 10 | 93 | 34 | 8450.7 | ||
| 52 | Male | Antrum | 3.0 | Mucosa | 10 | 120 | 16 | 8708.5 | ||
| Perforation | 1 (14.3%) | 54 * | Male | Cardia | 4.5 | Submucosa | 10 | 140 | 12 | 5934.4 |
| Adverse Events | Total N = 26 | Age | Sex | Location | Size (cm) | Infiltration | Estimated Blood Loss (mL) | Operative Time (min) | Hospital Duration (day) | Hospital Cost (USD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Delayed gastric emptying | 8 (30.9%) | 55 | Male | Antrum | 2.0 | Mucosa | 200 | 214 | 39 | 12,373.8 |
| 69 | Female | Body | 3.0 | Submucosa | 200 | 93 | 37 | 9534.3 | ||
| 85 | Male | Antrum | 2.0 | Mucosa | 100 | 135 | 37 | 16,754.6 | ||
| 64 | Male | Incisura angularis | 1.2 | Mucosa | 200 | 150 | 40 | 7747.8 | ||
| 66 | Female | Body | 1.0 | Submucosa | 100 | 295 | 40 | 11,897.8 | ||
| 54 | Male | Incisura angularis | 1.2 | Submucosa | 100 | 205 | 42 | 10,712.3 | ||
| 68 | Female | Antrum | 1.0 | Submucosa | 50 | 125 | 51 | 9484.9 | ||
| 63 | Male | Body | 3.0 | Submucosa | 100 | 246 | 14 | 5390.5 | ||
| Anastomotic leakage | 3 (11.5%) | 63 | Male | Body | 5.0 | Submucosa | 100 | 150 | 54 | 8917.2 |
| 71 | Female | Cardia | 3.5 | Submucosa | 200 | 317 | 36 | 9378.0 | ||
| 60 | Male | Antrum | 1.5 | Mucosa | 100 | 200 | 41 | 8457.5 | ||
| Anastomotic leakage + gastroparesis | 1 (3.8%) | 70 | Female | Body | 2.0 | Mucosa | 200 | 140 | 99 | 13,457.5 |
| Ileus | 4 (15.4%) | 61 | Male | Antrum | 3.0 | Submucosa | 100 | 265 | 47 | 13,814.6 |
| 37 | Male | Antrum | 1.5 | Mucosa | 100 | 180 | 23 | 5488.9 | ||
| 78 | Female | Antrum | 8.0 | Mucosa | 100 | 215 | 25 | 7170.1 | ||
| 67 | Male | Incisura angularis | 2.0 | Submucosa | 100 | 205 | 14 | 7371.2 | ||
| Bleeding | 2 (7.7%) | 63 | Male | Body | 2.5 | Mucosa | 100 | 154 | 44 | 6542.9 |
| 79 | Male | Cardia | 2.5 | Submucosa | 100 | 180 | 47 | 7887.4 | ||
| Infection | 3 (11.5%) | 69 | Male | Incisura angularis | 1.0 | Submucosa | 100 | 220 | 42 | 6533.3 |
| Severe infection ** | 78 | Male | Antrum | 5.0 | Mucosa | 100 | 155 | 28 | 21,170.6 | |
| Severe infection * | 60 | Male | Antrum | 2.0 | Submucosa | 300 | 235 | 25 | 21,101.8 | |
| Fever | 4 (15.4%) | 72 | Female | Cardia | 1.5 | Submucosa | 100 | 187 | 29 | 11,168.6 |
| 59 | Male | Cardia | 1.5 | Mucosa | 100 | 190 | 20 | 8965.7 | ||
| 67 | Male | Cardia | 2.0 | Mucosa | 200 | 189 | 46 | 11,228.6 | ||
| 70 | Female | Cardia | 2.5 | Submucosa | 200 | 305 | 29 | 9630.1 | ||
| Other | 1 (3.8%) | 86 | Male | Antrum | 2.0 | Mucosa | 200 | 162 | 28 | 9204.0 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Treatment method (ESD vs. surgery) | 0.48 (0.22–1.07) | 0.072 | 0.10 (0.04–0.26) | <0.001 ** |
| Age | 1.20 (1.15–1.25) | <0.001 ** | 1.24 (1.15–1.29) | <0.001 ** |
| Sex, male = 1 | 0.31 (0.09–1.03) | 0.055 | ||
| Cigarette, no = 1 | 1.60 (0.55–4.64) | 0.384 | ||
| Alcohol, no = 1 | 2.64 (0.63–11.15) | 0.286 | ||
| Positive family history | 22.01 (0.02–26.86) | 0.394 | ||
| Tumor location | 0.593 | |||
| Cardia | 1.00 | _ | ||
| Fundus | 0.32 (0.07–1.44) | _ | ||
| Body | 1.04 (0.44–2.47) | _ | ||
| Antrum | 1.24 (0.44–3.53) | _ | ||
| Incisura angularis or pylorus | 0.02 (0.27–1.54) | _ | ||
| Tumor size | 1.49 (1.22–1.82) | <0.001 ** | 1.37 (1.11–1.69) | 0.003 |
| Tumor morphology | 0.505 | |||
| Elevated | 1.00 | _ | ||
| Flat or depressed | 1.44 (0.50–4.15) | _ | ||
| Submucosal infiltration | 2.47 (1.12–1.81) | 0.025 | ||
| Tumor differentiation grade | ||||
| Well-differentiated | 1.00 | _ | ||
| Moderately differentiated | 1.68 (0.69–4.11) | 0.257 | ||
| Poorly differentiated | 5.02 (1.88–13.41) | 0.001 | 3.21 (1.13–9.10) | 0.022 |
| Ulceration, no = 1 | 2.50 (0.18–0.89) | 0.025 | ||
| Lymphovascular invasion, no = 1 | 1.97 (0.89–4.40) | 0.097 | ||
| Resection margin | ||||
| R0 resection | 1.00 | _ | ||
| R1 resection | 5.88 (0.06–1.47) | 0.001 | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Treatment method (ESD vs. surgery) | 0.53 (0.25–1.16) | 0.111 | ||
| Age | 1.18 (1.13–1.25) | <0.001 ** | 1.24 (1.18–1.30) | <0.001 ** |
| Sex, male = 1 | 0.30 (0.09–0.98) | 0.056 | ||
| Cigarette, no = 1 | 1.27 (0.48–3.35) | 0.624 | ||
| Alcohol, no = 1 | 2.75 (0.65–11.57) | 0.169 | ||
| Positive family history | 22.02 (0.02–23.49) | 0.385 | ||
| Tumor location | 0.602 | |||
| Cardia | 1.00 | _ | ||
| Fundus | 0.43 (0.25–1.56) | _ | ||
| Body | 0.32 (0.07–1.44) | _ | ||
| Antrum | 0.92 (0.38–2.26) | _ | ||
| Incisura angularis or pylorus | 1.24 (0.44–3.53) | _ | ||
| Tumor size | 1.52 (1.24–1.85) | <0.001 ** | 1.36 (1.07–1.73) | 0.012 |
| Tumor morphology | 0.853 | |||
| Elevated | 1.00 | _ | ||
| Flat or depressed | 1.40 (0.42–2.99) | _ | ||
| Submucosal infiltration | 2.20 (1.96–5.04) | 0.061 | ||
| Tumor differentiation grade | ||||
| Well-differentiated | 1.00 | _ | ||
| Moderately differentiated | 1.92 (0.76–4.88) | 0.170 | ||
| Poorly differentiated | 5.72 (2.07–15.79) | 0.001 | 3.41 (1.17–9.97) | 0.025 |
| Ulceration, no = 1 | 2.17 (0.20–1.04) | 0.062 | ||
| Lymphovascular invasion, no = 1 | 2.21 (0.96–5.09) | 0.063 | 9.61 (3.21–28.79) | <0.001 ** |
| Resection margin | ||||
| R0 resection | 1.00 | _ | ||
| R1 resection | 5.88 (0.06–0.50) | 0.001 | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p-Value | |
| Treatment method (ESD vs. surgery) | 0.31 (0.08–1.15) | 0.080 | 0.10 (0.02–0.46) | 0.003 |
| Age | 1.19 (1.11–1.27) | <0.001 ** | 1.24 (1.15–1.33) | <0.001 ** |
| Sex, male = 1 | 0.22 (0.03–1.73) | 0.151 | ||
| Cigarette, no = 1 | 2.99 (0.39–23.24) | 0.293 | ||
| Alcohol, no = 1 | 2.28 (0.57–3.41) | 0.431 | ||
| Positive family history | 21.99 (0.10–9.54) | 0.572 | ||
| Tumor location | 0.951 | |||
| Cardia | 1.00 | _ | ||
| Fundus | 0.01 (0.02–0.54) | _ | ||
| Body | 0.37 (0.13–1.24) | _ | ||
| Antrum | 0.77 (0.22–2.62) | _ | ||
| Incisura angularis or pylorus | 0.43 (0.05–3.47) | _ | ||
| Tumor size | 1.48 (1.10–2.00) | 0.010 | 1.38 (1.01–1.89) | 0.041 |
| Tumor morphology | 0.649 | |||
| Elevated | 1.00 | _ | ||
| Flat or depressed | 0.74 (0.20–2.73) | _ | ||
| Submucosal infiltration | 7.36 (2.33–23.21) | 0.001 | 5.75 (1.73–19.04) | 0.004 |
| Tumor differentiation grade | ||||
| Well-differentiated | 1.00 | |||
| Moderately differentiated | 7.82 (0.96–6.61) | 0.054 | ||
| Poorly differentiated | 19.40 (0.96–6.61) | 0.008 | ||
| Ulceration, no = 1 | 0.27 (0.09–0.86) | 0.027 | ||
| Lymphovascular invasion, no = 1 | 1.53 (0.41–5.67) | 0.144 | ||
| Resection margin | ||||
| R0 resection | 1.00 | _ | ||
| R1 resection | 3.22 (0.04–2.44) | 0.269 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, M.; Sheng, Y.; Wu, M.; Wang, S.; Zhang, K. Comparison between Endoscopic Submucosal Dissection and Surgery in Patients with Early Gastric Cancer. Cancers 2022, 14, 3603. https://doi.org/10.3390/cancers14153603
Qian M, Sheng Y, Wu M, Wang S, Zhang K. Comparison between Endoscopic Submucosal Dissection and Surgery in Patients with Early Gastric Cancer. Cancers. 2022; 14(15):3603. https://doi.org/10.3390/cancers14153603
Chicago/Turabian StyleQian, Meng, Yuan Sheng, Min Wu, Song Wang, and Kaiguang Zhang. 2022. "Comparison between Endoscopic Submucosal Dissection and Surgery in Patients with Early Gastric Cancer" Cancers 14, no. 15: 3603. https://doi.org/10.3390/cancers14153603
APA StyleQian, M., Sheng, Y., Wu, M., Wang, S., & Zhang, K. (2022). Comparison between Endoscopic Submucosal Dissection and Surgery in Patients with Early Gastric Cancer. Cancers, 14(15), 3603. https://doi.org/10.3390/cancers14153603






