Comparative Efficacy of Tyrosine Kinase Inhibitors and Antibody–Drug Conjugates in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A Systematic Review and Network Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
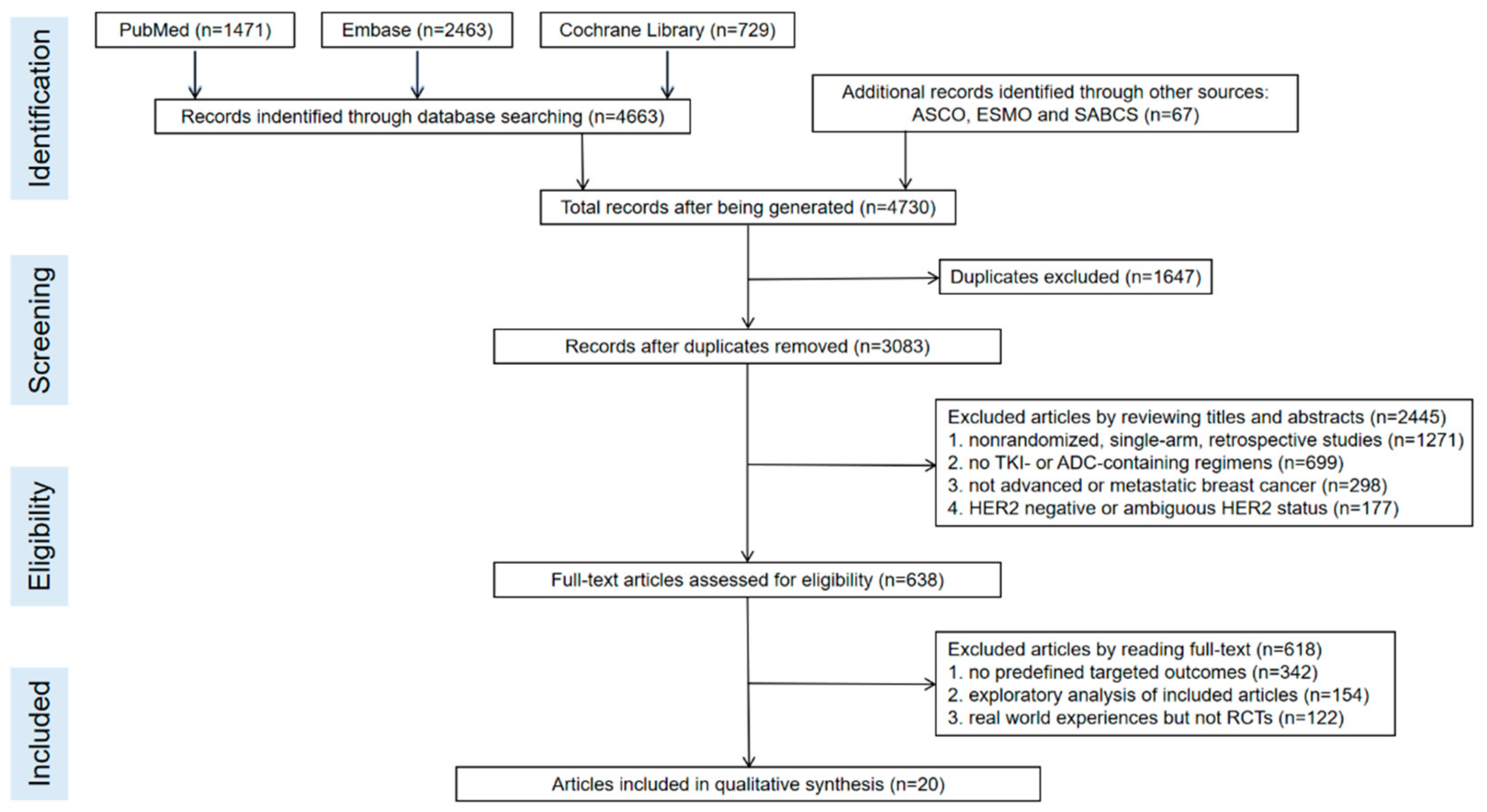
2.1. Search Strategy and Selection Criteria
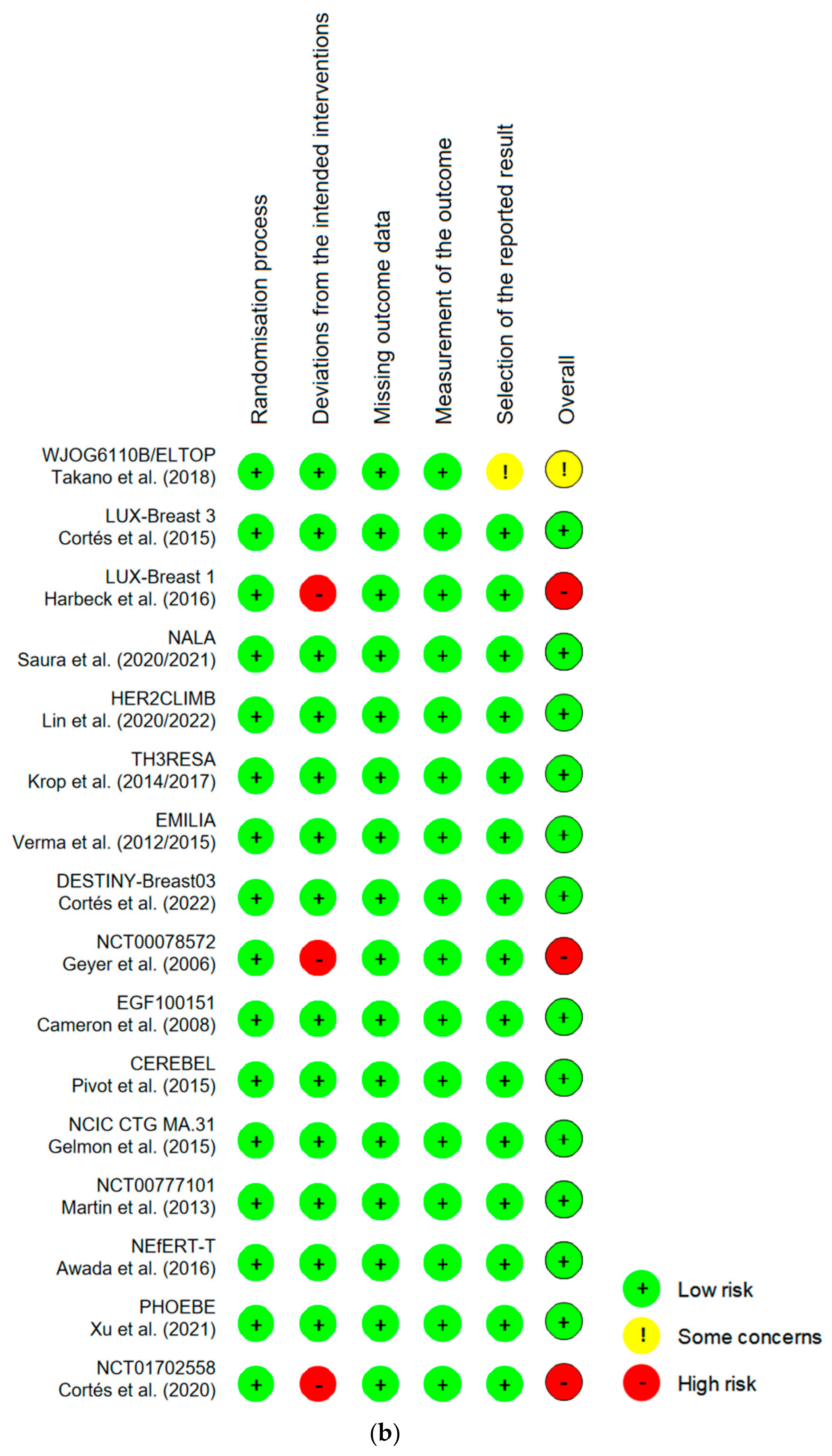
2.2. Data Extraction and Quality Assessment
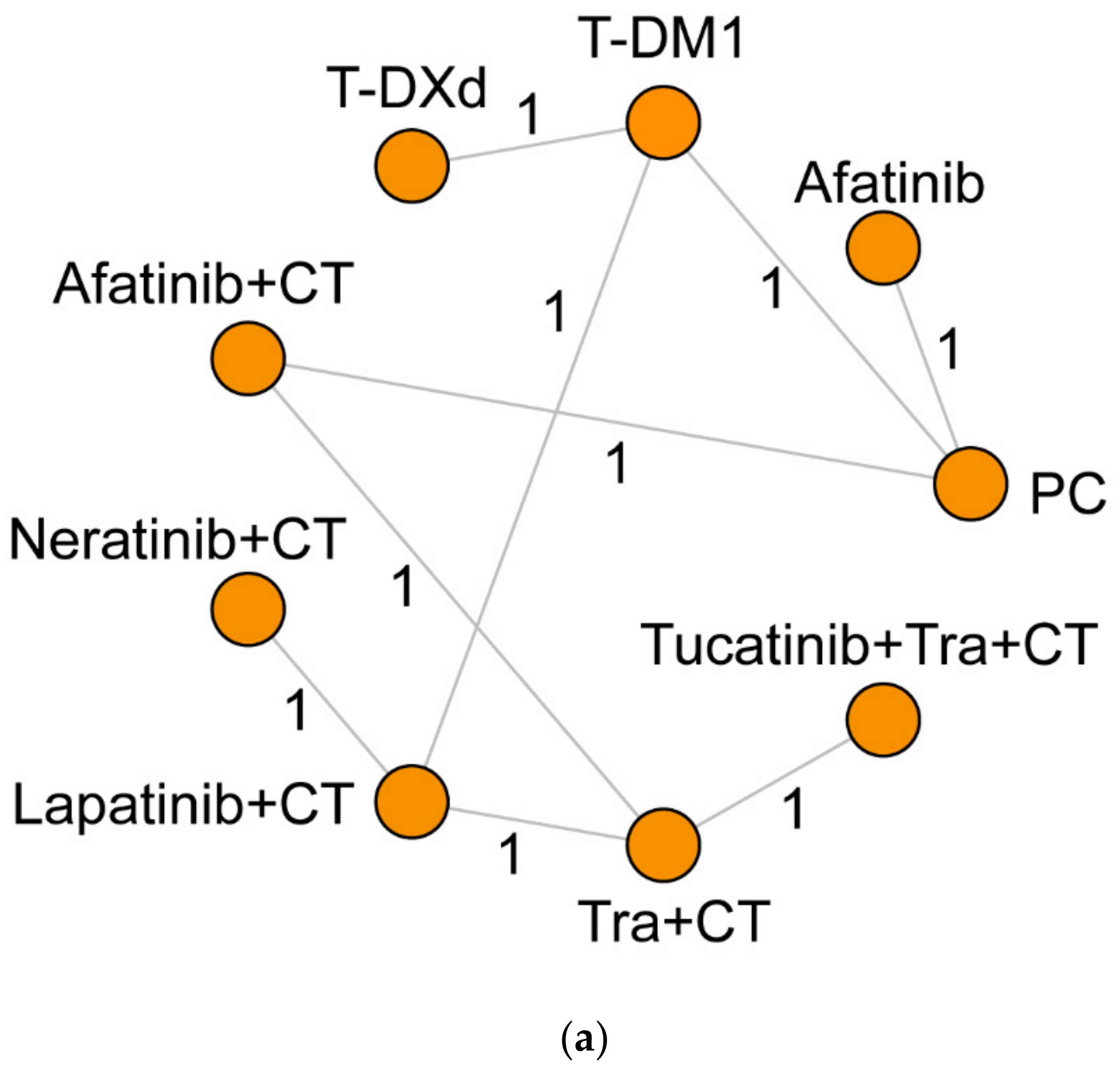
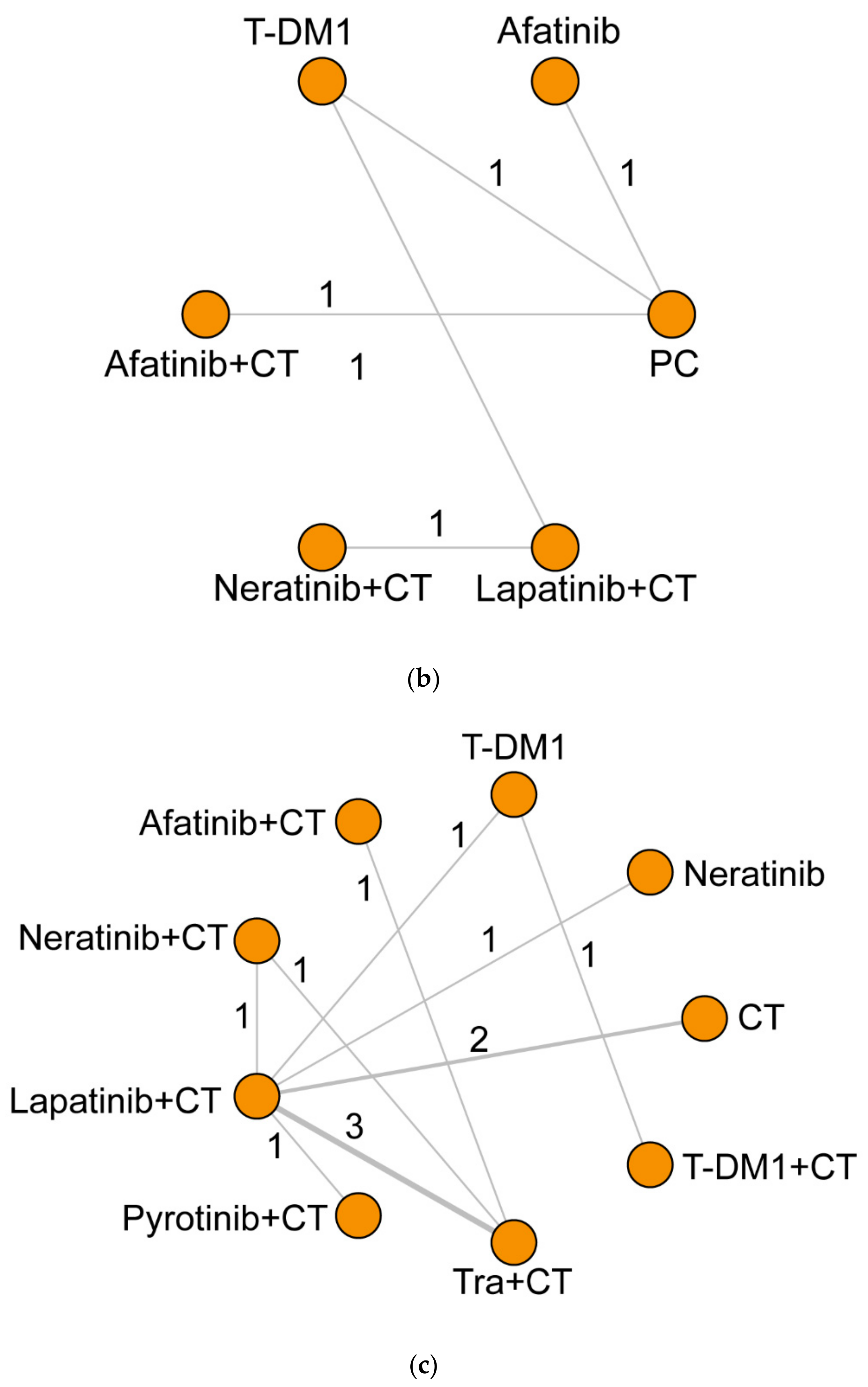
2.3. Data Synthesis and Statistical Analysis
3. Results
3.1. Characteristics of the Included Study
3.2. Survival Outcomes
3.3. CNS Recurrent Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of Brain Metastases. Curr. Oncol. Rep. 2011, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef]
- Watase, C.; Shiino, S.; Shimoi, T.; Noguchi, E.; Kaneda, T.; Yamamoto, Y.; Yonemori, K.; Takayama, S.; Suto, A. Breast Cancer Brain Metastasis-Overview of Disease State, Treatment Options and Future Perspectives. Cancers 2021, 13, 1078. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Warren, L.E.; Bellon, J.R.; Punglia, R.S.; Claus, E.B.; Lee, E.Q.; Wen, P.Y.; Haas-Kogan, D.A.; et al. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol. 2017, 3, 1069–1077. [Google Scholar] [CrossRef]
- Ramakrishna, N.; Temin, S.; Chandarlapaty, S.; Crews, J.R.; Davidson, N.E.; Esteva, F.; Giordano, S.H.; Gonzalez-Angulo, A.M.; Kirshner, J.J.; Krop, I.; et al. Recommendations on Disease Management for Patients With Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 2100–2108. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Angeli, E.; Nguyen, T.T.; Janin, A.; Bousquet, G. How to Make Anticancer Drugs Cross the Blood–Brain Barrier to Treat Brain Metastases. Int. J. Mol. Sci. 2019, 21, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steeg, P.S.; Camphausen, K.A.; Smith, Q.R. Brain metastases as preventive and therapeutic targets. Nat. Rev. Cancer 2011, 11, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Duchnowska, R.; Loibl, S.; Jassem, J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat. Rev. 2018, 67, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zou, Y.; Gao, T.; Xie, L.; Tan, D.; Xie, X. Therapeutic Landscape of Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. Cancer Control 2022, 29, 10732748221099230. [Google Scholar] [CrossRef] [PubMed]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: State of the art and future directions. Breast Cancer Res. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Pérez-García, J.M.; Batista, M.V.; Cortez, P.; Ruiz-Borrego, M.; Cejalvo, J.M.; de la Haba-Rodriguez, J.; Garrigós, L.; Racca, F.; Servitja, S.; Blanch, S.; et al. Trastuzumab Deruxtecan in Patients with Central Nervous System In-volvement from HER2-Positive Breast Cancer: The DEBBRAH Trial. Neuro-Oncology 2022, noac144. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, T.; Tsurutani, J.; Takahashi, M.; Yamanaka, T.; Sakai, K.; Ito, Y.; Fukuoka, J.; Kimura, H.; Kawabata, H.; Tamura, K.; et al. A randomized phase II trial of trastuzumab plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxanes: WJOG6110B/ELTOP. Breast 2018, 40, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Dieras, V.; Ro, J.; Barriere, J.; Bachelot, T.; Hurvitz, S.; Le Rhun, E.; Espié, M.; Kim, S.B.; Schneeweiss, A.; et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): A ran-domised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 1700–1710. [Google Scholar] [PubMed]
- Harbeck, N.; Huang, C.-S.; Hurvitz, S.; Yeh, D.-C.; Shao, Z.; Im, S.-A.; Jung, K.H.; Shen, K.; Ro, J.; Jassem, J.; et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): An open-label, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 357–366. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Saura, C.; Oliveira, M.; Trudeau, M.E.; Moy, B.; Delaloge, S.; Gradishar, W.; Kim, S.-B.; Haley, B.; Ryvo, L.; et al. Efficacy of Neratinib Plus Capecitabine in the Subgroup of Patients with Central Nervous System Involvement from the NALA Trial. Oncol. 2021, 26, e1327–e1338. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609, Erratum in 2020, 382, 586. [Google Scholar] [CrossRef]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619. [Google Scholar] [CrossRef]
- Curigliano, G.; Mueller, V.; Borges, V.; Hamilton, E.; Hurvitz, S.; Loi, S.; Murthy, R.; Okines, A.; Paplomata, E.; Cameron, D.; et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): Final overall survival analysis. Ann. Oncol. 2021, 33, 321–329. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.M.; Smitt, M.; Yu, R.; Leung, A.C.; Wildiers, H.; TH3RESA study collaborators. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Diéras, V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann. Oncol. 2014, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, D.; Casey, M.; Press, M.; Lindquist, D.; Pienkowski, T.; Romieu, C.G.; Chan, S.; Jagiello-Gruszfeld, A.; Kaufman, B.; Crown, J.; et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 2008, 112, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Manikhas, A.; Żurawski, B.; Chmielowska, E.; Karaszewska, B.; Allerton, R.; Chan, S.; Fabi, A.; Bidoli, P.; Gori, S.; et al. CEREBEL (EGF111438): A Phase III, Randomized, Open-Label Study of Lapatinib Plus Capecitabine Versus Trastuzumab Plus Capecitabine in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2015, 33, 1564–1573. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Boyle, F.M.; Kaufman, B.; Huntsman, D.G.; Manikhas, A.; Di Leo, A.; Martin, M.; Schwartzberg, L.S.; Lemieux, J.; Aparicio, S.; et al. Lapatinib or Trastuzumab Plus Taxane Therapy for Human Epidermal Growth Factor Receptor 2–Positive Advanced Breast Cancer: Final Results of NCIC CTG MA.31. J. Clin. Oncol. 2015, 33, 1574–1583. [Google Scholar] [CrossRef]
- Martin, M.; Bonneterre, J.; Geyer, C.E.; Ito, Y.; Ro, J.; Lang, I.; Kim, S.-B.; Germa, C.; Vermette, J.; Wang, K.; et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur. J. Cancer 2013, 49, 3763–3772. [Google Scholar] [CrossRef]
- Awada, A.; Colomer, R.; Inoue, K.; Bondarenko, I.; Badwe, R.A.; Demetriou, G.; Lee, S.C.; Mehta, A.O.; Kim, S.B.; Bachelot, T.; et al. Neratinib Plus Paclitaxel vs Trastuzumab Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer: The NEfERT-T Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1557–1564. [Google Scholar] [CrossRef]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef]
- Cortés, J.; Diéras, V.; Lorenzen, S.; Montemurro, F.; Riera-Knorrenschild, J.; Thuss-Patience, P.; Allegrini, G.; De Laurentiis, M.; Lohrisch, C.; Oravcová, E.; et al. Efficacy and Safety of Trastuzumab Emtansine Plus Capecitabine vs Trastuzumab Emtansine Alone in Patients With Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer: A Phase 1 and Ran-domized Phase 2 Trial. JAMA Oncol. 2020, 6, 1203–1209. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Gabos, Z.; Sinha, R.; Hanson, J.; Chauhan, N.; Hugh, J.; Mackey, J.R.; Abdulkarim, B. Prognostic Significance of Human Epidermal Growth Factor Receptor Positivity for the Development of Brain Metastasis After Newly Diagnosed Breast Cancer. J. Clin. Oncol. 2006, 24, 5658–5663. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Cancer 2019, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Pegram, M.D.; Lai, C.; Lacasia, A.; Stein, A.; Yoo, B.; Perez, E. Abstract OT3-01-04: An open-label, single-arm, phase II study of pertuzumab with high-dose trastuzumab for the treatment of central nervous system progression post-radiotherapy in patients with HER2-positive metastatic breast cancer (PATRICIA). Cancer Res. 2016, 76, OT3-01. [Google Scholar] [CrossRef]
- Morgan, A.J.; Giannoudis, A.; Palmieri, C. The genomic landscape of breast cancer brain metastases: A systematic review. Lancet Oncol. 2021, 22, e7–e17. [Google Scholar] [CrossRef]
- Hulsbergen, A.F.C.; Claes, A.; Kavouridis, V.K.; Ansaripour, A.; Nogarede, C.; Hughes, M.E.; Smith, T.R.; Brastianos, P.K.; Verhoeff, J.J.C.; Lin, N.U.; et al. Subtype switching in breast cancer brain metastases: A multicenter analysis. Neuro-Oncology 2020, 22, 1173–1181. [Google Scholar] [CrossRef]
- Schrijver, W.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low–Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Shih, J.-Y.; Su, W.-C.; Hsia, T.-C.; Tsai, C.-M.; Ou, S.-H.I.; Yu, C.-J.; Chang, G.-C.; Ho, C.-L.; Sequist, L.V.; et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol. 2012, 13, 539–548. [Google Scholar] [CrossRef]
- Montemurro, F.; Delaloge, S.; Barrios, C.; Wuerstlein, R.; Anton, A.; Brain, E.; Hatschek, T.; Kelly, C.M.; Peña-Murillo, C.; Yilmaz, M.; et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann. Oncol. 2020, 31, 1350–1358. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Ferraro, G.B.; Kodack, D.P.; Badeaux, M.; Shankaraiah, R.C.; Seano, G.; Kloepper, J.; Vardam, T.; Martin, J.D.; Naxerova, K.; et al. Preclinical Efficacy of Ado-trastuzumab Emtansine in the Brain Microen-vironment. J. Natl. Cancer Inst. 2016, 108, djv313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberst, L.; Bailleux, C.; Bachelot, T. Prevention of brain metastases in human epidermal growth factor receptor 2-positive breast cancer. Curr. Opin. Oncol. 2020, 32, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Nader-Marta, G.; Martins-Branco, D.; de Azambuja, E. How we treat patients with metastatic HER2-positive breast cancer. ESMO Open 2022, 7, 100343. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, T.; Cameron, D.; Palmieri, C. Central nervous system disease in phase III studies for advanced HER2 positive breast cancer: A review. Breast 2022, 63, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, R.K.; Siddiqui, J.A.; Mahapatra, S.; Batra, S.K.; Nasser, M.W. microRNAs Orchestrate Pathophysiology of Breast Cancer Brain Metastasis: Advances in Therapy. Mol. Cancer 2020, 19, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, B.; Burugu, S.; Leung, S.; Gao, N.; Virk, S.; Kos, Z.; Parulekar, W.R.; Shepherd, L.; Gelmon, K.A.; et al. Role of Cytotoxic Tumor-Infiltrating Lymphocytes in Predicting Outcomes in Metastatic HER2-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2017, 3, e172085. [Google Scholar] [CrossRef]

| Study | Author. Published Time | Phase | Regimen (No. of Patients) | Median PFS for BCBM (HR, 95% CI) | Median OS for BCBM (HR, 95% CI) | |
|---|---|---|---|---|---|---|
| Interventional Group | Control Group | |||||
| WJOG6110B/ELTOP [22] | Toshimi Takano et al., 2018.08 | II | Lapatinib + Capecitabine (6) | Trastuzumab + Capecitabine (7) | NA vs. NA 0.62 (0.18–2.17) | - |
| LUX-Breast 3 (a) * [23] | Javier Cortés et al., 2015.12 | II | Afatinib alone (40) | Physician’s Choice ** (43) | 11.9 w vs. 18.4 w 1.18 (0.72–1.93) | 57.7 w vs. 52.1 w 1.27 (0.72–2.21) |
| LUX-Breast 3 (b) * [23] | Javier Cortés et al., 2015.12 | II | Afatinib + Vinorelbine (38) | Physician’s Choice ** (43) | 12.3 w vs. 18.4 w 0.94 (0.57–1.54) | 37.3 w vs. 52.1 w 1.60 (0.93–2.76) |
| LUX-Breast 1 [24] | Nadia Harbeck et al., 2016.03 | III | Afatinib + Vinorelbine (43) | Trastuzumab + Vinorelbine (17) | NA vs. NA 1.32 (0.67–2.59) | - |
| NALA [25,26] | Cristina Saura et al., 2020.09/2021.08 | III | Neratinib + Capecitabine (51) | Lapatinib + Capecitabine (50) | 7.8 m vs. 5.5 m 0.66 (0.41–1.05) | 16.4 m vs. 15.4 m 0.90 (0.59–1.38) |
| HER2CLIMB [27,28,29] | Nancy U. Lin et al., 2020.02/2020.08/2022.03 | III | Tucatinib + Trastuzumab + Capecitabine (198) | Trastuzumab + Capecitabine (93) | 7.6 m vs. 5.4 m 0.48 (0.34–0.69) | NA vs. NA 0.60 (0.44–0.81) |
| TH3RESA [30] | Ian E Krop et al., 2014.06/2017.06 | III | T-DM1 (40) | Physician’s Choice ** (27) | 5.8 m vs. 2.9 m 0.47 (0.2–0.89) | 17.3 m vs. 12.6 m 0.62 (0.34–1.13) |
| EMILIA [17,31] | Sunil Verma et al., 2012.12/2015.01 | III | T-DM1 (45) | Lapatinib + Capecitabine (50) | 5.9 m vs. 5.7 m 1 (0.54–1.84) | 26.8 m vs. 12.9 m 0.38 (0.18–0.80) |
| DESTINY-Breast03 [16] | Javier Cortés et al., 2022.03 | III | T-Dxd (62) | T-DM1 (52) | 15.0 m vs. 5.7 m 0.38 (0.23–0.64) | - |
| Study | Author. Published Time | Phase | Regimen (No. of Patients) | Incidence of CNS Disease Progression | Baseline Characteristic | ||
|---|---|---|---|---|---|---|---|
| Interventional Group | Control Group | Interventional Group | Control Group | ||||
| NCT00078572 [32] | Charles E. Geyer et al., 2006.12 | III | Lapatinib + Capecitabine (163) | Capecitabine (161) | 4/163 | 11/161 | Non- or stable BCBM * |
| EGF100151 [33] | David Cameron et al., 2008.12 | III | Lapatinib + Capecitabine (198) | Capecitabine (201) | 4/198 | 13/201 | Non- or stableBCBM * |
| CEREBEL [34] | Xavier Pivot et al., 2015.05 | III | Lapatinib + Capecitabine (251) | Trastuzumab + Capecitabine (250) | 17/251 | 15/250 | No BCBM |
| WJOG6110B/ELTOP [22] | Toshimi Takano et al., 2018.08 | II | Lapatinib + Capecitabine (43) | Trastuzumab + Capecitabine (43) | 2/43 | 2/43 | Non- or stable BCBM * |
| NCIC CTG MA.31 [35] | Karen A. Gelmon et al., 2015.05 | III | Lapatinib + Taxane (242) | Trastuzumab + Taxane (219) | 40/270 | 48/267 | No BCBM |
| LUX-Breast 1 [24] | Nadia Harbeck et al., 2016.03 | III | Afatinib + Vinorelbine (339) | Trastuzumab + Vinorelbine (169) | 30/339 | 19/169 | Non- or stable BCBM * |
| NCT00777101 [36] | Miguel Martin et al., 2013.12 | II | Neratinib (117) | Lapatinib + Capecitabine (116) | 11/117 | 15/116 | Non- or stable BCBM * |
| NEfERT-T [37] | Ahmad Awada et al., 2016.12 | II | Neratinib + Paclitaxel (242) | Trastuzumab + Paclitaxel (237) | 20/242 | 41/237 | Non- or stable BCBM * |
| NALA [25] | Cristina Saura et al., 2020.09/2021.08 | III | Neratinib + Capecitabine (307) | Lapatinib + Capecitabine (314) | 70/307 | 92/314 | Non- or stable BCBM * |
| PHOEBE [38] | Binghe Xu et al., 2021.03 | III | Pyrotinib + Capecitabine (134) | Lapatinib + Capecitabine (132) | 3/134 | 3/132 | No BCBM |
| EMILIA [17,31] | Sunil Verma et al., 2012.12/2015.01 | III | T-DM1 (495) | Lapatinib + Capecitabine (496) | 19/495 | 11/496 | Non- or stable BCBM * |
| NCT01702558 [39] | Javier Cortés et al., 2020.08 | I/II | T-DM1 + Capecitabine (81) | T-DM1 (80) | 3/81 | 7/80 | Non- or stable BCBM * |
| PC | 1.17 (0.39, 3.57) | 0.43 (0.14, 1.33) | 0.17 (0.03, 0.82) | 0.93 (0.33, 2.63) | 0.28 (0.05, 1.66) | 0.43 (0.11, 1.71) | 0.70 (0.19, 2.69) | 0.34 (0.06, 1.89) |
| 0.80 (0.21, 2.96) | Afatinib | 0.37 (0.08, 1.80) | 0.14 (0.02, 1.00) | 0.80 (0.17, 3.62) | 0.24 (0.03, 1.94) | 0.37 (0.07, 2.08) | 0.60 (0.11, 3.43) | 0.29 (0.04, 2.25) |
| 1.60 (0.43, 6.09) | 2.03 (0.32, 13.41) | T-DM1 | 0.39 (0.12, 1.20) | 2.18 (0.57, 8.32) | 0.65 (0.14, 3.10) | 1.01 (0.35, 2.95) | 1.64 (0.39, 6.79) | 0.79 (0.13, 4.76) |
| - | - | - | T-DXd | 5.68 (0.93, 31.89) | 1.71 (0.24, 11.92) | 2.61 (0.54, 12.95) | 4.25 (0.68, 26.56) | 2.05 (0.24, 17.40) |
| 0.62 (0.17, 2.33) | 0.79 (0.12, 5.04) | 0.38 (0.06, 2.41) | - | Afatinib + CT | 0.30 (0.05, 1.86) | 0.46 (0.12, 1.90) | 0.76 (0.25, 2.20) | 0.37 (0.08, 1.62) |
| 0.68 (0.07, 6.89) | 0.85 (0.06, 12.35) | 0.42 (0.06, 2.76) | - | 1.08 (0.08, 15.65) | Neratinib + CT | 1.54 (0.49, 4.75) | 2.49 (0.44, 13.56) | 1.21 (0.16, 9.05) |
| 0.61 (0.09, 4.01) | 0.77 (0.08, 7.64) | 0.38 (0.09, 1.48) | - | 0.98 (0.10, 10.16) | 0.91 (0.25, 3.12) | Lapatinib + CT | 1.63 (0.45, 5.81) | 0.79 (0.15, 4.12) |
| - | - | - | - | - | - | - | Tra + CT | 0.49 (0.16, 1.46) |
| - | - | - | - | - | - | - | - | Tucatinib + Tra + CT |
| (a) PFS | |||
|---|---|---|---|
| Interventions | SUCRA Values (%) | ||
| T-DXd | 91.45 | ||
| Neratinib + CT | 76.12 | ||
| Tucatinib + Tra + CT | 69.59 | ||
| T-DM1 | 58.89 | ||
| Lapatinib + CT | 57.33 | ||
| Tra + CT | 36.04 | ||
| Afatinib + CT | 23.56 | ||
| PC | 20.71 | ||
| Afatinib | 16.31 | ||
| (b) OS | |||
| Interventions | SUCRA Values (%) | ||
| T-DM1 | 86.14 | ||
| PC | 61.50 | ||
| Afatinib | 46.32 | ||
| Neratinib + CT | 40.49 | ||
| Afatinib + CT | 33.04 | ||
| Lapatinib + CT | 32.51 | ||
| (c) Recurrent rate of CNS | |||
| Interventions | SUCRA Values (%) | ||
| All patients | No Baseline BCBM | Baseline Non- or Stable BCBM | |
| Neratinib + CT | 80.15 | - | 77.70 |
| Neratinib | 71.00 | - | 72.61 |
| T-DM1 + CT | 68.50 | - | 69.45 |
| Afatinib + CT | 56.49 | - | 50.57 |
| Lapatinib + CT | 52.79 | 67.77 | 56.16 |
| Pyrotinib + CT | 52.72 | 62.03 | - |
| Tra + CT | 38.75 | 58.11 | 35.51 |
| T-DM1 | 23.41 | 12.08 | 28.60 |
| CT | 6.19 | - | 9.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, H.; Han, Y.; Wu, Y.; Wang, J. Comparative Efficacy of Tyrosine Kinase Inhibitors and Antibody–Drug Conjugates in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A Systematic Review and Network Meta-Analysis. Cancers 2022, 14, 3372. https://doi.org/10.3390/cancers14143372
Wang Y, Xu H, Han Y, Wu Y, Wang J. Comparative Efficacy of Tyrosine Kinase Inhibitors and Antibody–Drug Conjugates in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A Systematic Review and Network Meta-Analysis. Cancers. 2022; 14(14):3372. https://doi.org/10.3390/cancers14143372
Chicago/Turabian StyleWang, Yan, Hangcheng Xu, Yiqun Han, Yun Wu, and Jiayu Wang. 2022. "Comparative Efficacy of Tyrosine Kinase Inhibitors and Antibody–Drug Conjugates in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A Systematic Review and Network Meta-Analysis" Cancers 14, no. 14: 3372. https://doi.org/10.3390/cancers14143372
APA StyleWang, Y., Xu, H., Han, Y., Wu, Y., & Wang, J. (2022). Comparative Efficacy of Tyrosine Kinase Inhibitors and Antibody–Drug Conjugates in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A Systematic Review and Network Meta-Analysis. Cancers, 14(14), 3372. https://doi.org/10.3390/cancers14143372






