Systemic Mastocytosis and Other Entities Involving Mast Cells: A Practical Review and Update
Abstract
:Simple Summary
Abstract
1. Introduction
2. Updates in Diagnosis and Subclassification of Systemic Mastocytosis
2.1. KIT Gene, Hot Spot Mutation (KIT D816V), and Variant Mutations: Current Standards and Suggested Approach for Testing
2.2. Serum Tryptase Levels
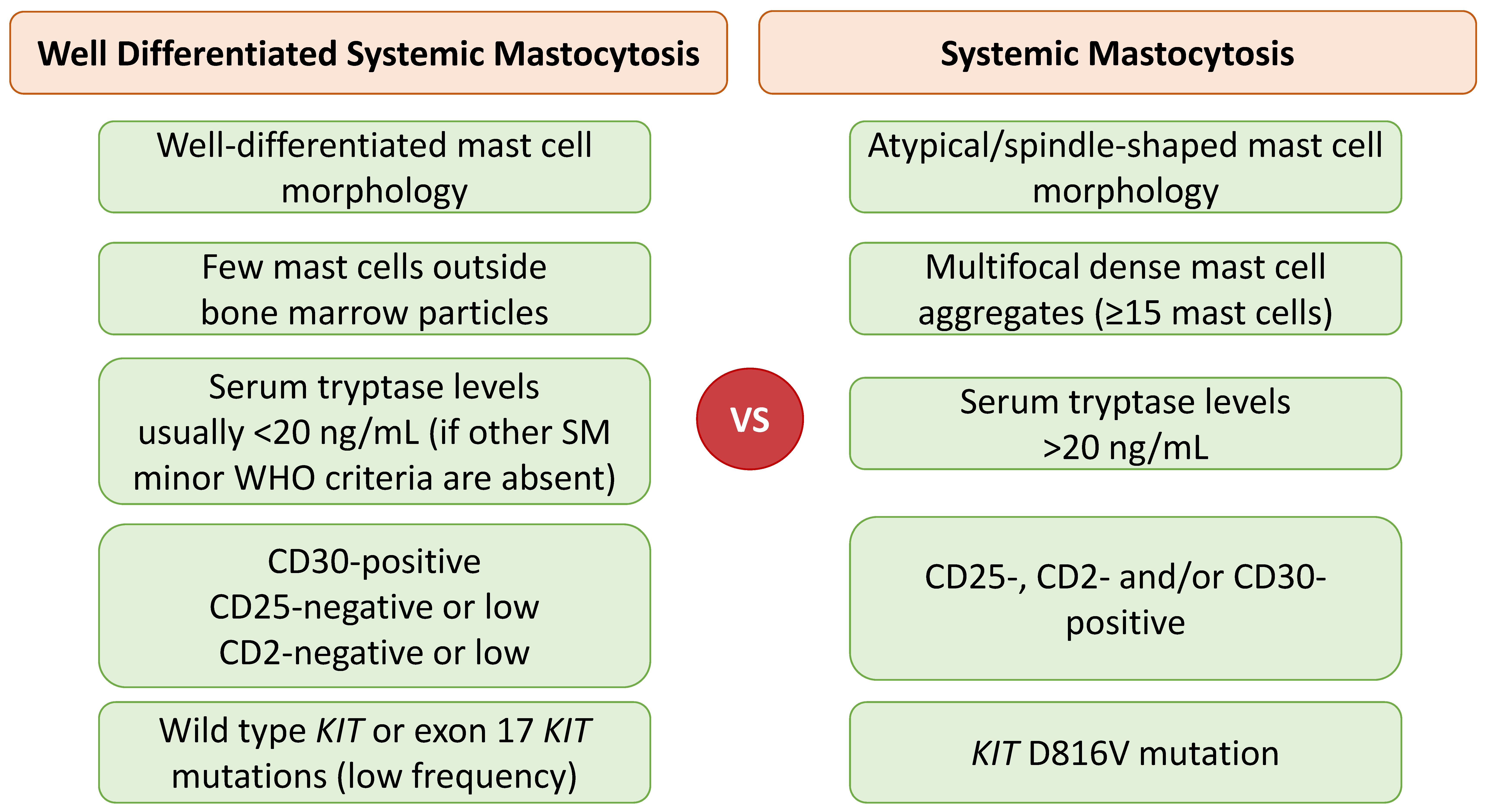
2.3. Well-Differentiated Systemic Mastocytosis (WDSM)
2.4. Bone Marrow Mastocytosis
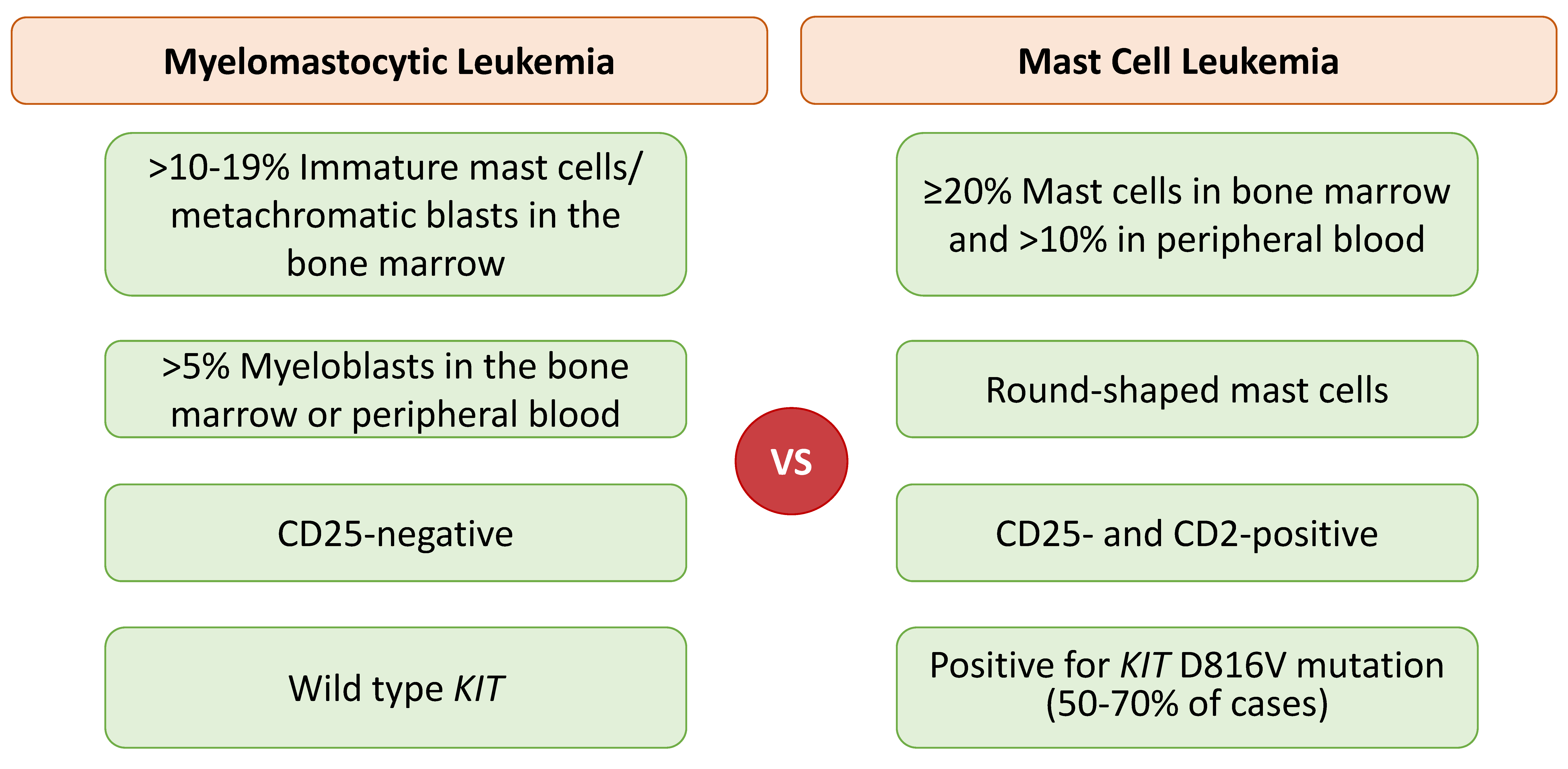
2.5. Mast Cell Leukemia: Chronic versus Acute
2.6. Myelomastocytic Leukemia (MML)
2.7. Mast Cell Activation Syndrome (MCAS)
2.8. Morphologic Variability of Mast Cells
3. Updates in Prognosis and Treatments for Systemic Mastocytosis
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich's visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Go, S.; Hatanaka, K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 1978, 52, 447–452. [Google Scholar] [CrossRef] [Green Version]
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horny, H.P.; Akin, C.; Arber, D.A.; Peterson, L.C.; Tefferi, A.; Metcalfe, D.D.; Bennett, J.M.; Bain, B.J.; Escribano, L.; Valent, P. Mastocytosis. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; Revised 4th ed.; IARC: Lyon, France, 2017; pp. 61–69. [Google Scholar]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Hochhaus, A. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Arock, M.; Hoermann, G.; Sotlar, K.; Hermine, O.; Sperr, W.R.; Hartmann, K.; Brockow, K.; Akin, C.; Triggiani, M.; Broesby-Olsen, S.; et al. Clinical impact and proposed application of molecular markers, genetic variants, and cytogenetic analysis in mast cell neoplasms: Status 2022. J. Allergy Clin. Immunol. 2022, 149, 1855–1865. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C.; et al. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. HemaSphere 2021, 5, e646. [Google Scholar] [CrossRef]
- Hoermann, G.; Sotlar, K.; Jawhar, M.; Kristensen, T.; Bachelot, G.; Nedoszytko, B.; Carter, M.C.; Horny, H.P.; Bonadonna, P.; Sperr, W.R.; et al. Standards of Genetic Testing in the Diagnosis and Prognostication of Systemic Mastocytosis in 2022: Recommendations of the EU-US Cooperative Group. J. Allergy Clin. Immunol. Pract. 2022. [Google Scholar] [CrossRef]
- Cruse, G.; Metcalfe, D.D.; Olivera, A. Functional deregulation of KIT: Link to mast cell proliferative diseases and other neoplasms. Immunol. Allergy Clin. N. Am. 2014, 34, 219–237. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, T.; Vestergaard, H.; Bindslev-Jensen, C.; Møller, M.B.; Broesby-Olsen, S.; Mastocytosis Centre Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am. J. Hematol. 2014, 89, 493–498. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Structure and regulation of Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem. Biophys. Res. Commun. 2005, 338, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S. Advanced systemic mastocytosis: The impact of KIT mutations in diagnosis, treatment, and progression. Eur. J. Haematol. 2013, 90, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalfe, D.D.; Mekori, Y.A. Pathogenesis and Pathology of Mastocytosis. Ann. Rev. Pathol. 2017, 12, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Erben, P.; Schwaab, J.; Metzgeroth, G.; Horny, H.P.; Jawhar, M.; Sotlar, K.; Fabarius, A.; Teichmann, M.; Schneider, S.; Ernst, T.; et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann. Hematol. 2014, 93, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, G.; Gleixner, K.V.; Dinu, G.E.; Kundi, M.; Greiner, G.; Wimazal, F.; Hadzijusufovic, E.; Mitterbauer, G.; Mannhalter, C.; Valent, P.; et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy 2014, 69, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Falchi, L.; Verstovsek, S. Kit Mutations: New insights and diagnostic value. Immunol. Allergy Clin. N. Am. 2018, 38, 411–428. [Google Scholar]
- Garcia-Montero, A.C.; Jara-Acevedo, M.; Teodosio, C.; Sanchez, M.L.; Nunez, R.; Prados, A.; Aldanondo, I.; Sanchez, L.; Dominguez, M.; Botana, L.M.; et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: A prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood 2006, 108, 2366–2372. [Google Scholar] [CrossRef]
- Escribano, L.; Alvarez-Twose, I.; Sanchez-Munoz, L.; Garcia-Montero, A.; Nunez, R.; Almeida, J.; Jara-Acevedo, M.; Teodosio, C.; Garcia-Cosio, M.; Bellas, C.; et al. Prognosis in adult indolent systemic mastocytosis: A long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J. Allergy Clin. Immunol. 2009, 124, 514–521. [Google Scholar] [CrossRef]
- Bodemer, C.; Hermine, O.; Palmerini, F.; Yang, Y.; Grandpeix-Guyodo, C.; Leventhal, P.S.; Hadj-Rabia, S.; Nasca, L.; Georgin-Lavialle, S.; Cohen-Akenine, A.; et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J. Investig. Dermatol. 2010, 130, 804–815. [Google Scholar] [CrossRef] [Green Version]
- Glenn, T.C. Field guide to next-generation DNA sequencers. Mol. Ecol. Resour. 2011, 11, 759–769. [Google Scholar] [CrossRef]
- Akin, C. Molecular diagnosis of mast cell disorders: A paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J. Mol. Diagn. 2006, 8, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotlar, K.; Fridrich, C.; Mall, A.; Jaussi, R.; Bultmann, B.; Valent, P.; Horny, H.P. Detection of c-kit point mutation Asp-816 → Val in microdissected pooled single mast cells and leukemic cells in a patient with systemic mastocytosis and concomitant chronic myelomonocytic leukemia. Leuk. Res. 2002, 26, 979–984. [Google Scholar] [CrossRef]
- Yavuz, A.S.; Lipsky, P.E.; Yavuz, S.; Metcalfe, D.D.; Akin, C. Evidence for the involvement of a hematopoietic progenitor cell in systemic mastocytosis from single-cell analysis of mutations in the c-kit gene. Blood 2002, 100, 661–665. [Google Scholar] [CrossRef]
- Sotlar, K.; Escribano, L.; Landt, O.; Mohrle, S.; Herrero, S.; Torrelo, A.; Lass, U.; Horny, H.P.; Bultmann, B. One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clamping and hybridization probes. Am. J. Pathol. 2003, 162, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Arock, M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano, L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.; et al. KIT mutation analysis in mast cell neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia 2015, 29, 1223–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, G.; Gurbisz, M.; Ratzinger, F.; Witzeneder, N.; Class, S.V.; Eisenwort, G.; Simonitsch-Klupp, I.; Esterbauer, H.; Mayerhofer, M.; Mullauer, L.; et al. Molecular quantification of tissue disease burden is a new biomarker and independent predictor of survival in mastocytosis. Haematologica 2020, 105, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Tzankov, A.; Duncavage, E.; Craig, F.E.; Kelemen, K.; King, R.L.; Orazi, A.; Quintanilla-Martinez, L.; Reichard, K.K.; Rimsza, L.M.; Wang, S.A.; et al. Mastocytosis. Am. J. Clin. Pathol. 2021, 155, 239–266. [Google Scholar] [CrossRef]
- Lim, K.H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield, J.H.; McClure, R.F.; Li, C.Y.; Pardanani, A. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood 2009, 113, 5727–5736. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Munoz, L.; Alvarez-Twose, I.; Garcia-Montero, A.C.; Teodosio, C.; Jara-Acevedo, M.; Pedreira, C.E.; Matito, A.; Morgado, J.M.; Sanchez, M.L.; Mollejo, M.; et al. Evaluation of the WHO criteria for the classification of patients with mastocytosis. Mod. Pathol. 2011, 24, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, S.A.; Konoplev, S.; Bueso-Ramos, C.E.; Thakral, B.; Miranda, R.N.; Jabbour, E.; Medeiros, L.J.; Kanagal-Shamanna, R. Well-differentiated systemic mastocytosis showed excellent clinical response to imatinib in the absence of known molecular genetic abnormalities: A case report. Medicine 2016, 95, e4934. [Google Scholar] [CrossRef]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef] [PubMed]
- Greiner, G.; Sprinzl, B.; Gorska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Lim, K.H.; Lasho, T.L.; Finke, C.M.; McClure, R.F.; Li, C.Y.; Tefferi, A. WHO subvariants of indolent mastocytosis: Clinical details and prognostic evaluation in 159 consecutive adults. Blood 2010, 115, 150–151. [Google Scholar] [CrossRef] [Green Version]
- Sperr, W.R.; El-Samahi, A.; Kundi, M.; Girschikofsky, M.; Winkler, S.; Lutz, D.; Endler, G.; Rumpold, H.; Agis, H.; Sillaber, C.; et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: A novel diagnostic approach and screen marker in clinical haematology. Eur. J. Clin. Investig. 2009, 39, 914–923. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; Jara-Acevedo, M.; Morgado, J.M.; Garcia-Montero, A.; Sanchez-Munoz, L.; Teodosio, C.; Matito, A.; Mayado, A.; Caldas, C.; Mollejo, M.; et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J. Allergy Clin. Immunol. 2016, 137, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.I. Hematologic aspects of mastocytosis: I: Bone marrow pathology in adult and pediatric systemic mast cell disease. J. Investig. Dermatol. 1991, 96, 47S–51S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, C.; Escribano, L.; Núñez, R.; García-Montero, A.; Angulo, M.; Orfao, A.; Metcalfe, D.D. Well-differentiated systemic mastocytosis: A new disease variant with mature mast cell phenotype and lack of codon 816 c-Kit mutations. J. Allergy Clin. Immunol. 2004, 113, S327. [Google Scholar] [CrossRef]
- de Melo Campos, P.; Machado-Neto, J.A.; Scopim-Ribeiro, R.; Visconte, V.; Tabarroki, A.; Duarte, A.S.; Barra, F.F.; Vassalo, J.; Rogers, H.J.; Lorand-Metze, I.; et al. Familial systemic mastocytosis with germline KIT K509I mutation is sensitive to treatment with imatinib, dasatinib and PKC412. Leuk. Res. 2014, 38, 1245–1251. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.C.; Bai, Y.; Kirshenbaum, A.S.; Fischer, E.R.; Simakova, O.; Bandara, G.; Scott, L.M.; Wisch, L.B.; Cantave, D.; Carter, M.C.; et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J. Allergy Clin. Immunol. 2014, 134, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; Smith, M.L.; Schultheis, B.; Fitzgibbon, J.; Lister, T.A.; Melo, J.V.; Cross, N.C.; Cavenagh, J.D. A novel K509I mutation of KIT identified in familial mastocytosis-in vitro and in vivo responsiveness to imatinib therapy. Leuk. Res. 2006, 30, 373–378. [Google Scholar] [CrossRef]
- Álvarez-Twose, I.; González, P.; Morgado, J.M.; Jara-Acevedo, M.; Sánchez-Muñoz, L.; Matito, A.; Mollejo, M.; Orfao, A.; Escribano, L. Complete response after imatinib mesylate therapy in a patient with well-differentiated systemic mastocytosis. J. Clin. Oncol. 2012, 30, e126–e129. [Google Scholar] [CrossRef] [PubMed]
- El Hussein, S.; Hu, S.; Fang, H.; Garces, S.; Muzzafar, T.; Wang, S.A.; Medeiros, L.J.; Bueso-Ramos, C.; Jelloul, F.Z. Well-differentiated systemic mastocytosis with associated myeloid sarcoma and myelodysplastic syndrome: Diagnostic challenges of an underrecognized entity. Leuk. Lymphoma 2022, 63, 235–238. [Google Scholar] [CrossRef]
- Sotlar, K.; Cerny-Reiterer, S.; Petat-Dutter, K.; Hessel, H.; Berezowska, S.; Mullauer, L.; Valent, P.; Horny, H.P. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod. Pathol. 2011, 24, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgado, J.M.; Perbellini, O.; Johnson, R.C.; Teodosio, C.; Matito, A.; Alvarez-Twose, I.; Bonadonna, P.; Zamo, A.; Jara-Acevedo, M.; Mayado, A.; et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology 2013, 63, 780–787. [Google Scholar] [CrossRef] [Green Version]
- Zanotti, R.; Tanasi, I.; Bernardelli, A.; Orsolini, G.; Bonadonna, P. Bone Marrow Mastocytosis: A Diagnostic Challenge. J. Clin. Med. 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, R.; Bonadonna, P.; Bonifacio, M.; Artuso, A.; Schena, D.; Rossini, M.; Perbellini, O.; Colarossi, S.; Chilosi, M.; Pizzolo, G. Isolated bone marrow mastocytosis: An underestimated subvariant of indolent systemic mastocytosis. Haematologica 2011, 96, 482–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanotti, R.; Bonifacio, M.; Lucchini, G.; Sperr, W.R.; Scaffidi, L.; van Anrooij, B.; Oude Elberink, H.N.; Rossignol, J.; Hermine, O.; Gorska, A.; et al. Refined diagnostic criteria for bone marrow mastocytosis: A proposal of the European competence network on mastocytosis. Leukemia 2022, 36, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Sotlar, K.; Sperr, W.R.; Reiter, A.; Arock, M.; Horny, H.P. Chronic mast cell leukemia: A novel leukemia-variant with distinct morphological and clinical features. Leuk. Res. 2015, 39, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Blatt, K.; Eisenwort, G.; Herrmann, H.; Cerny-Reiterer, S.; Thalhammer, R.; Mullauer, L.; Hoermann, G.; Sadovnik, I.; Schwarzinger, I.; et al. FLAG-induced remission in a patient with acute mast cell leukemia (MCL) exhibiting t(7;10)(q22;q26) and KIT D816H. Leuk. Res. Rep. 2014, 3, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Mital, A.; Piskorz, A.; Lewandowski, K.; Wasag, B.; Limon, J.; Hellmann, A. A case of mast cell leukaemia with exon 9 KIT mutation and good response to imatinib. Eur. J. Haematol. 2011, 86, 531–535. [Google Scholar] [CrossRef]
- Joris, M.; Georgin-Lavialle, S.; Chandesris, M.O.; Lhermitte, L.; Claisse, J.F.; Canioni, D.; Hanssens, K.; Damaj, G.; Hermine, O.; Hamidou, M. Mast Cell Leukaemia: C-KIT Mutations Are Not Always Positive. Case Rep. Hematol. 2012, 2012, 517546. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Lhermitte, L.; Suarez, F.; Yang, Y.; Letard, S.; Hanssens, K.; Feger, F.; Renand, A.; Brouze, C.; Canioni, D.; et al. Mast cell leukemia: Identification of a new c-Kit mutation, dup(501-502), and response to masitinib, a c-Kit tyrosine kinase inhibitor. Eur. J. Haematol. 2012, 89, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Arock, M.; Valent, P. Pathogenesis, classification and treatment of mastocytosis: State of the art in 2010 and future perspectives. Expert. Rev. Hematol. 2010, 3, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Berger, J.; Cerny-Reiterer, S.; Peter, B.; Eisenwort, G.; Hoermann, G.; Mullauer, L.; Mannhalter, C.; Steurer, M.; Bettelheim, P.; et al. Chronic mast cell leukemia (MCL) with KIT S476I: A rare entity defined by leukemic expansion of mature mast cells and absence of organ damage. Ann. Hematol. 2015, 94, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Akin, C.; Fumo, G.; Yavuz, A.S.; Lipsky, P.E.; Neckers, L.; Metcalfe, D.D. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood 2004, 103, 3222–3225. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Sotlar, K.; Sperr, W.R.; Escribano, L.; Yavuz, S.; Reiter, A.; George, T.I.; Kluin-Nelemans, H.C.; Hermine, O.; Butterfield, J.H.; et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): A consensus proposal. Ann. Oncol. 2014, 25, 1691–1700. [Google Scholar] [CrossRef]
- Horny, H.P.; Sotlar, K.; Reiter, A.; Valent, P. Myelomastocytic leukemia: Histopathological features, diagnostic criteria and differential diagnosis. Expert. Rev. Hematol. 2014, 7, 431–437. [Google Scholar] [CrossRef]
- Johnson, R.C.; Savage, N.M.; Chiang, T.; Gotlib, J.R.; Cherry, A.M.; Arber, D.A.; George, T.I. Hidden mastocytosis in acute myeloid leukemia with t(8;21)(q22;q22). Am. J. Clin. Pathol. 2013, 140, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Prokocimer, M.; Polliack, A. Increased bone marrow mast cells in preleukemic syndromes, acute leukemia, and lymphoproliferative disorders. Am. J. Clin. Pathol. 1981, 75, 34–38. [Google Scholar] [CrossRef]
- Varma, N.; Varma, S.; Wilkins, B. Acute myeloblastic leukaemia with differentiation to myeloblasts and mast cell blasts. Br. J. Haematol. 2000, 111, 991. [Google Scholar] [PubMed]
- Valent, P.; Akin, C.; Bonadonna, P.; Hartmann, K.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Siebenhaar, F.; Sperr, W.R.; Oude Elberink, J.N.G.; et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J. Allergy Clin. Immunol. Pract. 2019, 7, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Niedoszytko, M.; Triggiani, M.; Arock, M.; Brockow, K. Mast Cell Activation Syndromes: Collegium Internationale Allergologicum Update 2022. Int. Arch. Allergy Immunol. 2022, 183, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef]
- Valent, P.; Bonadonna, P.; Hartmann, K.; Broesby-Olsen, S.; Brockow, K.; Butterfield, J.H.; Triggiani, M.; Lyons, J.J.; Oude Elberink, J.N.G.; Arock, M.; et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int. Arch. Allergy Immunol. 2019, 180, 44–51. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Reiter, A.; Gotlib, J.; Sotlar, K.; Sperr, W.R.; Degenfeld-Schonburg, L.; Smiljkovic, D.; Triggiani, M.; et al. Drug-induced mast cell eradication: A novel approach to treat mast cell activation disorders? J. Allergy Clin. Immunol. 2022, 149, 1866–1874. [Google Scholar] [CrossRef]
- Pardanani, A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. Am. J. Hematol. 2021, 96, 508–525. [Google Scholar] [CrossRef]
- Valent, P.; Hartmann, K.; Schwaab, J.; Alvarez-Twose, I.; Brockow, K.; Bonadonna, P.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Personalized Management Strategies in Mast Cell Disorders: ECNM-AIM User's Guide for Daily Clinical Practice. J. Allergy Clin. Immunol. Pract. 2022. [Google Scholar] [CrossRef]
- Pardanani, A.; Shah, S.; Mannelli, F.; Elala, Y.C.; Guglielmelli, P.; Lasho, T.L.; Patnaik, M.M.; Gangat, N.; Ketterling, R.P.; Reichard, K.K.; et al. Mayo Alliance Prognostic System for mastocytosis: Clinical and hybrid clinical-molecular models. Blood Adv. 2018, 2, 2964–2972. [Google Scholar] [CrossRef] [Green Version]
- Pardanani, A.; Lasho, T.L.; Reichard, K.K.; Hanson, C.A.; Tefferi, A. World Health Organization class-independent risk categorization in mastocytosis. Blood Cancer J. 2019, 9, 29. [Google Scholar] [CrossRef]
- Gotlib, J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N. Engl. J. Med. 2016, 374, 2530–2541. [Google Scholar] [CrossRef] [PubMed]
- DeAngelo, D.J.; George, T.I.; Linder, A.; Langford, C.; Perkins, C.; Ma, J.; Westervelt, P.; Merker, J.D.; Berube, C.; Coutre, S.; et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia 2018, 32, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.K.; Gardino, A.K.; Kim, J.L.; Hodous, B.L.; Shutes, A.; Davis, A.; Zhu, X.J.; Schmidt-Kittler, O.; Wilson, D.; Wilson, K.; et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci. Transl. Med. 2017, 9, eaao1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose P, Verstovsek S: Avapritinib for Systemic Mastocytosis. Expert. Rev. Hematol. 2021, 14, 687–696. [CrossRef]
- Shomali, W.; Gotlib, J. The new tool “KIT” in advanced systemic mastocytosis. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, A.; George, T.I.; Gotlib, J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood 2020, 135, 1365–1376. [Google Scholar] [CrossRef]
- Baird, J.H.; Gotlib, J. Clinical validation of KIT inhibition in advanced systemic mastocytosis. Curr. Hematol. Malig. Rep. 2018, 13, 407–416. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Radia, D.H.; George, T.I.; Robinson, W.A.; Quiery, A.T.; Drummond, M.W.; Bose, P.; Hexner, E.O.; Winton, E.F.; Horny, H.P.; et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: The phase 1 EXPLORER trial. Nat. Med. 2021, 27, 2183–2191. [Google Scholar] [CrossRef]
- Gotlib, J.; Reiter, A.; Radia, D.H.; Deininger, M.W.; George, T.I.; Panse, J.; Vannucchi, A.M.; Platzbecker, U.; Alvarez-Twose, I.; Mital, A.; et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: Interim analysis of the phase 2 PATHFINDER trial. Nat. Med. 2021, 27, 2192–2199. [Google Scholar] [CrossRef]
- Pilkington, H.; Smith, S.; Roskell, N.; Iannazzo, S. Indirect treatment comparisons of avapritinib versus midostaurin for patients with advanced systemic mastocytosis. Future Oncol. 2022, 18, 1583–1594. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Gotlib, J.; Deininger, M.W.; DeAngelo, D.J.; Payumo, F.; Mensing, G.; George, T.I. A 3-part, phase 2 study of Bezuclastinib (CGT9486), an oral, selective, and potent KIT D816V inhibitor, in adult patients with nonadvanced systemic mastocytosis (NonAdvSM). Blood 2021, 138, 3642. [Google Scholar] [CrossRef]
- Zermati, Y.; De Sepulveda, P.; Feger, F.; Letard, S.; Kersual, J.; Casteran, N.; Gorochov, G.; Dy, M.; Ribadeau Dumas, A.; Dorgham, K.; et al. Effect of tyrosine kinase inhibitor STI571 on the kinase activity of wild-type and various mutated c-kit receptors found in mast cell neoplasms. Oncogene 2003, 22, 660–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, C.; Brockow, K.; D'Ambrosio, C.; Kirshenbaum, A.S.; Ma, Y.; Longley, B.J.; Metcalfe, D.D. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp. Hematol. 2003, 31, 686–692. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, S.; Metcalfe, D.D.; Akin, C.; Dimitrijevic, S.; Butterfield, J.H.; McMahon, G.; Longley, B.J. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood 2002, 99, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Twose, I.; Matito, A.; Morgado, J.M.; Sanchez-Munoz, L.; Jara-Acevedo, M.; Garcia-Montero, A.; Mayado, A.; Caldas, C.; Teodosio, C.; Munoz-Gonzalez, J.I.; et al. Imatinib in systemic mastocytosis: A phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget 2017, 8, 68950–68963. [Google Scholar] [CrossRef] [Green Version]
- Piris-Villaespesa, M.; Alvarez-Twose, I. Systemic mastocytosis: Following the tyrosine kinase inhibition roadmap. Front. Pharmacol. 2020, 11, 443. [Google Scholar] [CrossRef]
- Nakagomi, N.; Hirota, S. Juxtamembrane-type c-kit gene mutation found in aggressive systemic mastocytosis induces imatinib-resistant constitutive KIT activation. Lab. Investig. 2007, 87, 365–371. [Google Scholar] [CrossRef]
- Jawhar, M.; Schwaab, J.; Alvarez-Twose, I.; Shoumariyeh, K.; Naumann, N.; Lubke, J.; Perkins, C.; Munoz-Gonzalez, J.I.; Meggendorfer, M.; Kennedy, V.; et al. Mutation-Adjusted Risk Score for Advanced Systemic Mastocytosis. J. Clin. Oncol. 2019, 37, 2846–2856. [Google Scholar] [CrossRef]
- Jawhar, M.; Schwaab, J.; Naumann, N.; Horny, H.P.; Sotlar, K.; Haferlach, T.; Metzgeroth, G.; Fabarius, A.; Valent, P.; Hofmann, W.K.; et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood 2017, 130, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Chandesris, M.O.; Damaj, G.; Canioni, D.; Brouzes, C.; Lhermitte, L.; Hanssens, K.; Frenzel, L.; Cherquaoui, Z.; Durieu, I.; Durupt, S.; et al. Midostaurin in advanced systemic mastocytosis. N. Engl. J. Med. 2016, 374, 2605–2607. [Google Scholar] [CrossRef]
- Lubke, J.; Schwaab, J.; Naumann, N.; Horny, H.P.; Weiss, C.; Metzgeroth, G.; Kreil, S.; Cross, N.C.P.; Sotlar, K.; Fabarius, A.; et al. Superior efficacy of midostaurin over cladribine in advanced systemic mastocytosis: A Registry-Based Analysis. J. Clin. Oncol. 2022, 40, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Kittur, J.; Farrukh, F.; Begna, K.H.; Patnaik, M.M.; Al-Kali, A.; Elliott, M.A.; Reichard, K.K.; Gangat, N.; Pardanani, A. Cladribine therapy for advanced and indolent systemic mastocytosis: Mayo Clinic experience in 42 consecutive cases. Br. J. Haematol. 2022, 196, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Gleixner, K.V.; Mayerhofer, M.; Aichberger, K.J.; Derdak, S.; Sonneck, K.; Bohm, A.; Gruze, A.; Samorapoompichit, P.; Manley, P.W.; Fabbro, D.; et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: Comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood 2006, 107, 752–759. [Google Scholar] [CrossRef] [Green Version]
- Bohm, A.; Sonneck, K.; Gleixner, K.V.; Schuch, K.; Pickl, W.F.; Blatt, K.; Peter, B.; Herrmann, H.; Schernthaner, G.H.; Pehamberger, H.; et al. In vitro and in vivo growth-inhibitory effects of cladribine on neoplastic mast cells exhibiting the imatinib-resistant KIT mutation D816V. Exp. Hematol. 2010, 38, 744–755. [Google Scholar] [CrossRef]
- Penack, O.; Sotlar, K.; Noack, F.; Horny, H.P.; Thiel, E.; Notter, M. Cladribine therapy in a patient with an aleukemic subvariant of mast cell leukemia. Ann. Hematol. 2005, 84, 692–693. [Google Scholar] [CrossRef]
- Tefferi, A.; Li, C.Y.; Butterfield, J.H.; Hoagland, H.C. Treatment of systemic mast-cell disease with cladribine. N. Engl. J. Med. 2001, 344, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Kluin-Nelemans, H.C.; Oldhoff, J.M.; Van Doormaal, J.J.; Van’t Wout, J.W.; Verhoef, G.; Gerrits, W.B.; van Dobbenburgh, O.A.; Pasmans, S.G.; Fijnheer, R. Cladribine therapy for systemic mastocytosis. Blood 2003, 102, 4270–4276. [Google Scholar] [CrossRef]

| Cutaneous Mastocytosis (CM) * |
|---|
| Systemic Mastocytosis (SM) ** Bone Marrow Mastocytosis (BMM) Indolent Systemic Mastocytosis (ISM) Smoldering Systemic Mastocytosis (SSM) Aggressive Systemic Mastocytosis (ASM) SM with an Associated Hematologic Neoplasm (SM-AHN) Mast Cell Leukemia (MCL) |
| Mast Cell Sarcoma (MCS) |
| For Diagnosis of SM, at Least One Major and One Minor or Three Minor Criteria Are Required. |
|---|
| Major criterion: Multifocal dense infiltrates of mast cells (≥15 mast cells in aggregates) detected in sections of the bone marrow and/or other extracutaneous organ(s). |
| Minor criteria a. >25% of all mast cells are atypical cells (type I or type II) on bone marrow smears or are spindle-shaped in dense and diffuse mast cell infiltrates in BM or other extracutaneous organ(s). a b. Activating KIT point mutation(s) at codon 816 or in other critical regions of KIT b in the bone marrow or other extracutaneous organ(s). c. Mast cells in bone marrow, blood, or other extracutaneous organ(s) aberrantly express one or more of the following antigens: CD2, CD25, CD30. c d. Baseline serum tryptase concentration > 20 ng/mL in the absence of a myeloid AHN. d In the case of a known HαT, the tryptase level should be adjusted. e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hussein, S.; Chifotides, H.T.; Khoury, J.D.; Verstovsek, S.; Thakral, B. Systemic Mastocytosis and Other Entities Involving Mast Cells: A Practical Review and Update. Cancers 2022, 14, 3474. https://doi.org/10.3390/cancers14143474
El Hussein S, Chifotides HT, Khoury JD, Verstovsek S, Thakral B. Systemic Mastocytosis and Other Entities Involving Mast Cells: A Practical Review and Update. Cancers. 2022; 14(14):3474. https://doi.org/10.3390/cancers14143474
Chicago/Turabian StyleEl Hussein, Siba, Helen T. Chifotides, Joseph D. Khoury, Srdan Verstovsek, and Beenu Thakral. 2022. "Systemic Mastocytosis and Other Entities Involving Mast Cells: A Practical Review and Update" Cancers 14, no. 14: 3474. https://doi.org/10.3390/cancers14143474
APA StyleEl Hussein, S., Chifotides, H. T., Khoury, J. D., Verstovsek, S., & Thakral, B. (2022). Systemic Mastocytosis and Other Entities Involving Mast Cells: A Practical Review and Update. Cancers, 14(14), 3474. https://doi.org/10.3390/cancers14143474







