Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications
Abstract
:Simple Summary
Abstract
1. Introduction
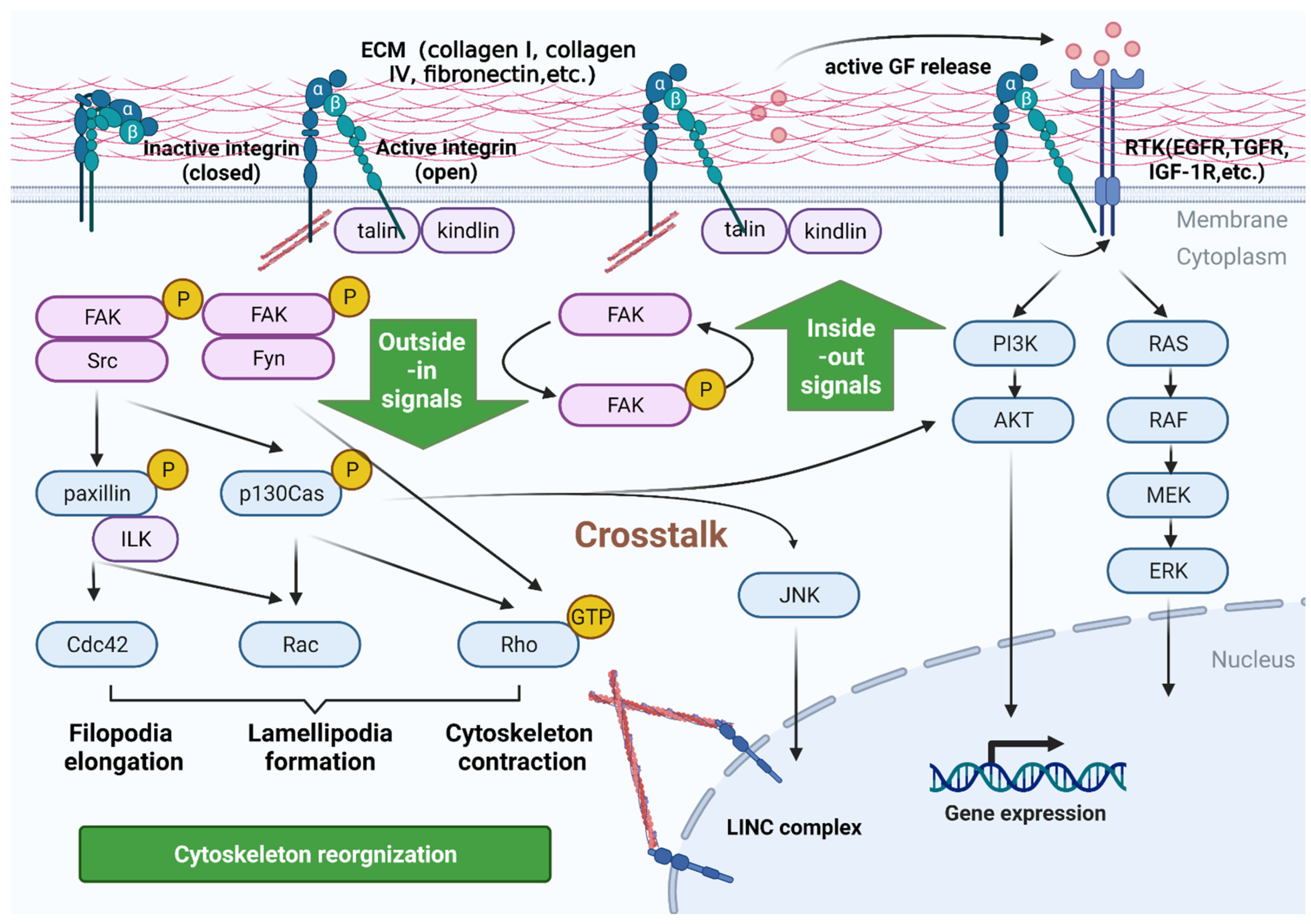
2. Signaling Pathways Mediated by Integrins in PC
2.1. Bidirectional Signaling Transduction Controls the Coupling of Ligands with Cytoskeleton and the Formation of Focal Adhesion
2.2. Integrins Are a Bidirectional Pressure Signal Transmitter
2.3. Crosstalk with Other Receptors in Inside-Out Signaling Pathways
2.4. Control Signaling by Anchoring and Regulating the Cytoskeleton
3. An Overview of Integrin β1
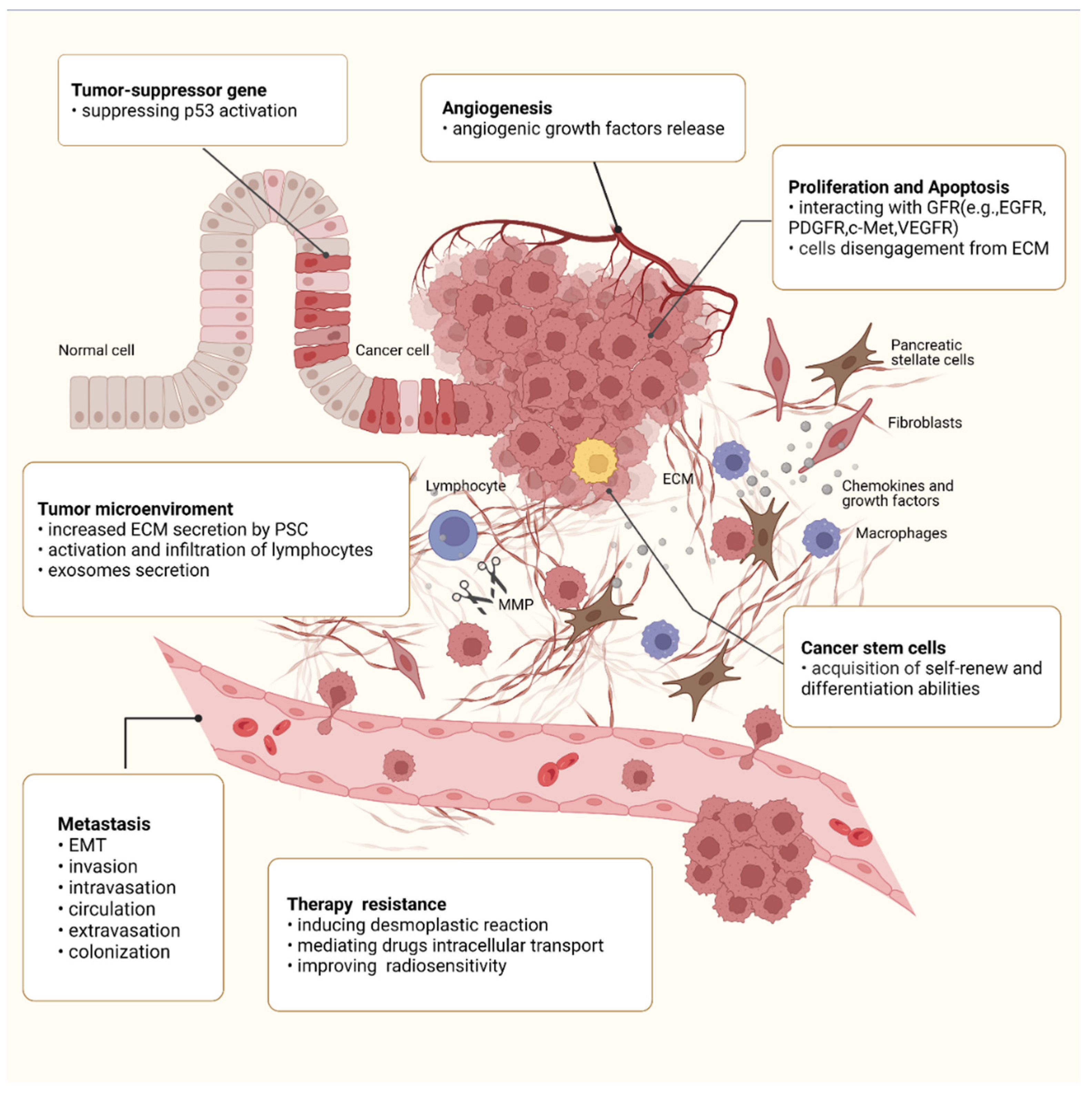
4. Roles of Integrin β1 in the Malignant Behaviors of PC
4.1. Integrin β1 and Proliferation-Related Signaling
4.2. Integrin β1 and Tumor Suppressor p53
4.3. Integrin β1 and Cell Apoptosis
4.4. Integrin β1 and Angiogenesis
4.5. Integrin β1 and Metastasis
4.6. Integrin β1 and Tumor Microenvironment (TME)
4.7. Integrin β1 and CSCs
4.8. Integrin β1 and Therapy
5. Clinical Significance of Integrin β1 in PC
6. Future Expectations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2140–2141. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthäi, E.; Carrato, A.; Earl, J.; Robbers, K.; van Mil, A.M.; Potjer, T.; et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Bachet, J.B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.L.; Ducreux, M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef]
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 603–605. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18, 14. [Google Scholar] [CrossRef]
- Nagakawa, Y.; Yi, S.Q.; Takishita, C.; Sahara, Y.; Osakabe, H.; Kiya, Y.; Yamaguchi, H.; Miwa, Y.; Sato, I.; Tsuchida, A. Precise anatomical resection based on structures of nerve and fibrous tissue around the superior mesenteric artery for mesopancreas dissection in pancreaticoduodenectomy for pancreatic cancer. J. Hepatobiliary Pancreat. Sci. 2020, 27, 342–351. [Google Scholar] [CrossRef]
- Incio, J.; Suboj, P.; Chin, S.M.; Vardam-Kaur, T.; Liu, H.; Hato, T.; Babykutty, S.; Chen, I.; Deshpande, V.; Jain, R.K.; et al. Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PLoS ONE 2015, 10, e0141392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Modi, S.; McGinn, O.; Zhao, X.; Dudeja, V.; Ramakrishnan, S.; Saluja, A.K. Impaired Synthesis of Stromal Components in Response to Minnelide Improves Vascular Function, Drug Delivery, and Survival in Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zienert, E.; Eke, I.; Aust, D.; Cordes, N. LIM-only protein FHL2 critically determines survival and radioresistance of pancreatic cancer cells. Cancer Lett. 2015, 364, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; He, X.; Wei, W.; Wang, J.; Zhang, T.; Shen, X. Tenascin-C induces resistance to apoptosis in pancreatic cancer cell through activation of ERK/NF-κB pathway. Apoptosis 2015, 20, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Jülich, D.; Cobb, G.; Melo, A.M.; McMillen, P.; Lawton, A.K.; Mochrie, S.G.; Rhoades, E.; Holley, S.A. Cross-Scale Integrin Regulation Organizes ECM and Tissue Topology. Dev. Cell 2015, 34, 33–44. [Google Scholar] [CrossRef] [Green Version]
- An, T.; Zhang, Z.; Li, Y.; Yi, J.; Zhang, W.; Chen, D.; Ao, J.; Xiao, Z.X.; Yi, Y. Integrin β1-Mediated Cell⁻Cell Adhesion Augments Metformin-Induced Anoikis. Int. J. Mol. Sci. 2019, 20, 1161. [Google Scholar] [CrossRef] [Green Version]
- Son, S.; Moroney, G.J.; Butler, P.J. β(1)-Integrin-Mediated Adhesion Is Lipid-Bilayer Dependent. Biophys. J. 2017, 113, 1080–1092. [Google Scholar] [CrossRef] [Green Version]
- Hohenester, E. Signalling complexes at the cell-matrix interface. Curr. Opin. Struct. Biol. 2014, 29, 10–16. [Google Scholar] [CrossRef]
- De Franceschi, N.; Hamidi, H.; Alanko, J.; Sahgal, P.; Ivaska, J. Integrin traffic—The update. J. Cell Sci. 2015, 128, 839–852. [Google Scholar] [CrossRef] [Green Version]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Bianchi, A. Click chemistry for the synthesis of RGD-containing integrin ligands. Molecules 2010, 15, 178–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.H. Integrin activation. BMB Rep. 2014, 47, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, D.; Pouwels, J.; De Franceschi, N.; Ivaska, J. Integrin inactivators: Balancing cellular functions in vitro and in vivo. Nat. Rev. Mol. Cell Biol. 2013, 14, 430–442. [Google Scholar] [CrossRef]
- Seguin, L.; Kato, S.; Franovic, A.; Camargo, M.F.; Lesperance, J.; Elliott, K.C.; Yebra, M.; Mielgo, A.; Lowy, A.M.; Husain, H.; et al. An integrin β₃-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 2014, 16, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desgrosellier, J.S.; Lesperance, J.; Seguin, L.; Gozo, M.; Kato, S.; Franovic, A.; Yebra, M.; Shattil, S.J.; Cheresh, D.A. Integrin αvβ3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Dev. Cell 2014, 30, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Shibue, T.; Brooks, M.W.; Inan, M.F.; Reinhardt, F.; Weinberg, R.A. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012, 2, 706–721. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Lee, W.C.; Aust, D.; Pilarsky, C.; Cordes, N. β8 Integrin Mediates Pancreatic Cancer Cell Radiochemoresistance. Mol. Cancer Res. 2019, 17, 2126–2138. [Google Scholar] [CrossRef]
- Moore, K.M.; Desai, A.; Delgado, B.L.; Trabulo, S.M.D.; Reader, C.; Brown, N.F.; Murray, E.R.; Brentnall, A.; Howard, P.; Masterson, L.; et al. Integrin αvβ6-specific therapy for pancreatic cancer developed from foot-and-mouth-disease virus. Theranostics 2020, 10, 2930–2942. [Google Scholar] [CrossRef]
- Turaga, R.C.; Sharma, M.; Mishra, F.; Krasinskas, A.; Yuan, Y.; Yang, J.J.; Wang, S.; Liu, C.; Li, S.; Liu, Z.R. Modulation of Cancer-Associated Fibrotic Stroma by An Integrin α(v)β(3) Targeting Protein for Pancreatic Cancer Treatment. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 161–179. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huveneers, S.; Danen, E.H. Adhesion signaling—Crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodniewicz, D.; Klemke, R.L. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim. Biophys. Acta 2004, 1692, 63–76. [Google Scholar] [CrossRef]
- Deakin, N.O.; Turner, C.E. Paxillin comes of age. J. Cell Sci. 2008, 121, 2435–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ten Klooster, J.P.; Jaffer, Z.M.; Chernoff, J.; Hordijk, P.L. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J. Cell Biol. 2006, 172, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Guilluy, C.; Swaminathan, V.; Garcia-Mata, R.; O’Brien, E.T.; Superfine, R.; Burridge, K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 2011, 13, 722–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, M.; Legate, K.R.; Zent, R.; Fässler, R. The tail of integrins, talin, and kindlins. Science 2009, 324, 895–899. [Google Scholar] [CrossRef]
- Xu, Z.; Isaji, T.; Fukuda, T.; Wang, Y.; Gu, J. O-GlcNAcylation regulates integrin-mediated cell adhesion and migration via formation of focal adhesion complexes. J. Biol. Chem. 2019, 294, 3117–3124. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Costell, M.; Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, S.; Siech, M.; Habisch, H.; Seufferlein, T.; Bachem, M.G. Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway. Br. J. Cancer 2014, 110, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Friedland, J.C.; Lee, M.H.; Boettiger, D. Mechanically activated integrin switch controls alpha5beta1 function. Science 2009, 323, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.W.; Roca-Cusachs, P.; Sheetz, M.P. Stretchy proteins on stretchy substrates: The important elements of integrin-mediated rigidity sensing. Dev. Cell 2010, 19, 194–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirmse, R.; Otto, H.; Ludwig, T. Interdependency of cell adhesion, force generation and extracellular proteolysis in matrix remodeling. J. Cell Sci. 2011, 124, 1857–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeltz, C.; Alam, J.; Liu, H.; Erusappan, P.M.; Hoschuetzky, H.; Molven, A.; Parajuli, H.; Cukierman, E.; Costea, D.E.; Lu, N.; et al. α11β1 Integrin is Induced in a Subset of Cancer-Associated Fibroblasts in Desmoplastic Tumor Stroma and Mediates In Vitro Cell Migration. Cancers 2019, 11, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcoux, N.; Vuori, K. EGF receptor mediates adhesion-dependent activation of the Rac GTPase: A role for phosphatidylinositol 3-kinase and Vav2. Oncogene 2003, 22, 6100–6106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, Y.; Takada, Y.K.; Fujita, M. Crosstalk between insulin-like growth factor (IGF) receptor and integrins through direct integrin binding to IGF1. Cytokine Growth Factor Rev. 2017, 34, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Sarker, F.A.; Prior, V.G.; Bax, S.; O’Neill, G.M. Forcing a growth factor response—Tissue-stiffness modulation of integrin signaling and crosstalk with growth factor receptors. J. Cell Sci. 2020, 133, jsc242461. [Google Scholar] [CrossRef]
- Dao, D.T.; Anez-Bustillos, L.; Adam, R.M.; Puder, M.; Bielenberg, D.R. Heparin-Binding Epidermal Growth Factor-Like Growth Factor as a Critical Mediator of Tissue Repair and Regeneration. Am. J. Pathol. 2018, 188, 2446–2456. [Google Scholar] [CrossRef] [Green Version]
- Malenica, I.; Adam, J.; Corgnac, S.; Mezquita, L.; Auclin, E.; Damei, I.; Grynszpan, L.; Gros, G.; de Montpréville, V.; Planchard, D.; et al. Integrin-α(V)-mediated activation of TGF-β regulates anti-tumour CD8 T cell immunity and response to PD-1 blockade. Nat. Commun. 2021, 12, 5209. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-β structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemper, M.; Schiecke, A.; Maar, H.; Nikulin, S.; Poloznikov, A.; Galatenko, V.; Tachezy, M.; Gebauer, F.; Lange, T.; Riecken, K.; et al. Integrin alpha-V is an important driver in pancreatic adenocarcinoma progression. J. Exp. Clin. Cancer Res. 2021, 40, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ye, H.; Ren, X.; Zheng, S.; Zhou, Q.; Chen, C.; Lin, Q.; Li, G.; Wei, L.; Fu, Z.; et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-β1/smad2/3 axis in pancreatic cancer. Cancer Lett. 2019, 459, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 363–378. [Google Scholar] [CrossRef] [Green Version]
- Carley, E.; Stewart, R.M.; Zieman, A.; Jalilian, I.; King, D.E.; Zubek, A.; Lin, S.; Horsley, V.; King, M.C. The LINC complex transmits integrin-dependent tension to the nuclear lamina and represses epidermal differentiation. eLife 2021, 10, e58541. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhao, J.; Sun, Y.; Li, D.; Meng, Z.; Wang, B.; Fan, P.; Liu, Z.; Jin, X.; Wu, H. Overexpressed ITGA2 promotes malignant tumor aggression by up-regulating PD-L1 expression through the activation of the STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 485. [Google Scholar] [CrossRef] [Green Version]
- Aksorn, N.; Chanvorachote, P. Integrin as a Molecular Target for Anti-cancer Approaches in Lung Cancer. Anticancer Res. 2019, 39, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Justo, B.L.; Jasiulionis, M.G. Characteristics of TIMP1, CD63, and β1-Integrin and the Functional Impact of Their Interaction in Cancer. Int. J. Mol. Sci. 2021, 22, 9319. [Google Scholar] [CrossRef]
- Pellinen, T.; Blom, S.; Sánchez, S.; Välimäki, K.; Mpindi, J.P.; Azegrouz, H.; Strippoli, R.; Nieto, R.; Vitón, M.; Palacios, I.; et al. ITGB1-dependent upregulation of Caveolin-1 switches TGFβ signalling from tumour-suppressive to oncogenic in prostate cancer. Sci. Rep. 2018, 8, 2338. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Mei, D.; Ding, Z. Caveolin-1 contributes to anoikis resistance in human gastric cancer SGC-7901 cells via regulating Src-dependent EGFR-ITGB1 signaling. J. Biochem. Mol. Toxicol. 2018, 32, e22202. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Deng, Z.; Sun, L. MicroRNA-3653 inhibits the growth and metastasis of hepatocellular carcinoma by inhibiting ITGB1. Oncol. Rep. 2019, 41, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Fukuto, H.S.; Brown, D.A.; Bliska, J.B.; London, E. Effects of host cell sterol composition upon internalization of Yersinia pseudotuberculosis and clustered β1 integrin. J. Biol. Chem. 2018, 293, 1466–1479. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, R.; Niwa, Y.; Simizu, S. Integrin β1 is an essential factor in vasculogenic mimicry of human cancer cells. Cancer Sci. 2018, 109, 2490–2496. [Google Scholar] [CrossRef]
- Beaty, B.T.; Sharma, V.P.; Bravo-Cordero, J.J.; Simpson, M.A.; Eddy, R.J.; Koleske, A.J.; Condeelis, J. β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol. Biol. Cell 2013, 24, 1661–1675. [Google Scholar] [CrossRef]
- Moreno-Layseca, P.; Streuli, C.H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef]
- Mohan, S.; Heitzer, E.; Ulz, P.; Lafer, I.; Lax, S.; Auer, M.; Pichler, M.; Gerger, A.; Eisner, F.; Hoefler, G.; et al. Changes in colorectal carcinoma genomes under anti-EGFR therapy identified by whole-genome plasma DNA sequencing. PLoS Genet. 2014, 10, e1004271. [Google Scholar] [CrossRef]
- Rao, T.C.; Ma, V.P.; Blanchard, A.; Urner, T.M.; Grandhi, S.; Salaita, K.; Mattheyses, A.L. EGFR activation attenuates the mechanical threshold for integrin tension and focal adhesion formation. J. Cell Sci. 2020, 133, jcs238840. [Google Scholar] [CrossRef]
- Vial, D.; McKeown-Longo, P.J. Epidermal growth factor (EGF) regulates α5β1 integrin activation state in human cancer cell lines through the p90RSK-dependent phosphorylation of filamin A. J. Biol. Chem. 2012, 287, 40371–40380. [Google Scholar] [CrossRef] [Green Version]
- Morozevich, G.E.; Kozlova, N.I.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Berman, A.E. Integrin α5β1 simultaneously controls EGFR-dependent proliferation and Akt-dependent pro-survival signaling in epidermoid carcinoma cells. Aging 2012, 4, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Petrás, M.; Lajtos, T.; Friedländer, E.; Klekner, A.; Pintye, E.; Feuerstein, B.G.; Szöllosi, J.; Vereb, G. Molecular interactions of ErbB1 (EGFR) and integrin-β1 in astrocytoma frozen sections predict clinical outcome and correlate with Akt-mediated in vitro radioresistance. Neuro Oncol. 2013, 15, 1027–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Chen, C.C.; Alpini, G.; Lau, L.F. CCN1 induces hepatic ductular reaction through integrin αvβ₅-mediated activation of NF-κB. J. Clin. Investig. 2015, 125, 1886–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, Y.; Zhang, Y.; Yang, H.; Wang, A.; Chen, X. The mechanism of CCN1-enhanced retinal neovascularization in oxygen-induced retinopathy through PI3K/Akt-VEGF signaling pathway. Drug Des. Devel. Ther. 2015, 9, 2463–2473. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Jung, K.; Baek, D.S.; Hong, S.S.; Kim, Y.S. Co-targeting of EGF receptor and neuropilin-1 overcomes cetuximab resistance in pancreatic ductal adenocarcinoma with integrin β1-driven Src-Akt bypass signaling. Oncogene 2017, 36, 2543–2552. [Google Scholar] [CrossRef]
- Ciriello, V.; Gudipati, S.; Stavrou, P.Z.; Kanakaris, N.K.; Bellamy, M.C.; Giannoudis, P.V. Biomarkers predicting sepsis in polytrauma patients: Current evidence. Injury 2013, 44, 1680–1692. [Google Scholar] [CrossRef]
- Lee, J.G.; Ahn, J.H.; Jin Kim, T.; Ho Lee, J.; Choi, J.H. Mutant p53 promotes ovarian cancer cell adhesion to mesothelial cells via integrin β4 and Akt signals. Sci. Rep. 2015, 5, 12642. [Google Scholar] [CrossRef] [Green Version]
- Savar, A.; Acin, S.; Gonzalez, C.L.; El-Sawy, T.; Mejia, O.; Li, Z.; Esmaeli, B.; Lacy-Hulbert, A.; El-Naggar, A.K.; McCarty, J.H.; et al. Loss of epithelial p53 and αv integrin cooperate through Akt to induce squamous cell carcinoma yet prevent remodeling of the tumor microenvironment. Oncogene 2015, 34, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Janouskova, H.; Maglott, A.; Leger, D.Y.; Bossert, C.; Noulet, F.; Guerin, E.; Guenot, D.; Pinel, S.; Chastagner, P.; Plenat, F.; et al. Integrin α5β1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012, 72, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Janouskova, H.; Dontenwill, M. Integrins and p53 pathways in glioblastoma resistance to temozolomide. Front. Oncol. 2012, 2, 157. [Google Scholar] [CrossRef] [Green Version]
- Renner, G.; Janouskova, H.; Noulet, F.; Koenig, V.; Guerin, E.; Bär, S.; Nuesch, J.; Rechenmacher, F.; Neubauer, S.; Kessler, H.; et al. Integrin α5β1 and p53 convergent pathways in the control of anti-apoptotic proteins PEA-15 and survivin in high-grade glioma. Cell Death Differ. 2016, 23, 640–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjonen, A.; Kaukonen, R.; Mattila, E.; Rouhi, P.; Högnäs, G.; Sihto, H.; Miller, B.W.; Morton, J.P.; Bucher, E.; Taimen, P.; et al. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. J. Clin. Investig. 2014, 124, 1069–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selivanova, G. Wild type p53 reactivation: From lab bench to clinic. FEBS Lett. 2014, 588, 2628–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, G.S.; Grundl, M.; Leychenko, A.; Reiter, S.; Young-Robbins, S.S.; Sulzmaier, F.J.; Caliva, M.J.; Ramos, J.W.; Matter, M.L. Bit-1 mediates integrin-dependent cell survival through activation of the NFκB pathway. J. Biol. Chem. 2011, 286, 14713–14723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchheit, C.L.; Weigel, K.J.; Schafer, Z.T. Cancer cell survival during detachment from the ECM: Multiple barriers to tumour progression. Nat. Rev. Cancer 2014, 14, 632–641. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.L.; Bian, K.; Wang, L.; Zhang, X.; Yan, B.; Jia, L.T.; Zhao, J.; Gammoh, N.; Yang, A.G.; et al. MicroRNA-26a promotes anoikis in human hepatocellular carcinoma cells by targeting alpha5 integrin. Oncotarget 2015, 6, 2277–2289. [Google Scholar] [CrossRef] [Green Version]
- Toricelli, M.; Melo, F.H.; Peres, G.B.; Silva, D.C.; Jasiulionis, M.G. Timp1 interacts with beta-1 integrin and CD63 along melanoma genesis and confers anoikis resistance by activating PI3-K signaling pathway independently of Akt phosphorylation. Mol. Cancer 2013, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, I.A.; Vermeulen, J.F.; Ercan, C.; Houthuijzen, J.M.; Saig, F.A.; Vlug, E.J.; van der Wall, E.; van Diest, P.J.; Vooijs, M.; Derksen, P.W. FER kinase promotes breast cancer metastasis by regulating α6- and β1-integrin-dependent cell adhesion and anoikis resistance. Oncogene 2013, 32, 5582–5592. [Google Scholar] [CrossRef] [Green Version]
- Schempp, C.M.; von Schwarzenberg, K.; Schreiner, L.; Kubisch, R.; Müller, R.; Wagner, E.; Vollmar, A.M. V-ATPase inhibition regulates anoikis resistance and metastasis of cancer cells. Mol. Cancer Ther. 2014, 13, 926–937. [Google Scholar] [CrossRef] [Green Version]
- Aslan, B.; Monroig, P.; Hsu, M.C.; Pena, G.A.; Rodriguez-Aguayo, C.; Gonzalez-Villasana, V.; Rupaimoole, R.; Nagaraja, A.S.; Mangala, S.; Han, H.D.; et al. The ZNF304-integrin axis protects against anoikis in cancer. Nat. Commun. 2015, 6, 7351. [Google Scholar] [CrossRef]
- Khalkar, P.; Díaz-Argelich, N.; Antonio Palop, J.; Sanmartín, C.; Fernandes, A.P. Novel Methylselenoesters Induce Programed Cell Death via Entosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M.; Takahashi, S.; Nishimura, R.; Kubota, A.; Kojima, H.; Ohta, T.; Hamada, J.; Kuramitsu, Y.; Maeda, H.; Miyashita, K.; et al. A Marine Carotenoid of Fucoxanthinol Accelerates the Growth of Human Pancreatic Cancer PANC-1 Cells. Nutr. Cancer 2022, 74, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Neyns, B.; Goldbrunner, R.; Schlegel, U.; Clement, P.M.; Grabenbauer, G.G.; Ochsenbein, A.F.; Simon, M.; Dietrich, P.Y.; et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 2712–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Eisele, G.; Wick, A.; Eisele, A.C.; Clément, P.M.; Tonn, J.; Tabatabai, G.; Ochsenbein, A.; Schlegel, U.; Neyns, B.; Krex, D.; et al. Cilengitide treatment of newly diagnosed glioblastoma patients does not alter patterns of progression. J. Neurooncol. 2014, 117, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janouskova, H.; Ray, A.M.; Noulet, F.; Lelong-Rebel, I.; Choulier, L.; Schaffner, F.; Lehmann, M.; Martin, S.; Teisinger, J.; Dontenwill, M. Activation of p53 pathway by Nutlin-3a inhibits the expression of the therapeutic target α5 integrin in colon cancer cells. Cancer Lett. 2013, 336, 307–318. [Google Scholar] [CrossRef]
- Hakanpaa, L.; Sipila, T.; Leppanen, V.M.; Gautam, P.; Nurmi, H.; Jacquemet, G.; Eklund, L.; Ivaska, J.; Alitalo, K.; Saharinen, P. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat. Commun. 2015, 6, 5962. [Google Scholar] [CrossRef]
- Hongu, T.; Funakoshi, Y.; Fukuhara, S.; Suzuki, T.; Sakimoto, S.; Takakura, N.; Ema, M.; Takahashi, S.; Itoh, S.; Kato, M.; et al. Arf6 regulates tumour angiogenesis and growth through HGF-induced endothelial β1 integrin recycling. Nat. Commun. 2015, 6, 7925. [Google Scholar] [CrossRef] [Green Version]
- Vitorino, P.; Yeung, S.; Crow, A.; Bakke, J.; Smyczek, T.; West, K.; McNamara, E.; Eastham-Anderson, J.; Gould, S.; Harris, S.F.; et al. MAP4K4 regulates integrin-FERM binding to control endothelial cell motility. Nature 2015, 519, 425–430. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ehling, M.; Kato, K.; Kanai, K.; van Lessen, M.; Frye, M.; Zeuschner, D.; Nakayama, M.; Vestweber, D.; Adams, R.H. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat. Commun. 2015, 6, 6429. [Google Scholar] [CrossRef] [Green Version]
- Herrlinger, K.R.; Diculescu, M.; Fellermann, K.; Hartmann, H.; Howaldt, S.; Nikolov, R.; Petrov, A.; Reindl, W.; Otte, J.M.; Stoynov, S.; et al. Efficacy, safety and tolerability of vidofludimus in patients with inflammatory bowel disease: The ENTRANCE study. J. Crohns Colitis 2013, 7, 636–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonell, W.S.; DeLay, M.; Jahangiri, A.; Park, C.C.; Aghi, M.K. β1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 2013, 73, 3145–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangiri, A.; Aghi, M.K.; Carbonell, W.S. β1 integrin: Critical path to antiangiogenic therapy resistance and beyond. Cancer Res. 2014, 74, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schluterman, M.K.; Chapman, S.L.; Korpanty, G.; Ozumi, K.; Fukai, T.; Yanagisawa, H.; Brekken, R.A. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Dis. Model. Mech. 2010, 3, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Naci, D.; Vuori, K.; Aoudjit, F. Alpha2beta1 integrin in cancer development and chemoresistance. Semin. Cancer Biol. 2015, 35, 145–153. [Google Scholar] [CrossRef]
- Eke, I.; Cordes, N. Focal adhesion signaling and therapy resistance in cancer. Semin. Cancer Biol. 2015, 31, 65–75. [Google Scholar] [CrossRef]
- Zhou, P.; Erfani, S.; Liu, Z.; Jia, C.; Chen, Y.; Xu, B.; Deng, X.; Alfáro, J.E.; Chen, L.; Napier, D.; et al. CD151-α3β1 integrin complexes are prognostic markers of glioblastoma and cooperate with EGFR to drive tumor cell motility and invasion. Oncotarget 2015, 6, 29675–29693. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ishihara, S.; Yasuda, M.; Nishioka, T.; Mizutani, T.; Ishikawa, M.; Kawabata, K.; Shirato, H.; Haga, H. Lung cancer cells that survive ionizing radiation show increased integrin α2β1- and EGFR-dependent invasiveness. PLoS ONE 2013, 8, e70905. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.C.; Coppolino, M.G. SNARE-dependent interaction of Src, EGFR and β1 integrin regulates invadopodia formation and tumor cell invasion. J. Cell Sci. 2014, 127, 1712–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, A.; Muharram, G.; Barrow-McGee, R.; Baghirov, H.; Rantala, J.; Kermorgant, S.; Ivaska, J. Distinct c-Met activation mechanisms induce cell rounding or invasion through pathways involving integrins, RhoA and HIP1. J. Cell Sci. 2014, 127, 1938–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, W.; Chen, C.; Dong, M.; Wang, G.; Zhou, J.; Song, H.; Li, Y.; Zhang, J.; Ding, S. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017, 8, e3147. [Google Scholar] [CrossRef] [PubMed]
- Borrirukwanit, K.; Pavasant, P.; Blick, T.; Lafleur, M.A.; Thompson, E.W. High threshold of β1 integrin inhibition required to block collagen I-induced membrane type-1 matrix metalloproteinase (MT1-MMP) activation of matrix metalloproteinase 2 (MMP-2). Cancer Cell Int. 2014, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Eibach, M.; Bartsch, J.W.; Dolga, A.M.; Schlomann, U.; Conrad, C.; Schieber, S.; Schilling, O.; Biniossek, M.L.; Culmsee, C.; et al. The metalloprotease-disintegrin ADAM8 contributes to temozolomide chemoresistance and enhanced invasiveness of human glioblastoma cells. Neuro Oncol. 2015, 17, 1474–1485. [Google Scholar] [CrossRef]
- Ashour, A.A.; Gurbuz, N.; Alpay, S.N.; Abdel-Aziz, A.A.; Mansour, A.M.; Huo, L.; Ozpolat, B. Elongation factor-2 kinase regulates TG2/β1 integrin/Src/uPAR pathway and epithelial-mesenchymal transition mediating pancreatic cancer cells invasion. J. Cell. Mol. Med. 2014, 18, 2235–2251. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Handa, A.; Tokunaga, T.; Tsuchida, T.; Lee, Y.H.; Kijima, H.; Yamazaki, H.; Ueyama, Y.; Fukuda, H.; Nakamura, M. Neuropilin-2 expression affects the increased vascularization and is a prognostic factor in osteosarcoma. Int. J. Oncol. 2000, 17, 291–295. [Google Scholar] [CrossRef]
- Yao, H.; Veine, D.M.; Livant, D.L. Therapeutic inhibition of breast cancer bone metastasis progression and lung colonization: Breaking the vicious cycle by targeting α5β1 integrin. Breast Cancer Res. Treat. 2016, 157, 489–501. [Google Scholar] [CrossRef]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3, e99911. [Google Scholar] [CrossRef] [Green Version]
- Grzesiak, J.J.; Tran Cao, H.S.; Burton, D.W.; Kaushal, S.; Vargas, F.; Clopton, P.; Snyder, C.S.; Deftos, L.J.; Hoffman, R.M.; Bouvet, M. Knockdown of the β(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. Int. J. Cancer 2011, 129, 2905–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelillo, C.; Bergamo, A.; Mollica, H.; Bestagno, M.; Sava, G. Colorectal Cancer Metastases Settle in the Hepatic Microenvironment Through α5β1 Integrin. J. Cell. Biochem. 2015, 116, 2385–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, M.; Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Berchtold, S.; Grünwald, B.; Krüger, A.; Reithmeier, A.; Hähl, T.; Cheng, T.; Feuchtinger, A.; Born, D.; Erkan, M.; Kleeff, J.; et al. Collagen type V promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Cancer Lett. 2015, 356, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Navab, R.; Strumpf, D.; To, C.; Pasko, E.; Kim, K.S.; Park, C.J.; Hai, J.; Liu, J.; Jonkman, J.; Barczyk, M.; et al. Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene 2016, 35, 1899–1908. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Ajani, J.A.; Sushovan, G.; Ochi, N.; Hwang, R.; Hafley, M.; Johnson, R.L.; Bresalier, R.S.; Logsdon, C.D.; Zhang, Z.; et al. Galectin-3 Mediates Tumor Cell-Stroma Interactions by Activating Pancreatic Stellate Cells to Produce Cytokines via Integrin Signaling. Gastroenterology 2018, 154, 1524–1537.e1526. [Google Scholar] [CrossRef]
- Anikeeva, N.; Steblyanko, M.; Fayngerts, S.; Kopylova, N.; Marshall, D.J.; Powers, G.D.; Sato, T.; Campbell, K.S.; Sykulev, Y. Integrin receptors on tumor cells facilitate NK cell-mediated antibody-dependent cytotoxicity. Eur. J. Immunol. 2014, 44, 2331–2339. [Google Scholar] [CrossRef]
- Jachetti, E.; Caputo, S.; Mazzoleni, S.; Brambillasca, C.S.; Parigi, S.M.; Grioni, M.; Piras, I.S.; Restuccia, U.; Calcinotto, A.; Freschi, M.; et al. Tenascin-C Protects Cancer Stem-like Cells from Immune Surveillance by Arresting T-cell Activation. Cancer Res. 2015, 75, 2095–2108. [Google Scholar] [CrossRef] [Green Version]
- Cantor, J.M.; Rose, D.M.; Slepak, M.; Ginsberg, M.H. Fine-tuning Tumor Immunity with Integrin Trans-regulation. Cancer Immunol. Res. 2015, 3, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Cai, W.; Zhang, Y.; Huang, C.; Zhang, H.; Liu, J.; Tang, K.; Xu, P.; Katirai, F.; Zhang, J.; et al. Innate immune cell-derived microparticles facilitate hepatocarcinoma metastasis by transferring integrin α(M)β₂ to tumor cells. J. Immunol. 2013, 191, 3453–3461. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.K.; Desai, N.S. Cancer Stem Cells: Acquisition, Characteristics, Therapeutic Implications, Targeting Strategies and Future Prospects. Stem Cell Rev. Rep. 2019, 15, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Gopalan, V.; Lam, A.K. Identification of Cancer Stem Cells in Esophageal Adenocarcinoma. Methods Mol. Biol. 2018, 1756, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiao, R.; Pan, S.; Yang, X.; Yuan, W.; Tu, Z.; Xu, M.; Zhu, Y.; Yin, Q.; Wu, Y.; et al. Uncovering the roles of long non-coding RNAs in cancer stem cells. J. Hematol. Oncol. 2017, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Lathia, J.; Liu, H.; Matei, D. The Clinical Impact of Cancer Stem Cells. Oncologist 2019, 25, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef]
- Prasad, S.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Srivastava, S.K. Cancer cells stemness: A doorstep to targeted therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165424. [Google Scholar] [CrossRef]
- Zheng, Y.; de la Cruz, C.C.; Sayles, L.C.; Alleyne-Chin, C.; Vaka, D.; Knaak, T.D.; Bigos, M.; Xu, Y.; Hoang, C.D.; Shrager, J.B.; et al. A rare population of CD24(+)ITGB4(+)Notch(hi) cells drives tumor propagation in NSCLC and requires Notch3 for self-renewal. Cancer Cell 2013, 24, 59–74. [Google Scholar] [CrossRef] [Green Version]
- Barnawi, R.; Al-Khaldi, S.; Majed Sleiman, G.; Sarkar, A.; Al-Dhfyan, A.; Al-Mohanna, F.; Ghebeh, H.; Al-Alwan, M. Fascin Is Critical for the Maintenance of Breast Cancer Stem Cell Pool Predominantly via the Activation of the Notch Self-Renewal Pathway. Stem Cells 2016, 34, 2799–2813. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Cho, K.H.; Jeong, K.J.; Park, Y.Y.; Kim, J.M.; Rha, S.Y.; Park, C.G.; Mills, G.B.; Cheong, J.H.; Lee, H.Y. Rab25 augments cancer cell invasiveness through a β1 integrin/EGFR/VEGF-A/Snail signaling axis and expression of fascin. Exp. Mol. Med. 2018, 50, e435. [Google Scholar] [CrossRef]
- Lahlou, H.; Sanguin-Gendreau, V.; Zuo, D.; Cardiff, R.D.; McLean, G.W.; Frame, M.C.; Muller, W.J. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc. Natl. Acad. Sci. USA 2007, 104, 20302–20307. [Google Scholar] [CrossRef] [Green Version]
- White, D.E.; Kurpios, N.A.; Zuo, D.; Hassell, J.A.; Blaess, S.; Mueller, U.; Muller, W.J. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 2004, 6, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schober, M.; Fuchs, E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-β and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 10544–10549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; He, J.; Liu, Y.; Simeone, D.M.; Lubman, D.M. Identification of glycoprotein markers for pancreatic cancer CD24+CD44+ stem-like cells using nano-LC-MS/MS and tissue microarray. J. Proteome Res. 2012, 11, 2272–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, A.; McMillan, R.H.; Chang, Y.T.; Penchev, V.R.; Rajeshkumar, N.V.; Maitra, A.; Goggins, M.G.; Eshelman, J.R.; Wolfgang, C.L.; Rasheed, Z.A.; et al. Direct Interactions With Cancer-Associated Fibroblasts Lead to Enhanced Pancreatic Cancer Stem Cell Function. Pancreas 2019, 48, 329–334. [Google Scholar] [CrossRef]
- Rasheed, Z.A.; Yang, J.; Wang, Q.; Kowalski, J.; Freed, I.; Murter, C.; Hong, S.M.; Koorstra, J.B.; Rajeshkumar, N.V.; He, X.; et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J. Natl. Cancer Inst. 2010, 102, 340–351. [Google Scholar] [CrossRef]
- Begum, A.; Ewachiw, T.; Jung, C.; Huang, A.; Norberg, K.J.; Marchionni, L.; McMillan, R.; Penchev, V.; Rajeshkumar, N.V.; Maitra, A.; et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS ONE 2017, 12, e0180181. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, T.M.A.; Di Molfetta, D.; Greco, M.R.; Koltai, T.; Alfarouk, K.O.; Reshkin, S.J.; Cardone, R.A. Tumor Microenvironment Features and Chemoresistance in Pancreatic Ductal Adenocarcinoma: Insights into Targeting Physicochemical Barriers and Metabolism as Therapeutic Approaches. Cancers 2021, 13, 6135. [Google Scholar] [CrossRef]
- Yang, D.; Shi, J.; Fu, H.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Yan, R.; Cai, Q. Integrinβ1 modulates tumour resistance to gemcitabine and serves as an independent prognostic factor in pancreatic adenocarcinomas. Tumour Biol. 2016, 37, 12315–12327. [Google Scholar] [CrossRef]
- Cannon, A.; Thompson, C.; Hall, B.R.; Jain, M.; Kumar, S.; Batra, S.K. Desmoplasia in pancreatic ductal adenocarcinoma: Insight into pathological function and therapeutic potential. Genes Cancer 2018, 9, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Manoukian, P.; Bijlsma, M.; van Laarhoven, H. The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth. Front. Cell Dev. Biol. 2021, 9, 743907. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Somer, B.G.; Park, J.O.; Li, C.P.; Scheulen, M.E.; Kasubhai, S.M.; Oh, D.Y.; Liu, Y.; Redhu, S.; Steplewski, K.; et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur. J. Cancer 2014, 50, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Alagesan, B.; Contino, G.; Guimaraes, A.R.; Corcoran, R.B.; Deshpande, V.; Wojtkiewicz, G.R.; Hezel, A.F.; Wong, K.K.; Loda, M.; Weissleder, R.; et al. Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer. Clin. Cancer Res. 2015, 21, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brannon, A., 3rd; Drouillard, D.; Steele, N.; Schoettle, S.; Abel, E.V.; Crawford, H.C.; Pasca di Magliano, M. Beta 1 integrin signaling mediates pancreatic ductal adenocarcinoma resistance to MEK inhibition. Sci. Rep. 2020, 10, 11133. [Google Scholar] [CrossRef]
- Petpiroon, N.; Bhummaphan, N.; Tungsukruthai, S.; Pinkhien, T.; Maiuthed, A.; Sritularak, B.; Chanvorachote, P. Chrysotobibenzyl inhibition of lung cancer cell migration through Caveolin-1-dependent mediation of the integrin switch and the sensitization of lung cancer cells to cisplatin-mediated apoptosis. Phytomedicine 2019, 58, 152888. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Ben-Josef, E.; Thomas, D.G.; Morgan, M.A.; Zalupski, M.M.; Khan, G.; Andrew Robinson, C.; Griffith, K.A.; Chen, C.S.; Ludwig, T.; et al. Caveolin-1 is Associated with Tumor Progression and Confers a Multi-Modality Resistance Phenotype in Pancreatic Cancer. Sci. Rep. 2015, 5, 10867. [Google Scholar] [CrossRef] [Green Version]
- Hehlgans, S.; Eke, I.; Storch, K.; Haase, M.; Baretton, G.B.; Cordes, N. Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells. Radiother. Oncol. 2009, 92, 362–370. [Google Scholar] [CrossRef]
- Cordes, N.; Frick, S.; Brunner, T.B.; Pilarsky, C.; Grützmann, R.; Sipos, B.; Klöppel, G.; McKenna, W.G.; Bernhard, E.J. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene 2007, 26, 6851–6862. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Deng, S.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2020, 13, 130. [Google Scholar] [CrossRef]
- Whitney, N.P.; Lamb, A.C.; Louw, T.M.; Subramanian, A. Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes. Ultrasound Med. Biol. 2012, 38, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, U.; Bashir, M.; Nabi, S.; Khanday, F.A. Epidermal growth factor receptor and integrins meet redox signaling through P66shc and Rac1. Cytokine 2021, 146, 155625. [Google Scholar] [CrossRef] [PubMed]
- Javadi, S.; Zhiani, M.; Mousavi, M.A.; Fathi, M. Crosstalk between Epidermal Growth Factor Receptors (EGFR) and integrins in resistance to EGFR tyrosine kinase inhibitors (TKIs) in solid tumors. Eur. J. Cell Biol. 2020, 99, 151083. [Google Scholar] [CrossRef] [PubMed]
- Leisewitz, A.V.; Zimmerman, E.I.; Huang, M.; Jones, S.Z.; Yang, J.; Graves, L.M. Regulation of ENT1 expression and ENT1-dependent nucleoside transport by c-Jun N-terminal kinase. Biochem. Biophys. Res. Commun. 2011, 404, 370–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binenbaum, Y.; Na’ara, S.; Gil, Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist. Updates 2015, 23, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Greenhalf, W.; Ghaneh, P.; Neoptolemos, J.P.; Palmer, D.H.; Cox, T.F.; Lamb, R.F.; Garner, E.; Campbell, F.; Mackey, J.R.; Costello, E.; et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J. Natl. Cancer Inst. 2014, 106, djt347. [Google Scholar] [CrossRef]
- Nordh, S.; Ansari, D.; Andersson, R. hENT1 expression is predictive of gemcitabine outcome in pancreatic cancer: A systematic review. World J. Gastroenterol. 2014, 20, 8482–8490. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Yang, J.; Cui, X.; Zhou, Z.; Zhan, H.; Ding, K.; Tian, X.; Yang, Z.; Fung, K.A.; et al. ZIP4 Increases Expression of Transcription Factor ZEB1 to Promote Integrin α3β1 Signaling and Inhibit Expression of the Gemcitabine Transporter ENT1 in Pancreatic Cancer Cells. Gastroenterology 2020, 158, 679–692.e671. [Google Scholar] [CrossRef]
- Mantoni, T.S.; Lunardi, S.; Al-Assar, O.; Masamune, A.; Brunner, T.B. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011, 71, 3453–3458. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.; Thomsen, A.; Follo, M.; Zamboglou, C.; Bronsert, P.; Mostafa, H.; Amen, A.; Mekawy, M.; Grosu, A.L.; Brunner, T.B. FAK inhibition radiosensitizes pancreatic ductal adenocarcinoma cells in vitro. Strahlenther. Onkol. 2021, 197, 27–38. [Google Scholar] [CrossRef]
- Al-Assar, O.; Demiciorglu, F.; Lunardi, S.; Gaspar-Carvalho, M.M.; McKenna, W.G.; Muschel, R.M.; Brunner, T.B. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother. Oncol. 2014, 111, 243–251. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Peng, W.; Li, Y.Y. Identification of novel candidate biomarkers for pancreatic adenocarcinoma based on TCGA cohort. Aging 2021, 13, 5698–5717. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Brentnall, T.A.; Pan, S.; Cooke, K.; Moyes, K.W.; Lane, Z.; Crispin, D.A.; Goodlett, D.R.; Aebersold, R.; Bronner, M.P. Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol. Cell. Proteom. 2007, 6, 1331–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Chiu, D.; Qin, D.; Niu, L.; Cai, J.; He, L.; Huang, W.; Xu, K. Detection and clinical significance of CD44v6 and integrin-β1 in pancreatic cancer patients using a triplex real-time RT-PCR assay. Appl. Biochem. Biotechnol. 2012, 167, 2257–2268. [Google Scholar] [CrossRef]
- Zhou, G.; Chiu, D.; Qin, D.; Niu, L.; Cai, J.; He, L.; Tan, D.; Xu, K. Expression of CD44v6 and integrin-β1 for the prognosis evaluation of pancreatic cancer patients after cryosurgery. Diagn. Pathol. 2013, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Chiu, D.; Qin, D.; Niu, L.; Cai, J.; He, L.; Huang, W.; Xu, K. The efficacy evaluation of cryosurgery in pancreatic cancer patients with the expression of CD44v6, integrin-β1, CA199, and CEA. Mol. Biotechnol. 2012, 52, 59–67. [Google Scholar] [CrossRef]
- Taniuchi, K.; Furihata, M.; Naganuma, S.; Sakaguchi, M.; Saibara, T. Overexpression of PODXL/ITGB1 and BCL7B/ITGB1 accurately predicts unfavorable prognosis compared to the TNM staging system in postoperative pancreatic cancer patients. PLoS ONE 2019, 14, e0217920. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef]
- Brown, C.W.; Amante, J.J.; Mercurio, A.M. Cell clustering mediated by the adhesion protein PVRL4 is necessary for α6β4 integrin-promoted ferroptosis resistance in matrix-detached cells. J. Biol. Chem. 2018, 293, 12741–12748. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.W.; Amante, J.J.; Goel, H.L.; Mercurio, A.M. The α6β4 integrin promotes resistance to ferroptosis. J. Cell Biol. 2017, 216, 4287–4297. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Y.; Zhang, C.; Zhan, M.; Jia, L.; Song, S.; Jiang, C.; Shen, M.; Shi, X. Fibronectin-Coated Metal-Phenolic Networks for Cooperative Tumor Chemo-/Chemodynamic/Immune Therapy via Enhanced Ferroptosis-Mediated Immunogenic Cell Death. ACS Nano 2022, 16, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liu, T.; Li, Y.; Lau, J.; Yang, Z.; Fan, W.; Zhou, Z.; Shi, C.; Ke, C.; Bregadze, V.I.; et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano 2018, 12, 11355–11365. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Plebanek, M.P.; Angeloni, N.L.; Vinokour, E.; Li, J.; Henkin, A.; Martinez-Marin, D.; Filleur, S.; Bhowmick, R.; Henkin, J.; Miller, S.D.; et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat. Commun. 2017, 8, 1319. [Google Scholar] [CrossRef]
- Anderson, R.L.; Balasas, T.; Callaghan, J.; Coombes, R.C.; Evans, J.; Hall, J.A.; Kinrade, S.; Jones, D.; Jones, P.S.; Jones, R.; et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 2019, 16, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.J.; Smith, J.A.; Rezniczek, G.A.; Pan, S.; Chen, R.; Brentnall, T.A.; Wiche, G.; Kelly, K.A. Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 19414–19419. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Armacki, M.; Polaschek, S.; Waldenmaier, M.; Morawe, M.; Ruhland, C.; Schmid, R.; Lechel, A.; Tharehalli, U.; Steup, C.; Bektas, Y.; et al. Protein Kinase D1, Reduced in Human Pancreatic Tumors, Increases Secretion of Small Extracellular Vesicles From Cancer Cells That Promote Metastasis to Lung in Mice. Gastroenterology 2020, 159, 1019–1035.e1022. [Google Scholar] [CrossRef]
- Casari, I.; Howard, J.A.; Robless, E.E.; Falasca, M. Exosomal integrins and their influence on pancreatic cancer progression and metastasis. Cancer Lett. 2021, 507, 124–134. [Google Scholar] [CrossRef]
- Almokadem, S.; Belani, C.P. Volociximab in cancer. Expert Opin. Biol. Ther. 2012, 12, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bell-McGuinn, K.M.; Matthews, C.M.; Ho, S.N.; Barve, M.; Gilbert, L.; Penson, R.T.; Lengyel, E.; Palaparthy, R.; Gilder, K.; Vassos, A.; et al. A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol. Oncol. 2011, 121, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besse, B.; Tsao, L.C.; Chao, D.T.; Fang, Y.; Soria, J.C.; Almokadem, S.; Belani, C.P. Phase Ib safety and pharmacokinetic study of volociximab, an anti-α5β1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer. Ann. Oncol. 2013, 24, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nwagwu, C.D.; Immidisetti, A.V.; Bukanowska, G.; Vogelbaum, M.A.; Carbonell, A.M. Convection-Enhanced Delivery of a First-in-Class Anti-β1 Integrin Antibody for the Treatment of High-Grade Glioma Utilizing Real-Time Imaging. Pharmaceutics 2020, 13, 40. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, W.D.; Hong, K.P.; Choi da, B.; Hong, J.W.; Kim, S.; Moon, Y.R.; Son, S.M.; Lee, O.J.; Lee, H.C.; et al. Novel monoclonal antibody against beta 1 integrin enhances cisplatin efficacy in human lung adenocarcinoma cells. J. Biomed. Res. 2016, 30, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Cianfrocca, M.E.; Kimmel, K.A.; Gallo, J.; Cardoso, T.; Brown, M.M.; Hudes, G.; Lewis, N.; Weiner, L.; Lam, G.N.; Brown, S.C.; et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br. J. Cancer 2006, 94, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Parvani, J.G.; Galliher-Beckley, A.J.; Schiemann, B.J.; Schiemann, W.P. Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β. Mol. Biol. Cell 2013, 24, 3449–3459. [Google Scholar] [CrossRef]
- Pan, B.; Guo, J.; Liao, Q.; Zhao, Y. β1 and β3 integrins in breast, prostate and pancreatic cancer: A novel implication. Oncol. Lett. 2018, 15, 5412–5416. [Google Scholar] [CrossRef] [Green Version]
- Moritz, M.N.O.; Merkel, A.R.; Feldman, E.G.; Selistre-de-Araujo, H.S.; Rhoades Sterling, J.A. Biphasic α2β1 Integrin Expression in Breast Cancer Metastasis to Bone. Int. J. Mol. Sci. 2021, 22, 6906. [Google Scholar] [CrossRef]
- Reynolds, A.R.; Hart, I.R.; Watson, A.R.; Welti, J.C.; Silva, R.G.; Robinson, S.D.; Da Violante, G.; Gourlaouen, M.; Salih, M.; Jones, M.C.; et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 2009, 15, 392–400. [Google Scholar] [CrossRef]
- Legler, D.F.; Wiedle, G.; Ross, F.P.; Imhof, B.A. Superactivation of integrin alphavbeta3 by low antagonist concentrations. J. Cell Sci. 2001, 114, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Tolomelli, A.; Galletti, P.; Baiula, M.; Giacomini, D. Can Integrin Agonists Have Cards to Play against Cancer? A Literature Survey of Small Molecules Integrin Activators. Cancers 2017, 9, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhu, J.; Negri, A.; Provasi, D.; Filizola, M.; Coller, B.S.; Springer, T.A. Closed headpiece of integrin αIIbβ3 and its complex with an αIIbβ3-specific antagonist that does not induce opening. Blood 2010, 116, 5050–5059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mould, A.P.; Craig, S.E.; Byron, S.K.; Humphries, M.J.; Jowitt, T.A. Disruption of integrin-fibronectin complexes by allosteric but not ligand-mimetic inhibitors. Biochem. J. 2014, 464, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Marsico, G.; Russo, L.; Quondamatteo, F.; Pandit, A. Glycosylation and Integrin Regulation in Cancer. Trends Cancer 2018, 4, 537–552. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, Y.; Sun, J.; Yang, T.; Zhi, D.; Zhang, E.; Zhong, F.; Zhen, Y.; Zhang, S.; Zhang, S. Integrin α(v)β(3)-targeted liposomal drug delivery system for enhanced lung cancer therapy. Colloids Surf. B Biointerfaces 2021, 201, 111623. [Google Scholar] [CrossRef]
- Gao, J.; Wang, S.; Dong, X.; Wang, Z. RGD-expressed bacterial membrane-derived nanovesicles enhance cancer therapy via multiple tumorous targeting. Theranostics 2021, 11, 3301–3316. [Google Scholar] [CrossRef]
- Shabana, A.M.; Kambhampati, S.P.; Hsia, R.C.; Kannan, R.M.; Kokkoli, E. Thermosensitive and biodegradable hydrogel encapsulating targeted nanoparticles for the sustained co-delivery of gemcitabine and paclitaxel to pancreatic cancer cells. Int. J. Pharm. 2021, 593, 120139. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, G.; Lang, L.; Li, F.; Fan, X.; Yan, X.; Yao, S.; Yan, W.; Huo, L.; Chen, L.; et al. Clinical Translation of a Dual Integrin αvβ3- and Gastrin-Releasing Peptide Receptor-Targeting PET Radiotracer, 68Ga-BBN-RGD. J. Nucl. Med. 2017, 58, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Liu, S. Radiolabeled Cyclic RGD Peptide Bioconjugates as Radiotracers Targeting Multiple Integrins. Bioconjug. Chem. 2015, 26, 1413–1438. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Wang, Y.; Lu, D.; Xu, X.; Zhou, X.; Zhang, H.; Zhang, T.; Zhu, H.; Yang, Z.; Wang, F.; et al. Clinical Translation of a (68)Ga-Labeled Integrin α(v)β(6)-Targeting Cyclic Radiotracer for PET Imaging of Pancreatic Cancer. J. Nucl. Med. 2020, 61, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, L.; Long, Y.; Fang, H.; Li, M.; Liu, Q.; Xia, X.; Qin, C.; Zhang, Y.; Lan, X.; et al. Synthesis and Preclinical Evaluation of a (68)Ga-Radiolabeled Peptide Targeting Very Late Antigen-3 for PET Imaging of Pancreatic Cancer. Mol. Pharm. 2020, 17, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, J.; Li, W.; Hao, X.; Hang, Q. Roles of Integrins in Gastrointestinal Cancer Metastasis. Front. Mol. Biosci. 2021, 8, 708779. [Google Scholar] [CrossRef]
- Gu, J.; Isaji, T.; Xu, Q.; Kariya, Y.; Gu, W.; Fukuda, T.; Du, Y. Potential roles of N-glycosylation in cell adhesion. Glycoconj. J. 2012, 29, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Isaji, T.; Sato, Y.; Zhao, Y.; Miyoshi, E.; Wada, Y.; Taniguchi, N.; Gu, J. N-glycosylation of the beta-propeller domain of the integrin alpha5 subunit is essential for alpha5beta1 heterodimerization, expression on the cell surface, and its biological function. J. Biol. Chem. 2006, 281, 33258–33267. [Google Scholar] [CrossRef] [Green Version]
- Isaji, T.; Sato, Y.; Fukuda, T.; Gu, J. N-glycosylation of the I-like domain of beta1 integrin is essential for beta1 integrin expression and biological function: Identification of the minimal N-glycosylation requirement for alpha5beta1. J. Biol. Chem. 2009, 284, 12207–12216. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.B.; Lee, I.; Kamar, M.; Akiyama, S.K.; Pierce, M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res. 2002, 62, 6837–6845. [Google Scholar]
- Sato, Y.; Isaji, T.; Tajiri, M.; Yoshida-Yamamoto, S.; Yoshinaka, T.; Somehara, T.; Fukuda, T.; Wada, Y.; Gu, J. An N-glycosylation site on the beta-propeller domain of the integrin alpha5 subunit plays key roles in both its function and site-specific modification by beta1,4-N-acetylglucosaminyltransferase III. J. Biol. Chem. 2009, 284, 11873–11881. [Google Scholar] [CrossRef] [Green Version]
- Isaji, T.; Gu, J.; Nishiuchi, R.; Zhao, Y.; Takahashi, M.; Miyoshi, E.; Honke, K.; Sekiguchi, K.; Taniguchi, N. Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and down-regulates cell adhesion and cell migration. J. Biol. Chem. 2004, 279, 19747–19754. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Isaji, T.; Im, S.; Fukuda, T.; Hashii, N.; Takakura, D.; Kawasaki, N.; Gu, J. β-Galactoside α2,6-sialyltranferase 1 promotes transforming growth factor-β-mediated epithelial-mesenchymal transition. J. Biol. Chem. 2014, 289, 34627–34641. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Isaji, T.; Im, S.; Fukuda, T.; Kameyama, A.; Gu, J. Expression of N-Acetylglucosaminyltransferase III Suppresses α2,3-Sialylation, and Its Distinctive Functions in Cell Migration Are Attributed to α2,6-Sialylation Levels. J. Biol. Chem. 2016, 291, 5708–5720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, Q.; Isaji, T.; Hou, S.; Wang, Y.; Fukuda, T.; Gu, J. A Key Regulator of Cell Adhesion: Identification and Characterization of Important N-Glycosylation Sites on Integrin α5 for Cell Migration. Mol. Cell. Biol. 2017, 37, e00558-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, Q.; Isaji, T.; Hou, S.; Im, S.; Fukuda, T.; Gu, J. Integrin α5 Suppresses the Phosphorylation of Epidermal Growth Factor Receptor and Its Cellular Signaling of Cell Proliferation via N-Glycosylation. J. Biol. Chem. 2015, 290, 29345–29360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, Q.; Isaji, T.; Hou, S.; Zhou, Y.; Fukuda, T.; Gu, J. N-Glycosylation of integrin α5 acts as a switch for EGFR-mediated complex formation of integrin α5β1 to α6β4. Sci. Rep. 2016, 6, 33507. [Google Scholar] [CrossRef] [Green Version]
- Kuo, T.C.; Wu, M.H.; Yang, S.H.; Chen, S.T.; Hsu, T.W.; Jhuang, J.Y.; Liao, Y.Y.; Tien, Y.W.; Huang, M.C. C1GALT1 high expression is associated with poor survival of patients with pancreatic ductal adenocarcinoma and promotes cell invasiveness through integrin α(v). Oncogene 2021, 40, 1242–1254. [Google Scholar] [CrossRef]
- Liang, C.; Fukuda, T.; Isaji, T.; Duan, C.; Song, W.; Wang, Y.; Gu, J. α1,6-Fucosyltransferase contributes to cell migration and proliferation as well as to cancer stemness features in pancreatic carcinoma. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129870. [Google Scholar] [CrossRef]
- Walser, M.; Umbricht, C.A.; Fröhli, E.; Nanni, P.; Hajnal, A. β-Integrin de-phosphorylation by the Density-Enhanced Phosphatase DEP-1 attenuates EGFR signaling in C. elegans. PLoS Genet. 2017, 13, e1006592. [Google Scholar] [CrossRef] [Green Version]
| No. | Integrin | Year | Cell Lines | Expression | Functions | Mechanism | Model Used | Reference PMID | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proliferation | Cell Cycle | Apoptosis | Angiogenesis | Adhesion | Migration | Invasion | CSC | Therapy Resistance | Tumor Growth | Tumor Metastasis | ECM Remodeling | ||||||||
| 1 | β1 | 2003 | MIA PaCa-2, BxPC-3 | up | + | + | GDNF/integrin β1 | cell | 12883269 | ||||||||||
| 2 | β1 | 2005 | SW1990, Capan-2 | up | + | + | + | GDNF/integrin β1 | cell | 15999351 | |||||||||
| 3 | α6β1 | 2006 | BxPC-3, Capan-2, SW1990 | up | + | + | + | IL-1α/integrin β1 and uPA/uPAR/Ras, ERK | cell | 16504015 | |||||||||
| 4 | β1 | 2006 | Panc-1 | up | + | CCK2/integrin β1/Src, PI3K | cell/animal | 16547500 | |||||||||||
| 5 | β1 | 2006 | Panc-1, BxPC-3 | up | + | integrin β1/FAK/B-catenin phosphorylation/-Lef/Tcf | cell | 16651417 | |||||||||||
| 6 | β1 | 2007 | PATU8902, MIA PaCa-2, Panc-1 | up | + | Cav-1/integrin β1/FAK | cell | 17471232 | |||||||||||
| 7 | β1 | 2007 | Capan-1 | up | + | p16INK4a/glycosylation/integrin β1 maturation | cell | 17535296 | |||||||||||
| 8 | β1 | 2008 | BxPC-3, Panc-1, SW1990 | up | + | integrin β1/ERK1/2 phosphorylation | cell | 17688882 | |||||||||||
| 9 | β1 | 2007 | Panc-1 | up | + | + | Np-1/integrin β1/FAK | cell | 17726369 | ||||||||||
| 10 | β1 | 2008 | Capan-1, Colo-357, AsPC-1, BxPC-3, MIA PaCa-2, Panc-1 | up | + | + | + | - | cell | 18754866 | |||||||||
| 11 | β1 | 2008 | HPDE6 | up | + | CX3CR1/integrin β1/FAK | cell/animal | 18974152 | |||||||||||
| 12 | β1 | 2009 | MIA PaCa-2 | up | + | + | + | cell | 19825166 | ||||||||||
| 13 | β1 | 2010 | bEnd.3, MEF | up | + | + | Fbln5/integrin β1/ROS | cell/animal | 20197418 | ||||||||||
| 14 | β1 | 2011 | FG, Colo-357 | up | + | + | + | + | + | - | cell/animal | 21491421 | |||||||
| 15 | β1 | 2011 | Panc-1, PSN-1, MIA PaCa-2 | up | + | PSC/integrin β1/FAK/radioresistance | cell/animal | 21558392 | |||||||||||
| 16 | β1 | 2011 | Panc-1 | up | + | FAP/ECM/integrin β1 | cell | 21668992 | |||||||||||
| 17 | β1 | 2011 | Panc-1, FG-Met2 | up | + | + | - | cell | 21678462 | ||||||||||
| 18 | β1 | 2011 | T3M4, BxPC-3, COLO-357 | up | + | + | + | + | DNp63a/EGFR, integrin β1/drug resistance | cell | 22053213 | ||||||||
| 19 | β1 | 2012 | Panc-1, AsPC-1 | up | + | integrin β1/Rho | cell | 22232555 | |||||||||||
| 20 | β1 | 2012 | Panc-1 | up | + | - | cell | 22335271 | |||||||||||
| 21 | β1 | 2012 | PT45-P1 | up | + | + | L1CAM/integrin β1/FAK/NF-κB/IL-1β/EMT | cell | 22764136 | ||||||||||
| 22 | β1 | 2013 | Capan-2, FG, Colo-357, Panc-1, Panc1-MUC1 | up | + | + | + | + | + | core 3 synthase/integrin β1/FAK | cell/animal | 23754791 | |||||||
| 23 | α2β1 | 2014 | Panc-1, UlaPaCa | up | + | + | integrin α2β1/FAK | cell | 24201748 | ||||||||||
| 24 | β1 | 2014 | Panc-1, AsPC-1,MIA PaCa-2 | up | + | + | + | + | p53/Myo10/integrin β1/filopodia-inducing | cell/animal | 24487586 | ||||||||
| 25 | β1 | 2014 | AsPC1, BxPC-3, CFPAC-1, Panc-1, SW1990 | up | + | + | + | + | integrin β1/FAK, AKT, and ERK/Gli-1/EMT | cell | 24720337 | ||||||||
| 26 | β1 | 2014 | MIA PaCa2, BXPC-3 ASPC-1, Panc-1 | up | GD3/integrin β1/FAK/AKT | cell | 24842107 | ||||||||||||
| 27 | β1 | 2014 | PANC-1, MIA PaCa-2 | up | + | + | eEF-2K/TG2/integrin β/Src/uPAR/MMP-2/EMT | cell | 25215932 | ||||||||||
| 28 | α2β1 | 2014 | BxPc-3, Capan-1, Panc-1 | up | + | + | + | integrin β1/FAK/ERK1/2 | cell/animal | 25336636 | |||||||||
| 29 | β1 | 2015 | AsPC-1, Panc-1, MIA PaCa-2 | up | + | + | + | EPAC1/PKC/integrin β1 trafficking and activation | cell/animal | 25385424 | |||||||||
| 30 | β1 | 2015 | AsPC-1, Capan-1, SU.86.86, PANC-1 | up | + | + | + | + | + | - | cell/animal | 25449434 | |||||||
| 31 | β1 | 2016 | ASPC-1, Panc-1, Suit-2 | up | + | + | PHLPP/AKT/integrin β1 | cell | 26760962 | ||||||||||
| 32 | β1 | 2016 | PSC | up | + | integrin β1/ECM/matrix remodeling | cell | 27170254 | |||||||||||
| 33 | β1 | 2016 | MIA PaCa-2, AsPC-1 | up | + | integrin β1/Cdc42, AKT | cell | 27289231 | |||||||||||
| 34 | β1 | 2017 | MIA PaCa-2, AsPC-1, BxPC-3, Panc-1, Capan-2,SW1990 | up | + | + | REGF receptor, neuropilin-1/integrin β1/Src-AKT bypass signaling | cell/animal | 27797376 | ||||||||||
| 35 | β1 | 2017 | Panc-1, L3.6pL, MIA PaCa2 | up | + | + | NR4A1/p300/Sp/integrin β1 | cell | 28418095 | ||||||||||
| 36 | β1 | 2017 | AsPC-1, BxPC-3, CFPAC1, Panc-1 | up | + | Fyn/P21-activated kinase 1/hnRNP E1/the alterative splicing of integrin β1. | cell/animal | 28560430 | |||||||||||
| 37 | β1 | 2017 | BxPC-3, Capan-1, MIA PaCa-2 | up | Y | + | + | + | integrin β1/FAK | cell/animal | 28692661 | ||||||||
| 38 | α2β1 | 2018 | Panc-1 | up | + | + | + | integrin β1/JNK, ERK kinases, Src | cell/animal | 28916526 | |||||||||
| 39 | β1 | 2017 | AsPC-1, BxPC-3, Panc-1 | up | + | + | + | integrin β1/EGFR/ERK/MAPK/EMT | cell/animal | 29072694 | |||||||||
| 40 | β1 | 2018 | PSC | up | + | + | + | + | GAL3/integrin β1/ILK/NF-kB/IL-8 | cell/animal | 29274868 | ||||||||
| 41 | β1 | 2018 | MIA PaCa-2, Capan-1, AsPC-1 | up | + | + | + | + | + | + | MUC4/integrin β1/FAK/ERK | cell/animal | 29777904 | ||||||
| 42 | β1 | 2018 | MIA PaCa-2 | up | + | + | VASP/integrin β1-FAK-YAP1/TAZ | cell/animal | 29872721 | ||||||||||
| 43 | β1 | 2018 | Panc-1, SW1990, MIA Paca-2 | up | + | + | + | miR-124/β1/phospho-FAK, phosphor-AKT, phospho-EEK1/2 | cell | 29988949 | |||||||||
| 44 | β1 | 2018 | Panc-1 | up | + | integrin β1/Cdc42 | cell | 30241340 | |||||||||||
| 45 | β1 | 2018 | AsPC-1 | up | + | integrin β1/Cdc42/p110b/PI3K | cell/animal | 30243721 | |||||||||||
| 46 | β1 | 2018 | Panc-1, PK59 | up | + | + | H19/integrin β1,CD24 | cell | 30410672 | ||||||||||
| 47 | β1 | 2019 | Capan-1,BxPC-3 | up | + | + | + | integrin β1/FAK/EMT | cell | 30747824 | |||||||||
| 48 | α11β1 | 2019 | myCAF | up | + | + | - | cell | 31159419 | ||||||||||
| 49 | α5β1 | 2019 | MIA PaCa-2, SW1990, CFPAC-1, PANC-1, AsPC-1, BxPC-3, Panc 03.27 | up | + | + | TGF-β/TFEB/RAB5A/α5β1 endocytosis | cell/animal | 31387632 | ||||||||||
| 50 | β1 | 2019 | MIA PaCa-2 | up | + | integrin β1/c-Myc degradation | cell | 31452837 | |||||||||||
| 51 | β1 | 2019 | SW1990, AsPC-1, Panc-1, BxPC-3 | up | + | + | + | miR-760/MOV10/integrin β1 | cell | 31693728 | |||||||||
| 52 | α3β1 | 2020 | AsPC-1, MIA PaCa-2 | up | + | + | + | + | ZIP4/ZEB/α3β1/JNK/ENT1/drug resistance | cell/animal | 31711924 | ||||||||
| 53 | β1 | 2020 | Panc-1, BxPC-3, MIA PaCa-2 | up | + | HLA-B/integrin β1 | cell | 32194036 | |||||||||||
| 54 | β1 | 2020 | PANC-1 | up | + | + | integrin β1 and Heparan Sulfate Dual-Targeting/YAP | cell | 32266811 | ||||||||||
| 55 | β1 | 2020 | iKras*p53* PC cells | up | + | integrin β1/Kras | cell | 32636409 | |||||||||||
| 56 | β1 | 2020 | Panc-1 | up | + | + | integrin β1/FAK, AKT, ERK1/2, NF-κB | cell | 33086527 | ||||||||||
| 57 | β1 | 2022 | Panc-1 | up | + | + | + | FxOH/integrin β1/FAK, paxillin, FYN, AKT, PPARγ | cell | 33590779 | |||||||||
| 58 | β1 | 2021 | Panc-1 | up | + | mi-16/integrin β1/PI3K/AKT | cell | 33591944 | |||||||||||
| 59 | β1 | 2021 | Panc-1, MIA PaCa-2 | up | + | + | + | hERG1/integrin β1 complex/AKT, HIF-1α | cell/animal | 34045227 | |||||||||
| 60 | β1 | 2022 | MIA PaCa-2 | up | + | - | cell | 34481933 | |||||||||||
| 61 | β1 | 2021 | MIA PaCa-2 | up | + | integrin β1/kindlin-2/TGF-β receptor 2/Smad2/3 | cell | 34638957 | |||||||||||
| 62 | β1 | 2022 | adipose-derived mesenchymal stem cells | up | + | + | + | Mucin 5AC/CD44-integrin β1/Rac1 | cell/animal | 35219699 | |||||||||
| 63 | β1 | 2021 | CF Pac-1, SW1990 | up | + | RAB5A/integrin β1/Cdc42 | cell | 33341673 | |||||||||||
| No | Year | Detection Method | Source | Expression | No. of Patients | Lymphatic Invasion | Distance Metastasis | TNM Stage | AUC a | Survival | Prognostic Marker | Reference PMID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2012 | PCR | PBMC b | up | 37 | + | + | + | 22382453 | |||
| 2 | 2012 | PCR | PBMC | up | 30 | + | + | 22695923 | ||||
| 3 | 2013 | PCR, ELISA | PBMC, plasma | up | 54 | + | + | + | DFS c | + | 24004467 | |
| 4 | 2016 | IHC | tissue | up | 63 | OS d, DFS | + | 27289231 | ||||
| 5 | 2017 | IHC | tissue | up | 68 | + | OS | + | 29072694 | |||
| 6 | 2018 | IHC | tissue | up | 30 | OS, DFS | + | 29988949 | ||||
| 7 | 2019 | IHC | tissue | up | 102 | + (combined with PODXL/BCL7B) | 31166991 | |||||
| 8 | 2020 | IHC | tissue | up | 93 | 31711924 | ||||||
| 9 | 2021 | public database | tissue | up | 178 | 0.8635 | OS, DFS | + | 33591944 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Peng, L.; Chen, Q.; Ye, Z.; Zhao, T.; Hou, S.; Gu, J.; Hang, Q. Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications. Cancers 2022, 14, 3377. https://doi.org/10.3390/cancers14143377
Li J, Peng L, Chen Q, Ye Z, Zhao T, Hou S, Gu J, Hang Q. Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications. Cancers. 2022; 14(14):3377. https://doi.org/10.3390/cancers14143377
Chicago/Turabian StyleLi, Jiajia, Liyao Peng, Qun Chen, Ziping Ye, Tiantian Zhao, Sicong Hou, Jianguo Gu, and Qinglei Hang. 2022. "Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications" Cancers 14, no. 14: 3377. https://doi.org/10.3390/cancers14143377
APA StyleLi, J., Peng, L., Chen, Q., Ye, Z., Zhao, T., Hou, S., Gu, J., & Hang, Q. (2022). Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications. Cancers, 14(14), 3377. https://doi.org/10.3390/cancers14143377






